Journal of Drug Metabolism & Toxicology
Open Access
ISSN: 2157-7609
ISSN: 2157-7609
Research Article - (2015) Volume 0, Issue 0
Wireless Fidelity (WIFI) is widely used in cell phones, computers and electronic devices to access the Internet. It is unclear whether or not the frequency range of the WIFI has any harmful biological effects. In this report, the effect of standard 2.45 GHz radio frequency source (Averaged whole body specific absorption rate (SAR) 0.01 W/ Kg, 24 hours daily for 40 successive days) on some oxidative stress parameters was examined in Wistar female rats. We found that exposure to WIFI decreased the malondialdehyde levels as well as the glutathione-S-transferase and catalase activities. Moreover, the activity of superoxide dismutase showed significant increase in the WIFIexposed group relative to the control group. Meanwhile, kidney functions were found to be unaffected. In addition, no significant histological alterations in rats’ kidneys were detected in WIFI-exposed group. These results indicate that WIFI exposure used in the present study implies oxidative alterations in rat kidneys, which does not result in severe consequences due to the effective activity of the antioxidant enzyme system of the rat kidneys.
Keywords: Radio-frequency; WIFI; Oxidative stress; Kidney; Wistar rat
CAT: Catalase; DHBS: 3, 5-Dichloro-2- Hydroxybenzene Sulfonic acid; DTNB: 5, 5′-Dithiobis 2-Nitrobenzoic acid; EMF: Electromagnetic Fields; EMR: Electromagnetic Radiation; GSH: Reduced Glutathione; GSH-PX: Glutathione Peroxidase; GST: Glutathione -S-Transferase; H & E: Hematoxylin and Eosin; IACUC: Institutional Animal Care and Use Committee; LPO: Lipid Peroxidation; MDA: Malondialdehyde; NO: Nitric Oxide; RF: Radiofrequency Radiation; ROS: Reactive Oxygen Species; SAR: Specific Absorption Rate; SOD: Superoxide Dismutase; WIFI: Wireless Fidelity; WLAN: Wireless Local Area Networks
Wireless fidelity (WIFI) network involves short-range communication between an access point and many personal devices (e.g., Computers, printers, gaming devices). Widely used laptop computers and wireless networks emit 2.45 GHz Microwave radiation, which may result in exposure in a low level electromagnetic fields (EMF) during data transport. They may affect organ systems in the body because of near field and chronic exposure. In many countries, wireless local area networks (WLAN) operate at 2.45 GHz frequency band.
The popularity of portable devices supporting this technology running at 2.45 GHz is continuously growing as it can be used worldwide at home, work, or near hotspots. The biological effects of electromagnetic radiation (EMR) and their results have become the theme of keen public debate.
During the last few years, many studies had reported scientific evidence on health impacts of electromagnetic radiation. In some of these studies, the observed biological effects were linked with a case of oxidative stress caused by an increase of reactive oxygen species (ROS) or stress proteins [1-3].
Devices that integrate wireless technology such as laptop computers and cellular phone telephones are often used near reproductive organs and kidneys and may cause harmful effects on the kidney and testis [4]. The kidneys perform the essential function of removing waste products from the blood and regulating the water fluid levels. The kidneys generate very high levels of ROS through their very highly aerobic metabolism and blood perfusion and have relatively poor enzymatic antioxidant defence systems [5]. Some recent studies reported that exposure to WIFI EMR induced oxidative stress and decreased the levels of antioxidants in the kidney and testis of experimental animals [6-8].
Some authors revealed that exposure to 2.45 GHz EMR causes an increase in lipid peroxidation levels and a reduction in the activity of enzymes that prevent or protect against lipid peroxidation in tissues. Gumral et al., 2009; Türker et al., 2011. Naziroglu et al. 2012 observed proliferative effects of 2.45 GHz EMR on human leukaemia cancer cells through the overproduction of reactive oxygen species (ROS) and Ca 2+ influx [9-11]. In addition, Aynali et al. (2013) showed that WIFI (2.45 GHz) EMR (for 1 h/day for 30 days) increased lipid peroxidation levels in laryngotracheal mucosal tissues of rats [12]. Moreover, Ceyhan et al. (2012) reported that EMR (2.45 GHz/1h/day for 28 days) increased glutathione peroxidase (GSH-PX levels) in skin tissues [13]. Gumral et al. (2009) reported that EMR (2.45 GHz/h/day for 28 days) decreased GSH-PX and reduced glutathione (GSH) levels in erythrocytes [9]. Recently, Salah et al. (2013) found that exposure of rats to EMR (2.45 GHz, 1 h/day during 21 consecutive days) induced a diabetes like status [14]. Moreover, they found that EMR decreased the activities of glutathione peroxidase, catalase and the superoxide dismutase and groups thiol amount respectively in liver and kidneys. Indeed, they revealed that exposure to EMR increased the malondialdehyde concentration in liver and kidneys. In contrast, Naziroglu et al. (2012) reported that EMR (2.45 GHz for 1, 2, 12 and 24 hrs) did not have effective influence on reduced glutathione (GSH) and GSH-PX levels in human leukaemia 60 cells [11].
The present study performed an experimental approach to investigate the effects of radiofrequency radiation (RF) (2.45 GHz, SAR 0.01 W/Kg, 24 hours / day for 40 consecutive days) emitted from indoor WIFI Internet access devices using 802.11. b wireless standards on oxidative stress parameters such as malondialdehyde (MDA), reduced glutathione (GSH), catalase (CAT), glutathione- Stransferase (GST), superoxide dismutase (SOD) and nitric oxide (NO) as well as histopathology and kidney function parameters such as urea and creatinine of female Wistar rats. Most previous studies have investigated the effects of 0.9-1.8 GHz on oxidative stress, however, there was a few investigations of the 2.45 GHz effects. Even if other reports of similar work exist, they showed different experimental setup than ours.
Animals
The experimental animals used in this study were growing female Wistar rats (average weight 100.88 ± 3.00, age 39 days at the beginning of the study) obtained from a local supplier. The animals were kept under fixed appropriate conditions of housing. Equal amount of food were given to all animals (irradiated and control groups). All animals received humane care in conformity with the guidelines of the Institutional Animal Care and Use Committee (IACUC), Faculty of Science, Cairo University (IACUC Permit Number: CUFSF Biophy 42 14, November 2014).
Chemicals
Phosphate buffer pH 7.4 (50 mM / L, Triton x 0.1 %, EDTA 0.5 mM) and kits for the determination of oxidative stress parameters were purchased from Bio diagnostic Co., Giza, Egypt. Kits used for measuring urea and creatinine levels were bought from Diamond Co., Cairo, Egypt.
Exposure system and experimental design
An indoor wireless access point communicating at 2.45 GHz was used as the radio frequency wave source. Rats of each group were divided into 2 cages, 6 rats per cage. For the WIFI-irradiated group, the access point from WIFI device (with 802.11.b mode and WPA2 network protection) integrated two omnidirectional antennas that were set up for internet broadcast via wireless at 2.45 GHz. The used access point was located midway between the 2 cages with a distance separation of 25 cm between each cage and the access point as viewed in (Figure 1). The control rats were put under the same status without applying RF. The averaged whole body specific absorption rate (SAR) was determined to be 0.01 W/Kg by using the finite difference time domain (FDTD) method, with the aid of the XFDTD Bio-pro software (version: 6.3.8.4, NY, USA).
Twenty four female Wistar rats were randomly divided into experimental (WIFI-irradiated group) and control groups, with twelve rats per group. Animals were housed in groups of six in cages at 25°C, under a 12:12 h light/dark cycle. Rats were kept 5 days before the start of the experiment for lab acclimatization. The WIFI-irradiated group was exposed to radiofrequency radiation (2.45 GHz) for 24 hours per day for 40 consecutive days. The control group rats were maintained in the same conditions as the irradiated rats except for radiation exposure. All rats were weighed at the beginning and on the day before decapitation. At the end of the experiment, all rats were sacrificed by sudden decapitation. Blood samples were collected and the serum was separated. The kidneys were removed, cleaned of fat, weighed and kept at -20°C until usage.
Histopathological assessment
After decapitation, the kidneys were fixed in buffered neutral 10% formalin, embedded in paraffin and sectioned at 8 micrometer thickness. The kidney sections were stained with hematoxylin and eosin (H & E) according to Banchroft et al. (1996) [15]. Slides were then examined through the light microscope (Zeiss, Germany) to investigate any histopathological lesions in rat kidneys.
Biochemical analysis
Tissue homogenization: Kidney tissues were homogenized in a motor-driven tissue Homogenizer with phosphate buffer (pH 7.4). Unbroken cells, cell debris and nuclei were sedimented at 7012 xg for 15 min, and the supernatant was pipetted into plastic tubes and stored at -20 oC until assayed.
Determination of lipid peroxidation: Lipid peroxidation was assayed by measuring the levels of malondialdehyde (MDA) in the kidney tissues. MDA was determined by measuring thiobarbituric reactive species using the method of Ruiz-Larrea et al. (1994) in which, the pink-coloured chromogen formed during the reaction of thiobarbituric acid with lipid peroxidation breakdown products was measured spectrophotometrically at 532 nm in a Helios Alpha Thermospectronic (UVA 111615, England) [16].
Determination of reduced glutathione (GSH) levels: The assay of reduced glutathione (GSH) levels was performed using Biodiagnostic kit No. GR 25 11, which is based on the spectrophotometric method of Beutler et al. (1963) [17]. It depends on the reduction of 5, 5′-dithiobis 2-nitrobenzoic acid (DTNB) with glutathione to produce a yellow colour of which the absorbance is measured at 405 nm in a Helios alpha thermospectronic (UVA 111615, England).
Determination of catalase (CAT) activity: Catalase activity was measured using Biodiagnostic Kit No.CA 25 17 which is based on the spectrophotometric method described by Aebi (1984) [18]. Briefly, the catalase reacts with a known quantity of hydrogen peroxide and the reaction is stopped after 1 min with catalase inhibitor. In the presence of peroxidase, the remaining hydrogen peroxide reacts with 3, 5-Dichloro-2-hydroxybenzene sulfonic acid (DHBS) and 4-aminophenazone to form a chromophore with the colour intensity inversely proportional to the activity of catalase in the sample. The absorbance of chromophore colour is read at 510 nm in a Helios alpha thermospectronic (UVA 111615, England).
Determination of Glutathione -S-Transferase (GST) activity: Glutatione-S-transferase (GST) activity was determined according to the method of Habig et al. (1974) [19]. Briefly, 0.4 ml potassium phosphate buffer (50 mmol / L; pH 6.5), 0.1 ml of supernatant, 1.2 mL water and 0.1 mL CDNB (1-chloro-2, 4 dinitrobenzene, 30 mmol / L) were added and incubated in a water bath at 37oC for 10 min. After incubation, 0.1 mL of reduced glutathione (30 mmol / L) was added. The change in absorbance was measured at 340 nm at one min interval.
Determination of Superoxide Dismutase (SOD) activity: Superoxide dismutase (SOD) activity was determined according to the method of Nishikimi et al. (1972) [20]. The assay for SOD levels was performed using Biodiagnostic kit No. SD 25 21. This assay relies on the ability of the enzyme to inhibit the phenazine methosulphatemediated reduction of nitroblue tetrazolium dye.
Determination of Nitric Oxide (NO) levels: The assay of nitric oxide was carried out using Biodiagnostic kit No. NO 25 33. This method is based on the spectrophotometric method of Montgomery and Dymock (1961) which depends on the measurement of endogenous nitrite concentration as an indicator of nitric oxide production [21]. The resulting azo dye has a bright reddish-purple colour whose absorbance is read at 540 nm in a Helios alpha thermospectronic (UVA 111615, England).
Determination of serum urea and creatinine levels
Serum urea was measured by the Berthelot enzymatic colorimetric method [22]. Serum creatinine was measured by Jaffe´ colorimetricend point method [22].
Statistical analysis
The number of animals used in this study was twenty four, according to statistical requirements to obtain significant results. Data of body weights, urea and creatinine levels and oxidative stress parameters were analyzed by independent samples T-test using origin software version 6.0. Percentage difference representing the percent of variation in the value with respect to the control was also computed.
% Difference (% D) = 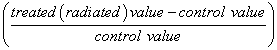
A p-value of < 0.05 was considered as statistically significant.
Effect of WIFI exposure on the rat’s body weights
Young rats were utilized in the current study because their growing organs may be more prone to the effects of EMR, similar to children and young adults who drop a big sum of time using mobile phone and WIFI devices at school and house. We divided the rats into two groups, one control and one treated group. We exposed the treated group for standard 2.45 GHz radio frequency source 24 hrs daily for 40 successive days. The effect of WIFI on body weight was examined. The present results indicate a significant increase in body weight gain in the rats of WIFI- irradiated group with respect to those of the control group and also a significant increase in body weight in both groups between the beginning of the experiment and the end of the experiment (Table 1).
| At the beginning of the experiment | At the end of the experiment | Change in body weight (percentage change from the initial weight) | Paired samples – T test (P-Value) between the beginning and the end of the experiment | |
|---|---|---|---|---|
| Control group | 108.5 ± 1.96 (12) |
146.25 ± 5.79 (12) |
34.79% | P=1.10x10-5* |
| WIFI-irradiated group | 94 ± 4.99 (12) |
161 ± 5.70 (12) |
71.28% | P=1.77x10-6* |
Values represent the mean ± S.E.M. The number of animals is shown between parentheses.
*: p < 0.05 significant.
N.B. At the beginning of the experiment, the average body weight of the irradiated group was lower than that of the control group, but this difference in body weight was not statistically significant between the 2 groups.
Table 1: Effects of WIFI exposure (24 hours per day, for 40 days, Frequency = 2450 MHz) on rat’s body weight.
Effect of WIFI exposure on some oxidative stress parameters in the kidneys of female Wistar rats
As shown in Table 2, the radio frequency radiation significantly reduces the levels of MDA and the activities of both CAT and GST in renal tissues compared to control group. Meanwhile, renal SOD recorded a significant increase in WIFI-exposed group relative to the control group. The GSH and NO levels showed non-significant changes in the WIFI-exposed group relative to the control group.
| Oxidative stress parameters | Control group | WIFI- irradiated group | Percentage difference (%D) |
Independent samples T-test (P-value) |
|---|---|---|---|---|
| MDA (nmol / g. tissue) |
44.25 ± 0.93 (6) |
16.20 ± 1.38 (6) |
-63.39% | 1.14 x 10-8* |
| GSH (mg / g. tissue) |
6.80 ± 0.45 (6) |
8.83 ± 1.08 (6) |
29.88% | 0.11 (n.s.) |
| Catalase (U/L) |
1.97 ± 0.12 (6) |
1.31 ± 0.25 (6) |
-50.38% | 0.04* |
| GST (U/g tissue) |
3.80 ± 0.18 (6) |
2.25 ± 0.28 (6) |
-40.79% | 9.43 x 10-4* |
| SOD (U/g tissue) |
319.61 ± 0.85 | 643.96 ±31.98 | 101.48% | 1.40 x 10-6* |
| NO (umol/g ) | 2.65 ± 0.23 | 2.22 ± 0.10 | -16.23% | 0.11 (n.s.) |
Values represent the mean ±S.E.M.
The number of animals is shown between parentheses.
% D: percentage difference in comparison to control group.
*: p < 0.05 significant.
n.s. non- significant
Table 2: Effect of WIFI exposure (F=2450 MHz, 24 hours daily for 40 days) on some oxidative and antioxidative stress parameters in the kidneys of female Wistar rats.
Effect of WIFI exposure on serum parameters (urea and creatinine levels of female Wistar rats)
Wireless fidelity (WIFI) radiation (2.45 GHz) induced significant decreases in urea levels when compared to control levels while creatinine levels showed non-significant alterations with respect to the control group (Table 3).
| Kidney function parameter | Control group | WIFI- irradiated group | Percentage difference (%D) |
Independent samples T-test (P-value) |
|---|---|---|---|---|
| Urea (mg / dL) |
65.36 ± 1.42 | 42.75 ± 2.44 | -34.59% | 1.16 x 10-5* |
| Creatinine (mol / L) |
28.19 ± 1.21 | 29.68 ± 0.40 | 5.29% | 0.27 (n.s.) |
Values represent the mean ±S.E.M.
The number of animals is shown between parentheses.
% D: percentage difference in comparison to control group.
*: p < 0.05 significant.
n.s. non- significant
Table 3: Effect of WIFI exposure on some kidney functions in female Wistar rats.
Histological findings
No significant histological alterations were detected in both WIFIexposed and control groups with normal histological architecture of glomeruli, renal tubules and interstitial tissue comprising the kidney (Figure 2).
Figure 2: Histological findings. a) Photomicrograph of irradiated group showing normal histological structure of glomeruli, renal tubules and interstitial tissue comprising renal cortex (H&E, X400). b) Photomicrograph of control group showing normal histological structure of renal cortex (H&E, X400).
Wireless fidelity (WIFI) network involves short-range communication between an access point and many personal devices (e.g., Computers, printers, gaming devices). Widely used laptop computers and wireless networks emit 2.45 GHz microwave radiation (MWR) which may result in exposure in a low level EMF during data transfer. They may affect organ systems in the body because of near field and chronic exposure. The purpose of the present study was to investigate the effect of radio frequency waves emitted from conventional WIFI devices on the rat kidney.
The results of the present study revealed that sub-chronic exposure to WIFI radiation (2.45 GHz/24h/day for 40 consecutive days) caused a significant increase in rat body weight with regard to the control group. Our results of body weight are inconsistent with a previous report that stated that the food consumption in electromagnetic radiation-exposed animals (2.45 GHz, 1h/day) is generally lower than that in sham exposed animals 005B [23]. Besides, our results of body weight are not in line with the results of Salah et al. (2013) who demonstrated that sub-chronic exposure to RF (2.45 GHz, 1 h/day during 21 successive days) resulted in a decrease in rat body weight [14]. This discrepancy in the rat body weight results may be attributed to the difference in the time of exposure and the experimental setup of these previously mentioned studies compared with our study. In a previous study done by Tsuji et al. (1996), they exposed BALB/c mice to 48 h of 5 Tesla static magnetic field and they found decreases in rats’ body weight [24]. The authors attributed this decrease in body weight to body fluid loss resulting from decreased food and water intake. The results of Tsuji et al., (1996) are not in line with our results, but, similarly, we can refer the present significance in rats’ body weights’ to changes in food intake or to metabolic variations [24].
An adverse effect of EMR on the kidney is described in many works which have recorded intertubular inflammation, glomerular atrophy, cytoplasmic vacuolation of the renal tubules and pycnotic nuclei [25-28]. Few studies have investigated the effect of 2.45 GHz EMR on the oxidative stress while there are many studies that have investigated the relation between mobile phones operating within a radiation range of 0.9–1.8 GHz frequency and oxidative stress [29-34]. Unfortunately, these previous studies didn’t reveal univocal results. Some studies showed that WIFI and mobile phone-induced EMR can generate oxidative stress in the testis and kidneys [5,7,8] while others suggested that EMR exposure does not affect oxidative stress markers such as MDA in rat testes [6].
In the present study, we have evaluated some of the oxidative stress markers as MDA, antioxidant markers such as GSH and NO in addition to antioxidant enzyme activities of SOD, CAT and GST in rat kidneys after exposure to WIFI (2.45 GHz, SAR 0.01 W/kg, 24 h/day during 40 consecutive days).
Superoxide dismutase (SOD) is considered to be the first line of defence against oxidative stress, it catalyses the dismutation of superoxide anions to molecular oxygen and H2O2, The latter is readily diffusible through biological membranes and may be converted to more highly reactive hydroxyl radical [35-37]. H2O2 which is the main product of the action of SOD dismutation is scavenged by peroxisomal CAT or glutathione peroxidase (GSH-PX) [38]. Catalase (CAT) is the main scavenger of H2O2 at high concentration [39], it plays the role of catalysing the conversion of H2O2 to H2O and molecular oxygen [40]. Hence, the present decrease in CAT activity in WIFI- exposed animals could be due to its exhaustion to overcome the overproduced H2O2 as a consequence of the significant increase in SOD activity.
Malondialdehyde (MDA) is the breakdown product of the major chain reactions leading to oxidation of polyunsaturated fatty acids and can be considered as a reliable marker for the oxidative stressmediated lipid peroxidation and oxidative damages in tissues [41-43]. Therefore, it could be suggested that the present decrease in the renal lipid peroxidation level in the WIFI-exposed group may be produced as a result of the concomitant significant increase in SOD activity according to the reported antioxidant properties of SOD that prevent lipid peroxidation. These results may provide an impression about the safe impact of WIFI radiation on rat kidney under the currently used experimental conditions. Our results are inconsistent with the results of Salah et al. (2013) who revealed that RF exposure (2.45 GHz, 1 h/ day during 21 consecutive days) led to a significant increase in MDA levels and a significant decrease in SOD activities [14]. The significant decrease in MDA levels that was detected in the present study was in line with the results of Kula et al. (1999) who reported a reduction in serum total lipids in the serum of steelworkers exposed to electromagnetic field (electric field strength of 20 V/m and magnetic field strength of 2 A/m) generated by induction heaters [44].
Glutathione-S-transferases (GSTs) are a family of enzymes that catalyse the addition of the tripeptide glutathione to endogenous and xenobiotic substances which have electrophilic functional groups. They play an important role in the detoxification and metabolism of many xenobiotic and endobiotic compounds [45-47]. The significant decrease of GST in the present study may be because there is no exposure to xenobiotic compounds, so no need for overexpression of GST activity. This decrease of GST detected in the present study helped to maintain normal levels of GSH (As GSH acts as a substrate for GST).
Therefore, the present increase in renal SOD activity and MDA content in addition to the present decrease in GST and catalase activities could indicate a protective mechanism exhibited by the antioxidative system of the rat kidney which may be mediated on one hand by an increase in antioxidant enzyme activity of SOD and by a decrease in lipid peroxidation on the other hand.
Both urea and creatinine are considered as kidney function markers .Supporting the above findings, the WIFI-exposed rats showed reductions in serum urea levels while creatinine levels showed nonsignificant alterations with respect to the control group. These results suggest that the kidney functions were not affected by WIFI exposure. In addition, no histopathological lesions were detected in the kidneys of rats exposed to WIFI radiation in the present study.
The changes in the production of MDA and the activity of SOD, CAT and GST imply oxidative alterations in rat kidneys upon WIFI exposure. However, no obvious histological changes were observed in rat kidney. These findings provide a clue that oxidative stress induced by WIFI exposure does not result in severe consequences due to the effective activity of the antioxidant enzyme system of the rat kidneys.
From the present results, it could be concluded that the exposure to a WIFI, at specific conditions employed in the present study (2.45 GHz/24h/day for 40 consecutive days) implies oxidative alterations in rat kidneys, which does not result in severe consequence due to the effective activity of the antioxidant enzyme system of the rat kidneys.
This research received no specific grant from any funding agency in the public, commercial, or not for profit sectors.
No conflict of interest
We thank Dr. Neveen A Noor, Associate professor at Zoology Department, Faculty of Science, Cairo University for reading the manuscript and for her comments that greatly improved the manuscript.