Fisheries and Aquaculture Journal
Open Access
ISSN: 2150-3508
ISSN: 2150-3508
Research Article - (2020)Volume 11, Issue 3
The effect of water physico-chemical characteristics on Myxobolus tilapiae loads and prevalence was investigated from May 2016 to May 2017 in 350 specimens of Oreochromis niloticus captured in MAPE dam, Adamawa region of Cameroon. The spores load and the fish condition factor were negatively correlated (r=-0.37; P<0.05). No significant (P>0.05) correlation was observed between the prevalence of Myxobolus tilapiae and water temperature, pH and nitrate concentration. However, the prevalence was significantly and positively correlated with the carbonate hardness (r= +0.14; P<0.001), and negatively correlated (r=-0.17; P<0.001) with water transparency. The optimum characteristics of water regarding transparency and carbonate hardness for reduced M. tilapiae infection and O. niloticus productivity need to be determined.
Myxobolus tilapiae; Water physico-chemical characteristics; Oreochromis niloticus; MAPE dam; Cameroon
Fish represents nearly 51% of animal proteins intake in Africa [1]. Unfortunately, fish production is impeded by many challenges, some of which are the deleterious effects of pathogens particularly Myxosporeans [2]. The world of Myxosporeans fauna is composed of about 2180 species gathered within 62 genera among which the genus Myxobolus Bütschli, 1882 is the most abundant with 792 species [2]. Myxobolus tilapiae is one of the Myxobolus species infecting various organs in O. niloticus.
In a given habitat, factors that influence the fish parasites fauna are both endogenous and exogenous [3]. Among the exogenous factors, water physico-chemical characteristics are important because its modification can disrupt the host - parasite equilibrium. As a result, fish not only stress but water can become more conducible to epizootics leading to massive fish deaths and important economic losses [4]. Thus, a better understanding and adequate monitoring of water physicochemical characteristics can help interrupting Myxobolus tilapiae life cycle since effective drugs against Myxosporeans are unavailable [5]. The study aimed at assessing the correlation between Myxobolus tilapiae loads as well as prevalence and the water physico-chemical characteristics in Oreochromis niloticus.
Study area
Fish were sampled in MAPE dam, Bankim subdivision (6°00’- 6°20’ NL/11°20’-11°40’ EL), Mayo-Banyo Division, Region of Adamawa – Cameroon (Figure 1). The average altitude is about 724m and the soil is a mixture of clay and sand. The climate is of tropical Sudano-Guinean type with two seasons: a long rainy season running from March to November and a short dry season from November to March. The annual average temperature is about 23°C and the rainfall varies between 1500 mm and 2000 mm [6].
Figure 1: Cameroon map showing the study area.
Fish and water sampling
A total of 350 fish specimens of Oreochromis niloticus (Figure 2) was harvested monthly from fishermen during the study period from May 2016 to May 2017. They were captured both at day and night using fish nets and fishing canes. Fish specimens were immediately stored at 10% formalin solution and transported to the laboratory for examination. During field collection of fish, water was equally sampled and analyzed in situ thrice a month between 8 and 9: am according to Rodier et al. [7]. The water physico-chemical characteristics as well as quantification materials are summarized in Table 1.
Figure 2: Photograph of host specimens (bar: 13 cm).
Table 1: Water physico-chemical characteristics quantification and materials.
| Physico-chemical characteristics | Units | Materials |
|---|---|---|
| Temperature | °C | Mercury thermometer |
| Transparency | cm | Secchi disc (30cm diameter) |
| pH | - | Portable pH- meter |
| Carbonate hardness | mg /l | Test strips |
| Nitrates | mg/l | Test strips |
Identification of Myxosporeans
Fish were identified as described by Stiassny et al. [8] and examined according to Abakar [9]. Thus, standard and total lengths were measured to the closest millimeter using a slide caliper of stainless brand. Fish were weighed using Sartorius electronic scale of 0.01 g accuracy and were sex-determined after dissection. The condition factor (K) was calculated using the following formula by Charles and Alan [10]:
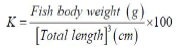
The condition factor expresses the health status or the wellbeing of fish at a given time [11]. As the animal grows, its condition factor or welfare increases [12]. The fish’s heath is good if k>1 and poor when k<1 [13]. The condition factor varies according to fish age, sex, season, the stage of development of the reproductive organs, the fullness of gut, type of food consumed, amount of fat reserve and degree of muscular development [10].
External organs (fins, skin, scales and eyes) and internal organs (gills, spleen, kidneys, intestines, gall bladder, stomach and gonads) were examined with naked eyes, and then with stereoscopic microscope using the 10X lens to look for the cysts. As for kidneys, spleen and gonads, three smears were made per organ (anterior, medium and posterior regions) and examined at a total magnification of 1000X with a light microscope for spores. For each smear, spores were counted in 40 microscope fields. Cysts were crushed between slide and cover glass in a drop of distilled water and their contents were identified with the light microscope using 100X lens. Spores were fixed using methanol, stained with May-Grünwald-Giemsa and snapped with digital camera (Canon Ixus brand). Species were identified according to Lom and Arthur (1989) [14].
Parasitological parameters studied
The prevalence (Pr) of infection expressed in percentage was monthly determined and defined as the number of host species infected by Myxobolus tilapiae during a given sampling month divided by the number examined [15]. The parasite load (spores or cysts load) was the sum total of spores or cysts harbored by the host during a given month of sampling.
Statistical analysis
The comparison of prevalence was performed using the Chisquare (X2) test while the Spearman correlation coefficient was determined to look for a possible relationship between the parasitological parameters and the water physico-chemical characteristics. The error probability for analysis was P<0.05.
Results are illustrated in Figures 3-4 and Tables 2-3.
Figure 3: Myxobolus tilapiae spore and cyst micrographs. A: Myxobolus tilapiae stained spore (x1200) B: Myxobolus tilapiae cyst (white spot) imbedded in the gill filaments (x10).
Figure 4: Regression line of O. niloticus condition factor (K) as a function of Myxobolus tilapiae spores load (x).
Table 2: Monthly variation of water physico-chemical characteristics presented as mean ± standard deviation.
| Months | pH | Temperature (°C) | Transparency (cm) | Nitrates (mg/l) | Carbonate hardness (mg/l) |
|---|---|---|---|---|---|
| May-16 | 7.4 ± 0.1 | 22.0 ± 1.5 | 151.0 ± 2.3 | 10.2 ± 1.5 | 107.4 ± 8.9 |
| Jun-16 | 8.2 ± 0.0 | 24.0 ± 2.2 | 149.0 ± 1.4 | 10.0 ± 0.5 | 110.9 ± 3.6 |
| Jul-16 | 8.0 ± 0.6 | 21.0 ± 0.6 | 153.0 ± 5.7 | 10.0 ± 1.1 | 116.3 ± 7.2 |
| Aug-16 | 8.9 ± 0.8 | 20.0 ± 1.6 | 148.0 ± 0.0 | 10.1 ± 0.9 | 80.5 ± 0.1 |
| Sep-16 | 6.7 ± 0.4 | 23.0 ± 4.0 | 145.0 ± 7.1 | 7.5 ± 3.5 | 107.4 ± 0.0 |
| Oct-16 | 6.4 ± 0. 2 | 27.0 ± 3.2 | 150.0 ± 1.2 | 5.0 ± 0.2 | 107.4 ± 7.2 |
| Nov-16 | 6.6 ± 0.5 | 26.0 ± 1.8 | 185.0 ± 0.0 | 7.0 ± 1.5 | 143.2 ± 0 .0 |
| Dec-16 | 6.2 ± 0.2 | 24.7 ± 1.9 | 146.0 ± 1.5 | 8.3 ± 2.9 | 89.5 ± 16.1 |
| Jan-17 | 6.3 ± 0.2 | 22.0 ± 0.9 | 151.0 ± 2.1 | 10.0 ± 2.3 | 80.5 ± 10.7 |
| Feb-17 | 6.6 ± 0.4 | 25.5 ± 2.5 | 160.0 ± 3.6 | 17.5 ± 3.5 | 53.7 ± 0.0 |
| Mar-17 | 6.1 ± 0.0 | 25.5 ± 1.2 | 148.0 ± 1.3 | 5.0 ± 0.5 | 44.75 ± 3.6 |
| Apr-17 | 6.5 ± 0.6 | 23.0 ± 0.3 | 150.0 ± 1.0 | 6.0 ± 1.2 | 71.6 ± 5.37 |
| May-17 | 6.3 ± 0.7 | 24.0 ± 1.4 | 158.0 ± 2.5 | 7.5 ± 1.6 | 53.7 ± 1.8 |
Table 3: Correlation between the physico-chemical characteristics of water and Myxobolus tilapiae loads.
| Physico-chemical characteristics of water | |||||
|---|---|---|---|---|---|
| pH | Temperature (°C) | Transparency (cm) | Nitrates (mg/l) | Carbonate hardness (mg/l) | |
| r1 | -0.06 | -0.09 | -0.29 | 0.02 | -0.03 |
| P | 0.734 | 0.654 | 0.142 | 0.909 | 0.881 |
| r2 | 0.03 | -0.01 | -0.08 | 0 .10 | 0.03 |
| P | 0.723 | 0.942 | 0.437 | 0.322 | 0.8 |
r1 and r2: correlation coefficients relevant to cyst and spore loads respectively; P: error probability
Monthly variation of water physico-chemical characteristics
The Monthly variation of water physico-chemical characteristics is summarized in Table 2.
Correlation between spores load and O. niloticus condition factor
The effect of Myxobolus tilapiae on O. niloticus health could be evaluated by calculating the correlation coefficient between the parasite load and the fish condition factor (K). The spores (Figure 3) load of Myxobolus tilapiae was negatively correlated (r=-0.37; P<0.05) with O. niloticus condition factor. The regression equation line (Figure 4) according to the arbitrary adopted linear model was K=-0.03x+2.07 Where K and x stood for the condition factor and spores load respectively.
Correlation between the prevalence, parasite load and water physico-chemical characteristics
The prevalence varied with the physico-chemical characteristics of water but no significant correlation was noticed (P>0.05) with the temperature, pH and nitrates concentration. However, the prevalence was significantly and positively correlated with the carbonate hardness (r=+0.14; P<0.001), and negatively correlated (r=-0.17; P<0.001) with water transparency. The correlation coefficients between the physico-chemical characteristics of water and parasite loads (Table 3) were too low and insignificant (P>0.05).
The fluctuation of the water physico-chemical characteristics throughout the study period may be due to the fact that water was sampled during different seasons. In fact, the seasonal impact on these water characteristics is mostly due to variation in the weather characteristics (rain, sunshine regimen, temperature) which also affect the characteristics of water. The prevalence of Myxobolus tilapiae was significantly and negatively correlated (r=-0.17) with the transparency and positively correlated (r=+0.14) with carbonate hardness. The increase in the carbonate hardness probably affects the biology of oligochaetes (definitive hosts of M. tilapiae ) by speeding up its proliferation in the water. The carbonates may equally encourage rapid planktons and algae growth. As spores are known to stick on planktons and algae, fish therefore easily get infected when feeding on these planktons and algae [9]. Probably the increase in water transparency reduces the extrusion of the polar capsule filament of the spore, thus prevents the parasite to anchor to the host tissue.
Nounagnon et al. [16] studied the correlations between the prevalence of gill’s myxosporeans in Tilapia zillii and Sarotherodon melanotheron melanotheron from Lake Nokoué (Bénin). They observed that the prevalence of Myxobolus dossoui, Myxobolus zillii and Myxobolus beninnensis was not correlated with pH, salinity and water temperature. These results are in accordance with our own work. Anu [17] revealed that in aquaculture in Punja-India, the prevalence of Myxosporean infections significantly increased with the water temperature
Our result also agrees with the observations of Siau [18] in Tunisia who thought that myxosporean prevalence depends mainly on the host species, its size and origin and not on water pH and temperature. Martins et al. [19] showed that in fish ponds, water quality did not interfere with the prevalence of myxosporean parasites. The spores load of Myxobolus tilapiae was negatively correlated (r=-0.37; P<0.05) with O. niloticus condition factor showing that the higher the spores load, the less healthy were fish. Myxobolus tilapiae depressed the host health probably by destroying vital organs such as gills, kidneys, spleen and liver [20].
The prevalence of Myxobolus tilapiae was significantly and positively correlated with the carbonate hardness while negatively correlated with water transparency. Spores load was negatively correlated with the fish condition factor. Thus, M. tilapiae probably depressed O. niloticus health and its appearance in ponds should be controlled. It is necessary to look for an adequate monitoring of those water physico-chemical characteristics in order to interrupt the Myxobolus tilapiae life cycle.
Citation: Fonkwa G, Kouam KM, Tchuinkam T, Tomedi EM, Tchoumboue. (2020) Effect of water physico-chemical characteristics on Myxobolus tilapiae, a Myxosporean parasite of the fish Oreochromis niloticus at MAPE dam in Adamawa region of Cameroon. Fish Aqua J 11:278. doi: 10.35248/2150-3508.20.11.278
Received: 27-Apr-2020 Accepted: 24-Jul-2020 Published: 31-Jul-2020 , DOI: 10.35248/2150-3508.20.11.278
Copyright: © 2020 Fonkwa G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.