Journal of Pollution Effects & Control
Open Access
ISSN: 2375-4397
ISSN: 2375-4397
Research Article - (2019)Volume 7, Issue 3
Nanoparticles (Np), as single particles with diameters smaller than 100 nm, represent a subgroup of nanomaterials. Their production and consumption have exponentially increased in recent years since they are used in the consumer products by different industrial sectors, for example, agriculture, construction, cosmetic, food and medicine. Nevertheless, researches on contamination caused by nanoproducts in aquatic environments are still scarce. Titanium dioxide (TiO2) has been used in various cosmetic applications due its ability to absorb UV light. Np-TiO2 in sunscreens has the same composition as the larger white TiO2 particles, but in nanoscale TiO2 is transparent, being more cosmetically elegant. The aim of this work was to evaluate the effect of TiO2 nanoparticles (TiO2- Np) on microalgae Chattonella subsalsa exposed to different concentrations of a sunscreen formulation. C. subsalsa was exposed for 30 minutes and 96 hours to 10, 25 and 50 mgL-1 of TiO2-Np under visible light and analyzed by photographic records, cell counting. The oxidative stress was monitored by specific activity of catalase. In this study, C. subsalsa did not form cell clusters but showed significant increase in catalase production after 96 hours of treatment at concentrations higher than 25 mgL-1. Such observations suggest higher resistance of these microalgae to the exposure to the sunscreen formulation based on TiO2-Np when compared to other algae already described in the literature.
TiO2; Nanoparticles; Sunscreens; Chattonella subsalsa; Guanabara Bay
The release of chemical substances in the environment is one of the most alarming contemporary problems. Many of these products cause effects on the atmosphere by reducing natural protections or generate toxic effects on microorganisms that are essential for environmental balance, especially those at the base of the food chain.
Although ultraviolet (UV) radiation is important for vitamin D synthesis, excessive exposure to radiation can cause a variety of harmful effects on unprotected skin, which undergoes a number of chemical and morphological reactions, such as the development of squamous cell carcinomas and premature aging [1].
Due to the recognition of the harmful effects that UV radiation causes to human health, researchers have sought to develop products that protect the human body from excess UV radiation, such as photo protective products. Titanium dioxide (TiO2) belongs to the class of inorganic or physical active ingredient in sunscreens, able to reflect and disperse visible and UV radiation. Nonetheless, from the point of view of cosmetic science, sunscreens might be inconvenient since the formulations based on TiO2 tend to be opaque on the skin [2].
Nanoparticles based on TiO2 (TiO2-Np) are used in a wide range of products, such as sunscreens, paints, paper, plastics and food [2]. When the photo protectors based on nanometric forms of TiO2 were produced in the 90s [3,4] it was possible to reduce the particle size up to 10-50 nm, corresponding to 50-90% of its original size. TiO2-Np in several sunscreens formulations reflects UV radiation in a more efficient way when they are between 60- 120 nm in diameter [5,6]. As result, the TiO2-Np sunscreens are less opaque [7] and maintain the protection against UV radiation [2]. Thus, this allowed the development of more stable and safer formulations, making them more accepted by consumers.
Recent reports suggest a direct risk to human health through penetration of TiO2-Np across the skin and its distribution throughout the body by the circulatory system [8], demonstrating the cytotoxic and genotoxic potential of TiO2-Np in human skin cells [9] and in fibroblasts [10]. In addition, TiO2 was reported to cause chronic pulmonary inflammation in rats and to induce a pro-inflammatory effect in cultured human endothelial cells [11].
In Brazil, there is still no legal regulation on the industrial application of TiO2-Np. The use of such nanoparticles in cosmetics is allowed by Brazilian regulatory agencies without considering the risks of these materials. Despite several studies demonstrating toxic effects of products based on TiO2-Np, the European Commission (EC) allows the manufacturing of sunscreens containing TiO2- Np [12]. In accordance with Regulation 2016/621 that regulates the cosmetic products, both zinc oxide and titanic dioxide are authorized except for in products subject to inhalation by the end user (due to the possible risk to the lungs).
Nevertheless, the consumption of Np-products such as sunscreens in recreation and day-to-day activity results in their release in aquatic environments. In this sense, several questions about the environmental impacts associated with the large scale production and quotidian use of TiO2-Np remain unanswered [13]. Moreover, reports showing hazardous effects of sunscreens containing TiO2- Np to environmental health are scarce. For Jeon and co-workers, about 40% of the initial concentration of Np in the sunscreens is released in water [14]. Mueller and Nowack found values around 0.7-16 μgL-1 of TiO2-Np in water, being higher than those values of Predicted No Effect Concentration (PNEC) [15].
Adams and co-workers suggested that the smaller particles are probably more toxic due to their larger relative surface, resulting in higher bioavailability [16]. Evidences of toxic effects of manufactured nanoparticles have been described in the literature. For Hund-Rinke and Simon, microbial membranes are significantly compromised, even under environmentally-predicted concentrations of TiO2-Np for surface water [17]. In algae, Abe and co-workers showed that TiO2-Np induced several effects, highlighting their cell agglomeration [13].
Regarding the intracellular generation of reactive oxygen species (ROS), TiO2-Np generates harmful effects and high sensitivity on microbial communities, with unknown implications for ecosystem functioning and health. In Daphnia magna, a planktonic crustacean, long-term exposure to TiO2-Np causes growth retardation and mortality [18]. In addition, severe oxidative effects were observed in the zebra fish liver after treatment with TiO2-Np at 50 mgL-1 [19]. The toxic potential is usually attributed to the photo catalytic properties of TiO2-Np, inducing the generation of ROS in the presence of UV light [20].
The impact of TiO2-Np on phytoplankton communities is a used parameter to better understand the interaction between Np and the environment. In this context, this study aimed to evaluate the effects of different doses of a sunscreen formulation based on TiO2- Np on Chattonella subsalsa a significant species of phytoplankton population from Guanabara Bay (Rio de Janeiro, Brazil).
C. subsalsa belongs to the class Raphidophyceae, has an elliptical shape, brownish colour and slightly flattened morphology presenting two flagella, with about 20 μm [21]. This alga shows a large number of chloroplasts (at least six) in the peripheral layer of their cell bodies [22]. Moreover, algal bloom of the genus Chattonella sp. is consistently reported on events of death of fish populations, phenomenon intensified by eutrophication processes [23]. In order to increase the knowledge about the toxicity of Np- TiO2, the cell growth, the cluster formation and the measurement of catalase activity were evaluated.
Cell isolation and cell culture: Non-axenic cultures of microalgae Chattonella subsalsa were obtained from Guanabara Bay (22° 50’40’’S 43° 12’40’’W), Rio de Janeiro, Brazil, in December, 2015. Cells were cultivated in marine Guillard F/2 media (35% 0 salinity) made with filtered (0.22 mm) natural seawater, being autoclaved prior to inoculation [24]. The algae were maintained in a germinating camera (BOD incubator with photoperiod- Electrolux Cienlab, BR) with 100 mE/m2/s irradiance at 22°C. The photoperiod to which the algae were submitted was 16 hours light, 8 hours dark. Cells (the initial number of 2.6*104/sample), at exponential growth were exposed to various concentrations of TiO2-Np under cultivation conditions. The assays were performed in triplicate.
Experimental procedures: C. subsalsa, a representative species of phytoplankton from Brazilian coast, was exposed for 30 minutes (acute effect) and 96 hours (chronic effect) to 10, 25 and 50 mgL- 1 of TiO2-Np (Eusolex T-aqua ®) diluted in F/2 medium under visible light.
After the treatments, the samples were photographed at a determined distance (5.5cm), using a digital still camera (Sony DSC-W350D, USA).
TiO2-Np morphology: The morphology was assessed through transmission electron microscopy (TEM-Morgagni 268 D, FEI, USA) (Figure 1).
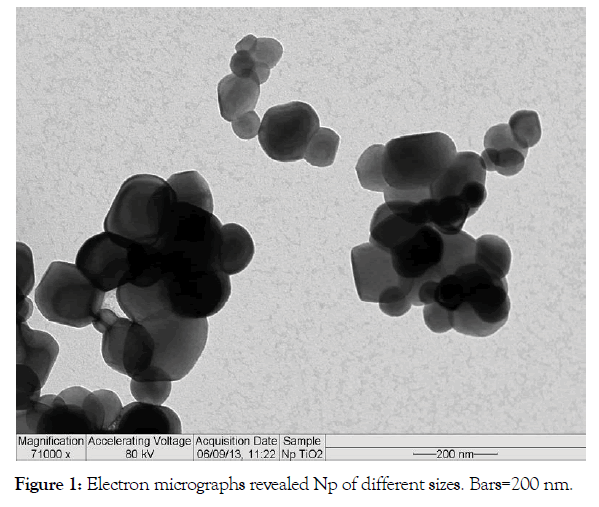
Figure 1: Electron micrographs revealed Np of different sizes. Bars=200 nm.
Catalase (CAT) activity: After treatment, the samples were sonicated (Sonicator Ultra Cleaner 1600A, Unique, BR) at the potency 4, three times for 10 seconds, with a two-second interval between the sonications. The samples were then incubated in a microplate with hydrogen peroxide (H2O2) diluted in phosphate buffer (PBS) (0.16%) at a proportion of 3 μL of sample/97 μL of the peroxide solution. CAT activity was evaluated spectrophotometrically (Flex 3 station, using the software Softmax 5) by measuring the consumption of H2O2 at 240 nm; 0, 30 and 60 seconds after incubation. The mensuration of CAT activity was made using the extinction coefficient 0.0436 mM-1 cm-1 [25]. The results were expressed as U/mg protein.
Statistical analysis: All methods described were performed in triplicate and statistically analyzed in the software Graph Pad Prism (version 4), using non-parametric one-way ANOVA tests with Turkey post-tests.
Flocculation effect
Several reports of flocculation phenomena are found in planktonic organisms exposed to TiO2-Np during different periods of exposure [13,26,27]. Surprisingly, C. subsalsa algae were not trapped in cell clusters (Figure 2), even after the 30 minutes treatment with the highest concentrations (50 mgL-1) of the TiO2-Np formulation.
Figure 2:Macroscopic view of the cell after 30 minutes of the interaction of Chattonella subsalsa submitted to the sunscreen formulation based on TiO2-Np.
Cell counting
Cell counting was performed to evaluate cell growth after 30 minutes and 96 hours of exposure to the TiO2-Np formulation.
After 30 minutes of treatment, C. subsalsa did not show a significant reduction of the number of cells at any of the concentrations (Figure 3A). On the other hand, after 96 hours, the number of cells was reduced 11.6 times in comparison to control cells at 10 mgL-1 TiO2-Np, 16.3 times at 25 mgL-1and 28.1 times at the maximum concentration tested (50 mgL-1). The growth inhibition was observed at concentrations of 10, 25 and 50 mgL-1 of TiO2-Np when compared to the control, having EC50 of 87.8 mgL-1 (Figure 3B).
Figure 3:Effect of TiO2-Np on microalgae, after 30 minutes (A) and 96 hours (B) At 30 minutes, no significant decrease in the cells was observed, while at 96 hours, 25 mg.L-1 of TiO2-Np was enough to induce a significant reduction in the number of cells (p>0.05).
Catalase production
The catalase activity did not show significant changes after 30 minutes of interaction with TiO2-Np (Figure 4A). However, after 96 hours, the catalase levels increased significantly at both 25 and 50 mgL-1 of TiO2-Np (Figure 4B).
Figure 4:Measurement of the catalase activity. At 30 minutes (A) the amount of catalase showed no significant change. At 96 hours (B) the catalase activity increased at 25 and 50 mg.L-1 of TiO2-Np in comparison with control cells.
In contrast to Tetratelmis sp. [13], the flocculation phenomenon was not detected in C. subsalsa after treatment with TiO2-Np. On the other hand, at 10 mgL-1 of TiO2-Np, the alga swimming was seemingly reduced (data not shown). For Daphnia Magna, swimming was significantly modified after 72 hours at doses greater than 0.1 mgL-1 of TiO2-Np, revealing a gradual increase in toxicity with 100% immobilization and 90% mortality at 500 mgL-1 of TiO2-Np [18].
Considering the number of free C. subsalsa, no significant reduction in the number of cells was detected after 30 minute treatment with the formulation containing TiO2-Np. This result was expected since C. subsalsa do not form cell clusters, but they exhibited significant growth after 96 hour treatment with TiO2-Np. However, the number of cells was modestly increased at concentrations higher than 10 mgL-1 when compared to control. Previous studies noticed that after treatment with TiO2-Np occurred inhibition of cell growth and lethality with high variability of EC50 and LC50, respectively [28-30]. In D. magna, treatment with TiO2-Np revealed that LC50 varies between 5.5 mgL-1 and 20.000 mgL-1[29]. However, the correlation between the primary size of nanoparticles and the obtained effect are not clear [30].
The results showed that cell growth of C. subsalsa reduced when compared to the control, being EC50 of 87.8 mgL-1 of TiO2-Np. For Tetraselmis sp., the growth cell was smaller (EC50 of 26.29 mgL- 1) [13], indicating that Tetraselmis sp. is more susceptible to TiO2-Np when compared to C. salsa. Hund-Rinke and Simon pointed out that the growth inhibition of the green alga Desmodemus subspicatus after interaction with TiO2-Np may not be caused by the intrinsic toxicity of Np [17]. Another factor that could affect the cellular susceptibility was the adsorption of nutrients by Np, such as zinc and phosphorus that could limit the availability of the algae, impairing their survival [31].
Several enzymes are involved in reactive oxygen species (ROS) detoxification. An indirect way of analyzing the generation of ROS is the quantification of antioxidant enzymes produced in response to oxidative stress. In this work, the response to ROS generation was analyzed through the production of catalase, which degrades hydrogen peroxide and used as a biomarker for a large group of xenobiotic [32] in fish and other organisms in the monitoring of polluted areas [33]. In C. subsalsa, no significant difference in catalase activity was observed after 30 minutes of the treatment when compared to the control. After 96 hours, a significant increase in the catalase activity at concentrations of 25 and 50 mgL- 1 TiO2-Np was observed. These results are in contrast to Tetraselmis sp., which revealed no significant alteration of the catalase activity [13]. Oxidative stress causes several reactions, such as degradation of amino acids and fats with the production of free radicals and hydrogen peroxides that are highly reactive and harmful to cells [33]. Miller e co-workers showed that TiO2-Np generated an increase in ROS concentration when exposed to solar radiation [34].
In this study, increased levels of catalase as well as cell growth and the absence of cell clusters after the treatments with TiO2-Np suggest that C. subsalsa possess resistance to the formulation with TiO2-Np when compared to Tetraselmis sp., indicating different profiles in the response to Np exposure of both algae from Guanabara Bay.
The low susceptibility of C. subsalsa may be an alert since these algae are related to the phenomenon of the red tide that affects the quality of the seawater, and causes death of marine organisms due to the increase of CO2. This leads to imbalance for aquatic ecosystem.
Although the use of nanoparticles is promising in several areas, including the medical and cosmetic areas, it is possible that new technologies will have unexpected consequences, both helpful and harmful. Thus, studies are necessary to improve the knowledge about the effects of nanoparticles in aquatic environments in order to public awareness of the use of nanotechnologies in addition to regulating products containing nanomaterials, as they may interfere with the ecological balance, indirectly affecting human welfare.
The authors would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Citation: Dias da Cunha RL, Franco da Silva Abe AS, Salomon PS, Gitirana LB (2019) Effect of Titanium Dioxide Nanoparticles on the Microalgae Chattonella subsalsa. J Pollut Eff Cont 7.236.
Received: 11-May-2019 Accepted: 30-Jul-2019 Published: 06-Aug-2019
Copyright: © 2019 Dias da Cunha RL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.