Original Article - (2019)Volume 5, Issue 1
Effect of Temperature, pH, Carbon and Nitrogen Sources on Extracellular Protease Production by Four Geobacillus Species Isolated from Maha Oya Geothermal Springs in Sri Lanka
DMSU Dissanayaka and IVN Rathnayake*Abstract
Thermophilic bacteria are biotechnologically important group of microorganisms due to their capability to produce a variety of thermostable enzymes, such as proteases. The aim of the present study was the optimization of protease production by thermophilic bacteria isolated from hot springs (54°C-55.5°C) in Maha Oya, Sri Lanka. Four isolates of thermophilic bacteria that belong to the genera Geobacillus, which were previously been isolated and identified, were used in the present investigation to study their protease production. Among the four isolates, Geobacillus toebii (DMBUK 107191) reached a maximum protein concentration with a level of 858 μg/mL and Geobacillus kaustophilus (DMBUK 107161) showed maximum protease activity of 2.232 units/mL (55°C, pH 7). Studies on the result of temperature and pH on protease activity discovered that the extreme protease activity was detected at 60°C over a pH range of 6-8. The isolates utilized several carbon and nitrogen sources for the production of proteases. Among the various carbon sources used, bacteria showed maximum protease activity in the presence of sucrose and fructose. Furthermore, bacteria showed a maximum yield of protease activity when gelatin was used as the nitrogen source.
Keywords
Thermophilic bacteria; Protease; Temperature; pH; Carbon source; Nitrogen source.
Introduction
Most of these thermophilic bacteria which are living in hot springs produce enzymes with high thermo stability such as amylase, protease, chitinase, cellulase, lipase etc. These have wider applications in pharmaceutical, food, detergent, leather, diagnostics, waste management and metal recovery industries. Thermostable proteases usually do not get denature at high temperatures; hence it is the superior benefit in industrial application [1]. At the Russian academy of science, a group of Moscow scientists studied the microbial ecology of high temperature oil field in Kazakhstan. They observed the new genus Geobacillus, physiologically and morphologically closely related to the phylogenetic tree of the genus Bacillus [2]. The Geobacillus is a potential source of biotechnological applications [3]. Alpha-arabinofuranosidase (US05434071), Acetate kinase (US05610045), Alpha-amylase (US05824532, US05849549), Arabino furanoside (US05491087), Biological indicator for sterilization (US05073488, US05223401, US05252484, US05418167) are some sections of U.S.A. patents for Geobacillus products or process (Zeigler 2001).
Bacillus stearothermophilus was first revealed in the year 1920 [4]. Then in 2001, it was reclassified under genus Geobacillus bacillus together with thermoglucosidasius [2]. They produce many heat stable enzymes and protease used in artificial aspartame production. Furthermore, it is used in the biotechnological industries to check the progress of sterilization cycles of equipment [5].
Proteases can break peptide bonds and protease assay used to determine the activity of proteases. The strong activation of protease gives through the high released amount of tyrosine from casein as well as proteases withstand at high temperature and also have the capability to hydrolyse numerous peptide bonds due to that protease can be used in various functions [6]. Proteases also offer a valuable target in many therapeutic settings such as Alzheimer's cancer, and viral infection. MMP-9 is a matrix metallopeptidase; it plays a role in angiogenesis and it is a therapeutic target for cancer. Proteases are relevant in drug target class because of their significance in the pathology of disease [7]. Protease enzyme is used in many food industries. In bakery industry, endo and exo-proteases from Aspergillus oryzae are used to modify wheat gluten by proteolysis and are used for the production of bread, baked goods, crackers and waffles [8].
This study aims to report optimization of protease production by selected Geobacillus species isolated from hot springs in Maha Oya. Isolates were studied to investigate their optimum growth parameters of temperature, pH and also to investigate the optimum activity of protease as well as to study the effect of temperature, pH, carbon sources and nitrogen sources for the protease production.
Materials and Methods
Chemicals
All the chemicals, media and medium components used in this study were of analytical grade, obtained from Sigma Chemicals (USA), HiMedia Laboratories (India).
Bacterial cultures
Four bacterial cultures Geobacillus kaustophilus (DMBUK 107161), Geobacillus toebii (DMBUK 107191), Geobacillus stearothermophilus type I (DMBUK 107233) and Geobacillus stearothermophilus type II (DMBUK 107233) were previously isolated, identified and deposited at the Departmental Culture Collection, Department of Microbiology, University of Kelaniya, Sri Lanka and used in the present study. Freeze-dried cultures of Geobacillus species were revived and cells were re-suspended and the content of ampoules was poured into nutrient agar plates. The plates were incubated at 55°C for 48 h. The pure cultures were stored as slant cultures in the refrigerator at 4°C for further investigations.
Estimation of the temperature tolerance of bacteria
Serial dilution technique and colony count method were used in the quantitative analysis of bacteria at various temperatures (30°C-70°C) and pH (6,7,8) respectively [9].
Screening for proteolytic activity
The ability of the bacterial isolates to hydrolyse casein by extracellular caseinases was studied using casein hydrolysis method [10].
Preparation of the crude enzyme using thermophilic bacteria
The isolated thermophilic bacteria were inoculated in protease specific medium broth [11]. The medium was dispensed in 5 mL amounts in test tubes and tubes were sterilized by autoclaving at 121°C for 15-20 min. The sterilized protease specific medium broth was cooled and inoculated with 5% inoculum of 48 h old cultures of the isolate. The cultures were maintained at 55°C at 140 rpm for 48 h in shaking incubator. After the fermentation period, each fermentation broth was centrifuged at 10,000 rpm at 4°C for 15 min. The clear supernatant was used as the crude enzyme preparation.
Estimation of protein concentration
The concentration of protein in the crude cell- free extract was estimated by Lowry’s method [12].
Assay of protease enzyme activity
Protease assay was performed by modified method of Anson with casein as the substrate [13].
Protease assay for test reagents
Test tubes were taken and labelled them all and 5 mL of 0.65% casein solution was added, equilibrated in a water bath at 37°C for 15 min, and 1 mL enzyme solution was added. The test samples were mixed by swirling and incubated at 37°C for exactly 10 min. The protease activity and production of tyrosine started during this incubation time. After incubation, 5 mL of the Trichloroacetic Acid reagent (TCA) was added to each test tube to stop the reaction. Then 1 mL amount of enzyme solution was added to each tube. After all, tubes were mixed by swirling and incubated at 37°C for 30 min. Test samples were filtered through Whatman number 50 filter paper to remove any insoluble matter from the samples. After pipetting out, 2 mL of test filtrate to the new test tube and 5 mL of sodium carbonate reagent was added, followed by 1 mL of Folin ’ s reagent immediately afterward, and then mixed by swirling and incubated at 37°C for 30 min.
Then the samples were kept to cool to room temperature. Samples were filtered through Whatman filter papers and immediately prior to record the reading using the spectrophotometer, Thermo scientific Multiskan GO at 660 nm.
Effect of temperature on protease production
Protease specific medium broth (pH 7) was inoculated with 5% inoculum of an actively growing respective thermophilic bacterial culture and incubated at different temperatures, like 40°C, 55°C, 60°C in an orbital shaker at 120 rpm for 48 h. After incubation period broth samples were centrifuged at 10,000 rpm at 4°C for 15 min. Finally, the protease activity was assayed using supernatant by following the procedure indicated in a protease assay for test reagents.
Effects of pH on protease production
The effect of pH on protease production by the bacterial isolates was determined by growing the thermophilic bacteria in protease specific medium broth at varying pH values, pH 6, pH 7, and pH 8. The growth medium was inoculated with 5% inoculum of isolated microorganisms. The inoculated culture flasks of varying pH values were incubated at 55°C for 48 h. After incubation, broths were centrifuged at 10,000 rpm at 4°C for 15 min. The supernatant was used to measure protease production through assay of protease.
Effect of carbon sources on protease production
The sterilized protease specific medium broth (pH 7) was prepared with various carbon sources. These carbon sources were used to replace the carbon source (glucose) available in the protease specific medium broth. The culture tubes were inoculated with 5% inoculum on different carbon sources and incubated at 55°C for 48 h on a rotary shaker at 120 rpm. The resulting culture broth was centrifuged at 10,000 rpm at 4°C for 15 min. Finally, the protease activity was measured by assay of protease as per the procedure given in section of protease assay for test reagents.
Effect of nitrogen sources protease production
The sterilized protease specific medium broth (pH 7) was prepared with different nitrogen sources (sodium nitrate, urea and gelatin). These nitrogen sources were used instead of the nitrogen source (peptone) contained in the original protease medium broth. The prepared broth cultures were inoculated with 5% inoculum of the respective thermophilic bacterial culture and incubated at 55°C for 48 h on a rotary shaker at 120 rpm. After incubation period broth samples were centrifuged at 10,000 rpm at 4°C for 15 min. The protease activity measured by assay of protease as per the procedure previously described in the text.
Calculating enzyme activity
The absorbance of the samples was measured by a spectrophotometer at the wavelength of 660 nm. Absorbance values for the standards, standard blank and different test samples were recorded. After collecting all of the data, a standard curve was created.
Unit definition
Hydrolysed casein one unit produces colour that, equivalent to 1.0 μmol (181 μg) of tyrosine per minute at pH 7.5 at 37°C (coloured by Folin and Ciocalteu’s Phenol Regent) [14].
Statistical analysis
The protease assay data analysis was carried out using minitab 17 statistical software, and one way Analysis of Variance (ANOVA) method was used. Randomized Complete Black Design was used to determine the effect of various treatments on protease production and Turkey ’ s pairwise comparison was used to determine the significant difference among the groups of treatments.
Results and Discussion
Reviving the thermophilic bacterial cultures
Freeze-dried cultures are revived on nutrient agar medium at 55°C for 48 h given in the following Table 1.
| Revived | Thermophilic | Freeze-dried thermophilic bacteria species |
|---|---|---|
| Bacterial Culture Sample numbers | ||
| Type I (DMBUK 107233) | Geobacillus stearothermophilus | |
| Type II (DMBUK 107233) | Geobacillus stearothermophilus | |
| DMBUK 107161 | Geobacillus kaustophilus | |
| DMBUK 107191 | Geobacillus toebii | |
Table 1: Revived thermophilic Bacterial cultures.
All the Geobacillus bacterial cultures showed very good to excellent growth between 40°C to 60°C but at 80°C the culture growths were inhibited. The bacterial cultures of Geobacillus stearothermophilus type I (DMBUK 107233), Geobacillus stearothermophilus type II (DMBUK 107233), Geobacillus toebii (DMBUK 107191) and Geobacillus kaustophilus (DMBUK 107161) were showing their optimum growth at 40°C, 55°C, 55°C, 55°C respectively.
Effect of temperature on thermophilic bacterial growth
Temperature tolerance of Geobacillus bacterial cultures are given in the following Table 2.
| Sample numbers | 30°C | 40°C | 50°C | 55°C | 60°C | 70°C | 80°C |
|---|---|---|---|---|---|---|---|
| Type I (DMBUK 107233) | + | + | + | + | + | + | - |
| Type II (DMBUK 107233) | + | + | + | + | + | + | - |
| DMBUK 107161 | - | + | + | + | + | + | - |
| DMBUK 107191 | + | + | + | + | + | - | - |
Table 2: Temperature tolerance of Geobacillus bacterial cultures.
As these isolates withstand at high temperature (above 40°C), their metabolic functions may be active due to their physiological adaptations. Temperature effects on organisms at the two ways. One is when the temperature rises, chemical and enzymatic reactions proceed at a faster rate and the growth rate increases. Another way is when the temperature rises, proteins are invisibly damaged. As a molecular adaptation thermophiles are much more heat stable because of their intracellular enzymes and some of factors such as salts, high protein concentration, coenzymes, substrates, activators and general stabilizers [15].
Effect of pH on the growth of thermophilic bacteria
The number of colonies present in each Geobacillus sample varied in the pH range between 6-8.
According to results, almost all of these cultures were showed excellent growth at pH 6, 7 and 8 and the results are given in the Table 3. The bacterial culture GeoBacillus stearothermophilus type I (DMBUK 107233) showed its highest growth at pH 6, whereas GeoBacillus stearothermophilus type II (DMBUK 107233), Geobacillus toebii (DMBUK 107191) and Geobacillus kaustophilus (DMBUK 107161) showed their highest growth at pH 7. According to the previous literature the pH values of the Maha Oya hot water spring was ranging between pH 7.4 and pH 7.6 [9,16]. So pH 7 was showing good growth of isolates. Each organism has a particular pH range, which is possible to grow. Most neutral environments have pH values between 5-9 and most microorganisms have pH optimal in this range. High [H+] ions are required for thermophiles to stabilize the cytoplasmic membrane, but it dissolves when the pH value is raised to neutrality [17].
| Sample numbers | pH 6 (Log CFU/mL) | pH 7 (Log CFU/mL) | pH 8 (Log CFU/mL) |
|---|---|---|---|
| Type I (DMBUK 107233) | 9.075547 | 7.680336 | 8.113943 |
| Type II (DMBUK 107233) | 6.4843 | 7.127105 | 9.230449 |
| DMBUK 107161 | 7.870989 | 4.053078 | 9.672098 |
| DMBUK 107191 | 6.989895 | 8.068186 | 7.09691 |
Table 3: Geobacillus sample varied in the pH range between 6-8.
Proteolytic activity of the thermophilic bacteria
Isolates were screened for the protease activity on the casein agar plates. The results obtained are presented in the Table 4. Selected isolates were used to further experimental studies.
| Inhibition zone (mm) of isolates | Zone of Inhibition (mm) Isolates |
|---|---|
| Type I - DMBUK 107233 | 11 |
| Type II - DMBUK 107233 | 15 |
| DMBUK 107191 | 24 |
| DMBUK 107161 | 22 |
Table 4: The proteolytic activity of the thermophilic bacteria.
Quantification of protein content
The protein level of the crude enzyme of each isolate was estimated by Lowry’s method. Using absorbance values results a standard curve plotted and the protein concentration of each thermophilic bacterium was deduced using the standard curve.
The standard curve and the protein concentration present in each isolate are presented in the Figure 1.
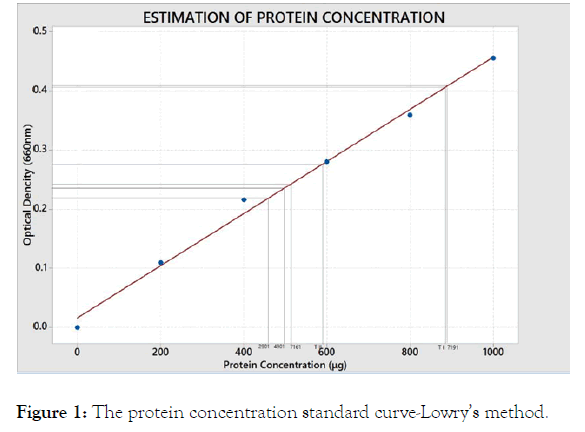
Figure 1. The protein concentration standard curve-Lowry’s method.
Tyrosine standard curve
The tyrosine standard curve was prepared by taking a different concentration of L-tyrosine standard solution.
Protease activity of thermophilic bacteria
Protease activities of the bacterial isolates were determined at 55°C and pH 7 as controlled samples. Protease activities of thermophilic bacterial isolates are shown in Figure 2.
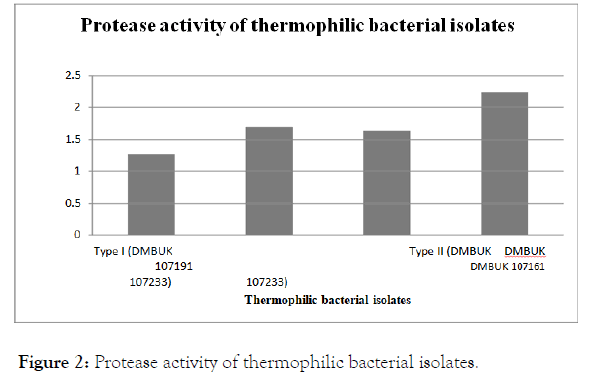
Figure 2. Protease activity of thermophilic bacterial isolates.
As shown in Figure 2, the protease activity was highest in Geobacillus kaustophilus (DMBUK 107161), its protease activity was 2.2320 units/mL and 1.2708 units/mL was shown the lowest protease activity by GeoBacillus stearothermophilus type I (DMBUK 107233).
Effect of temperature on protease production
The crude enzyme samples were tested for the effect of temperature on protease production.
Protease activity at temperatures 40°C, 55°C and 60°C are presented in Figure 3.
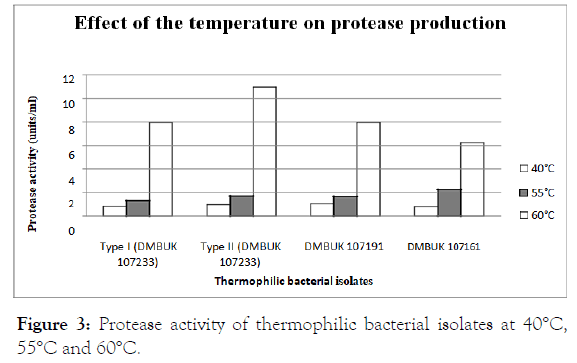
Figure 3. Protease activity of thermophilic bacterial isolates at 40°C, 55°C and 60°C.
The temperature was a critical parameter affecting the bacterial growth and protease enzyme production. Therefore, Geobacillus isolates were grown at temperature 40°C, 55°C and 60°C. The statistical method Analysis of Variance (ANOVA) was used to compare the effect of temperature on protease production. Then “Tukey”- test was applied to determine the significant difference among the 3 groups of temperatures. (55°C, 40°C), (60°C, 40°C) and (60°C, 55°C) shown various effects on protease production. According to data analysis of the present study shows the maximum activity of protease at 60°C and the minimum protease activity at 40°C as well as Geobacillus stearothermophilus type II (DMBUK 107233) shows the maximum protease activity among the Geobacillus isolates. Previous study of thermophilic protease producing
Geobacillus isolates were grown optimally at a temperature between 60°C-62°C [18]. Another report showed highest protease activity was found at growth temperature 60°C-70°C for B. stearothermophilus [11].
Effect of pH on protease production
The crude enzyme samples were tested for the effect of pH on protease production. Protease activity at pH 6, pH 7 and pH 8 are given in Figure 4.
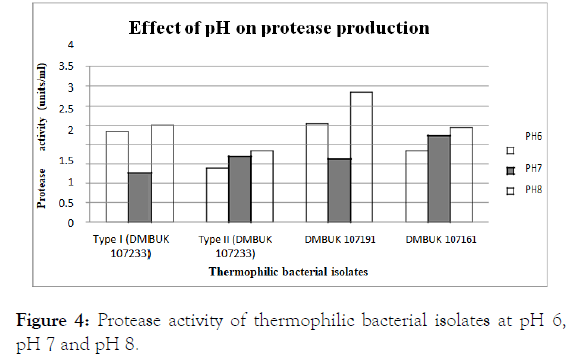
Figure 4. Protease activity of thermophilic bacterial isolates at pH 6, pH 7 and pH 8.
The pH is an important parameter for the growth of the thermophilic bacteria because metabolic reactions are active in the bacterial cell and it leads to the production of protease with respect to pH, it was evident that the thermophilic bacterial isolates were able to grow and produce protease over a selected pH values of 6, 7 and 8 [19]. According to Figure 4, selected Geobacillus isolates were shows pH 8 was effect to protease production, thus ANOVA was applied to compare the effect of pH on the protease production; therefore the conclusion was that the effects of selected pH levels on protease production are equal so, One-Way ANOVA was applied. Therefore, at pH 6,7,8; protease activities were not affected. So the protease activity was not affected at the pH range used in this study. Previous studies showed optimum pH of the enzyme was found to be 8. Thereafter, incubation of crude enzyme solution was carried out for 24 h at pH 5.5, 8 and 9 respectively for protease producing thermophilic Bacillus sp. [20].
Effect of carbon source on protease production
The different carbon sources fructose, mannitol and sucrose on the cells growth and protease production was investigated by replacing the carbon sources in protease specific medium. Protease activity in the presence of fructose, mannitol and sucrose are given in Figure 5.
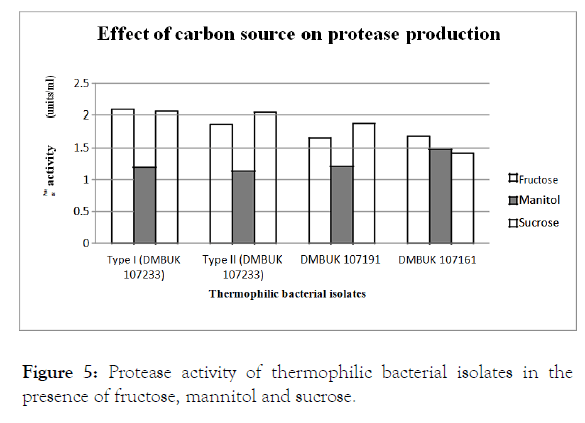
Figure 5. Protease activity of thermophilic bacterial isolates in the presence of fructose, mannitol and sucrose.
The results indicated that different carbon sources have a different impact on the production of protease from isolates. All tested carbon sources supported the growth of isolates. According to Figure 5; GeoBacillus stearothermophilus type I, Geobacillus kaustophilus were shows maximum protease production with fructose and GeoBacillus stearothermophilus type II, Geobacillus toebii were shows that maximum effect with the Sucrose. The effect of carbon sources on protease production in Geobacillus isolates was analysed using ANOVA statistical method and belongs to the results, Tukey pairwise comparisons used for selected carbon sources as 3 pairs of carbon groups. Protease production was affected when (mannitol, fructose) and (sucrose, mannitol) groups were used as carbon sources but (sucrose, fructose) group did not affect the protease production. Therefore the best carbon source, sucrose and fructose both were given the maximum effect on protease production of isolates. These results are in accordance with some previous studies which showed the addition of most carbon sources in the peptone medium failed to improve the protease production [21].
Effect of nitrogen source on protease production
The selected nitrogen sources as sodium nitrate, urea and gelatin on the thermophilic bacterial growth and protease production was evaluated replacing the nitrogen sources present in protease specific medium.
Protease activity in sodium nitrate, urea and gelatin are presented in Figure 6.
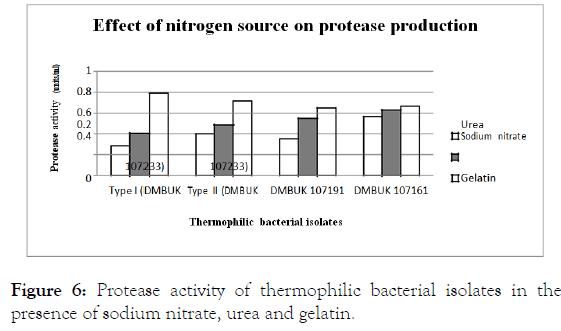
Figure 6. Protease activity of thermophilic bacterial isolates in the presence of sodium nitrate, urea and gelatin.
The maximum protease production analysed using ANOVA method and the selected nitrogen sources on protease production were different, thus Tukey ’ s test was applied to determine significant difference among the groups of nitrogen sources; therefore the effect of selected nitrogen sources on protease production was also different. The 3 groups of nitrogen (sodium nitrate, gelatin), (urea, gelatin) and (urea, sodium nitrate) shows various effects on protease production. The results indicated among the nitrogen sources used, several organic sources supported both growth and enzyme production by isolates, with maximum yields in medium containing gelatin followed by urea and sodium nitrate. Previous study showed sodium nitrate, ammonium salts and amino acids as sole nitrogen sources interfered with protease formation and protease production was enhanced in the presence of Sodium nitrate [21].
Conclusion
The present study was based on optimization of protease production by four isolates of thermophilic bacteria belonging to the genera Geobacillus from Maha Oya hot springs in Sri Lanka. According to the results obtained, it can be concluded that the Geobacillus thermophilic bacterial isolates were able to grow at temperatures between 30°C-70°C, but excellent growth was observed between 40°C-50°C. Geobacillus isolates shows excellent growth at pH 6,7,8 accordingly. Based on the results, this study was carried out to investigate the temperature, pH, carbon sources and nitrogen sources effects on protease production in selected Geobacillus isolates. According to statistical analysis, effect of temperature on protease production analysed with 3 groups of temperatures as, (55°C, 40°C); (60°C, 40°C) and (60°C, 55°C) can affect production of protease. Thus, maximum activity of protease observed at 60°C. But ANOVA for pH values showed that, pH6, pH7 and pH8 have no effect on protease production. So, it can be concluded that the maximum protease activity can be detected at pH 6, pH 7 or pH 8 with that Geobacillus condition.
Effect of carbon source on protease activity of bacteria showed variable results. The statistical analysis of pairwise comparison shows significant difference between the results of the pairs of (mannitol, fructose) and (sucrose, mannitol). So, that pairs can effect on protease production, but no significant difference between (sucrose, fructose) group thus, it cannot effect on protease production. Therefore, finally observed sucrose and fructose were shown the maximum protease activity on Geobacillus isolates. According to the statistical data analysis there was no significant difference between the results of the pairs of nitrogen sources, (sodium nitrate, gelatin), (urea, gelatin) and (urea, sodium nitrate). Therefore, pairs of nitrogen sources cannot effect on protease production, but the maximum protease activity can be observed as gelatin from the selected Geobacillus isolates.
Acknowledgment
I wish to thank with deep respect for the valuable guidance and encouragements given by Dr. I.V.N. Rathnayake (Senior lecturer, Department of Microbiology, University of Kelaniya).
References
- Coolbear T, Daniel RM, Morgan HW. The enzymes from extreme thermophiles: bacterial sources, thermostabilities and industrial relevance. In: Enzymes and Products from Bacteria Fungi and Plant Cells. Advances in Biochemical Engineering/Biotechnology. Springer, Berlin, Heidelberg. 1992;45:57-98.
- Nazina TN, Tourova T, Poltaraus AB, Novikova EV, Grigoryan AA, Ivanova A, et al. Taxonomic study of aerobic thermophilic bacilli: descriptions of Geobacillus subterraneus gen. nov., sp. nov. and Geobacillus uzenensis sp. nov. from petroleum reservoirs and transfer of Bacillus stearothermophilus, Bacillus thermocatenulatus, Bacillus thermoleovorans, Bacillus kaustophilus, Bacillus thermodenitrificans to Geobacillusas the new combinations G. stearothermophilus, G. th. Int J Syst Evol Microbiol. 2001;51(2):433-446.
- Zeigler DR. The genus Geobacillus-Introduction and strain catalog. (7th edn) Catalog of strains. 2001;3:1-23.
- Donk PJ. A highly resistant thermophilic organism. J Bacteriol. 1920;5(4):373.
- Lemieux P, Sieber R, Osborne A, Woodard A. Destruction of spores on building decontamination residue in a commercial autoclave. Appl Environ Microbiol. 2006;72(12):7687-7693.
- Sevinc N, Demirkan ES. Production of protease by Bacillus sp. N-40 isolated from soil and its enzymatic properties. J Biol Environ Sci. 2011;5(14):95-103.
- Alemu FM, Yalew AW, Fantahun M, Ashu EE. Antiretroviral therapy and pregnancy outcomes in developing countries: a systematic review. Int J MCH AIDS. 2015;3(1):31-43.
- Sawant R, Nagendran S. Protease: an enzyme with multiple industrial applications. World J Pharm Sci. 2014;3:568-579.
- Perera Isiri, Rathnayake IVN. Preliminary characterization of bacteria isolated from hot springs in MahaOya,Sri Lanka. Proceedings of the International Conference on Multidisciplinary Approaches –ICMA. 2014.
- Olajuyigbe FM, Ajele JO Production dynamics of extracellular protease from Bacillus species. Afri J Biotechnol. 2005;4(8):776.
- Vijayalakshmi TM, Murali R. Isolation and screening of Bacillus subtilis isolated from the dairy effluent for the production of protease. Int J Curr Microbiol App Sci. 2015;4(12):820-827.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265-275.
- Anson ML. The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. J Gen Physiol. 1938;22(1):79-89.
- Folin O, Ciocalteu V. On tyrosine and tryptophane determinations in proteins. J Biol Chem. 1927;73(2):627-650.
- Ward OP, Moo-Young M. Thermostable enzymes. Biotechnol Adv. 1988;6(1):39-69.
- Fonseka GM. Geothermal systems in Sri Lanka and exploitation of geothermal energy. J Geol Soc. 1994;5:127-133.
- Ward DM, Weller R, Shiea J, Castenholz RW, Cohen Y. Hot spring microbial mats: Anoxygenic and oxygenic mats of possible evolutionary significance. In: Cohen Y & E Rosenburg (Eds.) Microbial mats: physiological ecology of benthic microbial communities. American Society for Microbiology, Washington. 1989:3-15.
- Hawumba JF, Theron J, Brozel VS. Thermophilic protease-producing Geobacillus from Buranga hot springs in Western Uganda. Curr Microbiol. 2002;45(2):144-150.
- Sumantha A, Larroche C, Pandey A. Microbiology and industrial biotechnology of food-grade proteases: a perspective. Food Technol Biotechnol. 2006;44(2):211.
- Nascimento WCAD, Martins MLL. Production and properties of an extracellular protease from thermophilic Bacillus sp. Braz J Microbiol. 2004;35(1-2):91-96.
- Rahman RNZRA, Basri M, Salleh AB. Thermostable alkaline protease from Bacillus stearothermophilus F1; Nutritional factors affecting protease production. Ann Microbiol. 2003;53(2):199-210.
Author Info
DMSU Dissanayaka and IVN Rathnayake*Citation: DMSU Dissanayaka, IVN Rathnayake (2019) Effect of temperature, pH, carbon and nitrogen sources on extracellular protease production by four Geobacillus species isolated from Maha Oya geothermal springs in Sri Lanka. Appli Microbiol Open Access 5:160. doi: 10.35248/2471-9315.19.5.160
Received: 25-May-2019 Accepted: 08-Jul-2019 Published: 17-Jul-2019
Copyright: © 2019 DMSU Dissanayaka, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.