Journal of Sleep Disorders & Therapy
Open Access
ISSN: 2167-0277
ISSN: 2167-0277
Research Article - (2021)Volume 10, Issue 1
Background: Obesity without metabolic syndrome is known as metabolically healthy obesity (MHO). The influence of MHO on apnea severity and sleep parameters in patients with obstructive sleep apnea (OSA) remains unknown. To determine whether MHO affect the apnea severity and sleep parameters, such as apnea-hypopnea index (AHI) and arousal index, in OSA patients with non-obesity, MHO, and metabolically unhealthy obesity (MUHO).
Methods: A total of 266 men with OSA, aged between 20 and 65 years, were enrolled in this single-center, retrospective study. After excluding patients on hypnotics, antidepressants, or anticonvulsants, a total of 180 patients were included. The clinical data, AHI, and arousal index were examined in OSA patients with non-obesity, MHO, and MUHO.
Results: The arousal index showed a significant difference in patients with MHO compared to those with MUHO (MHO vs MUHO: 28.6 ± 15.5 vs. 40.2 ± 21.1, respectively, p<0.01). There was no significant difference in the arousal index in OSA patients between the non-obese and MHO groups. AHI indicated a significant increase in patients with MHO compared to non-obesity (non-obesity vs MHO: 22.5 ± 13.7 vs. 38.4 ± 22.8, respectively, p<0.01).
Conclusions: In OSA patients with MHO and MUHO, there were no significant differences in apnea severity, but the arousal index in OSA patients with MHO was lower than MUHO. The results demonstrate that the arousals during sleep may be due to the simple correlation with apnea appearance and metabolically unknown factors.
Metabolically healthy obesity (MHO); Metabolic syndrome (MetS); Obstructive sleep apnea (OSA); Apnea-hypopnea index (AHI); Arousal index
Obesity increases the risk of metabolic syndrome (MetS), but a few obese people do not have metabolic abnormalities [1,2]. Persons with a high body mass index (BMI) are classified as obese but without metabolic abnormalities, they can be classified under metabolically healthy obesity (MHO) [3,4]. However, there are no universally accepted criteria for MHO identification. It was reported in a previous study that obese persons without MetS did not have an excess risk of myocardial infarction and heart failure 5). In another study, MHO patients were reported to have a higher risk for cardiovascular disease (CVD) than metabolically healthy normal body weight people [5-7]. Patients with obstructive sleep apnea (OSA) are also reported to have an increased risk for CVD [8]. The study indicated that the increased risk for CVD was dependent on the apnea severity, apnea-hypopnea index (AHI). Consequently, examination of the effect of apnea severity on CVD risk in OSA patients with MHO is necessary for adequate clinical management. However, no study has reported the differences in sleep parameters in OSA patients between MHO and metabolically unhealthy obesity (MUHO).
According to the American Academy of Sleep Medicine (AASM) manual, arousal is defined as an abrupt shift of electroencephalographic frequency during sleep [9]. Repetitive arousals increase sympathetic nerve activity in patients with OSA [10,11], and the arousal index represents a sympathetic nerve activity [11,12]. Therefore, it is important to examine the sympathetic activity in patients with OSA because of the possibility of increased cardiovascular comorbidity. In addition, arousals lead to sleep fragmentation and result in poor sleep quality [12,13]. In MetS, insulin resistance and adipokine secretion from visceral fat triggers sympathetic nerve activation [14]. Increased arousal index has been reported in patients with OSA and MetS [15]. However, no study on the differences in arousal index in OSA patients with MHO and MUHO has been reported.
This study was conducted to evaluate the differences in apnea severity and arousal index in OSA patients with MHO and MUHO.
Participants
A flow chart of the study is shown in Figure 1. This study enrolled a total of 725 patients who visited the Fukuoka Urasoe Clinic between April 2019 and March 2020 with the chief complaints of daytime sleepiness, apnea, and/or loud snoring. A total of 266 male patients, aged between 20 and 65 years with more than 5 of AHI, were recruited in this study. All patients underwent diagnostic polysomnography (PSG). Only men were included to eliminate the influence of sex-related differences, such as sex hormones. A total of 86 patients, including 24 patients lacking data, 60 patients taking hypnotics and/or antidepressants, and two patients taking anticonvulsants that might influence the AHI score and arousal index were excluded [16]. In 60 patients taking hypnotics and/ or antidepressants, there are 7 patients who have insomnia, 51 patients who have depression, 2 patients who have both insomnia and depression. Two patients taking anticonvulsants have epilepsy. A total of 180 patients were analyzed in this study. The number of patients in the present study was 180, indicating that the sample size is sufficient for the analysis.
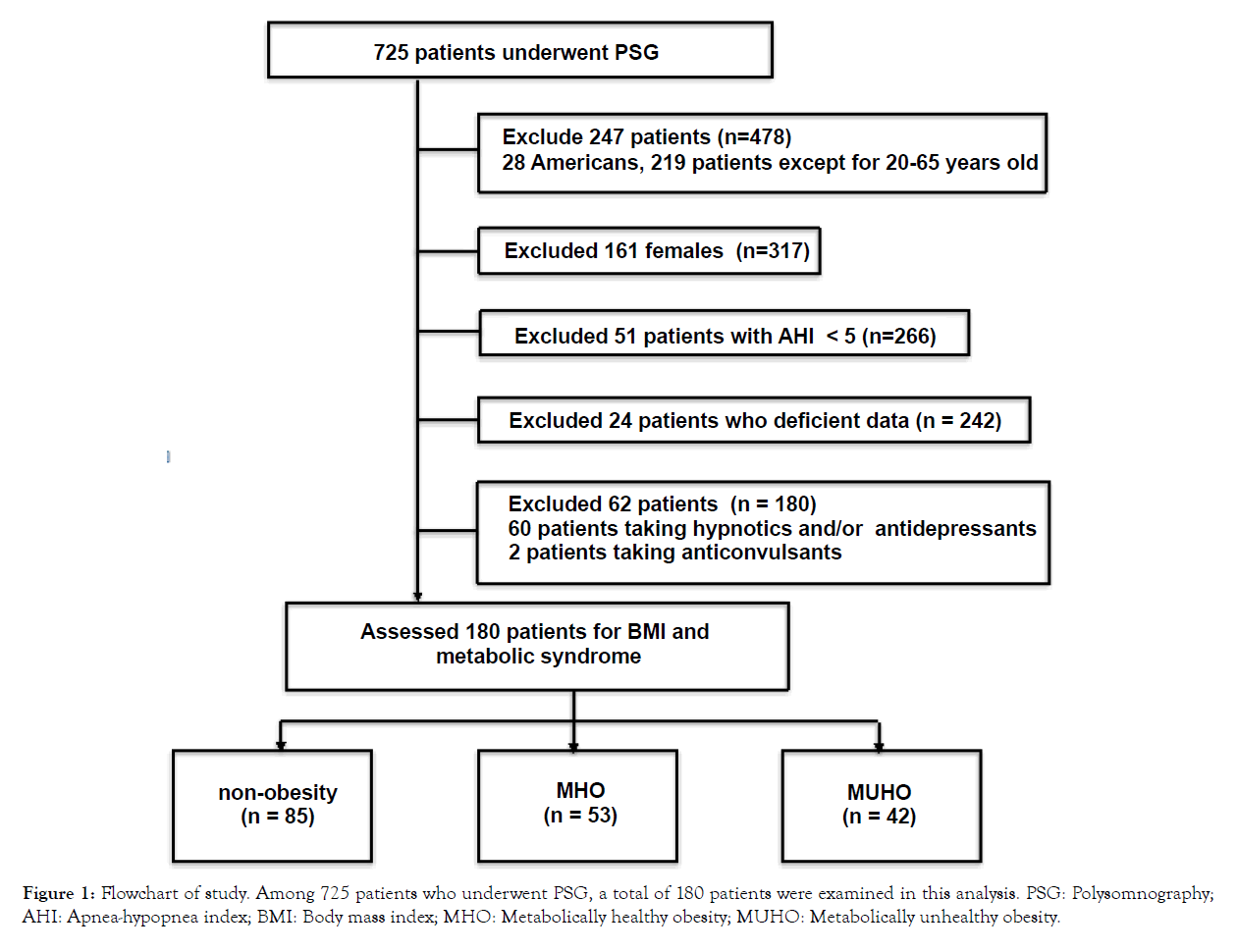
PSG: Polysomnography; AHI: Apnea-hypopnea index; BMI: Body mass index; MHO: Metabolically healthy obesity; MUHO: Metabolically unhealthy obesity.
Figure 1: Flowchart of study. Among 725 patients who underwent PSG, a total of 180 patients were examined in this analysis.
According to the criteria and classification of obesity in Japan and Asia-Oceania [17], patients with a BMI <25 were defined as non-obese and patients with a BMI ≥ 25 were defined as obese. Diagnosis of MetS was made according to the definition and criteria for metabolic syndrome [18] for Japanese, for those with abdominal obesity with waist circumstance at an umbilical level more than 85 cm, and who had any two of the following three abnormalities: a systolic blood pressure of more than 130 mm Hg and/or a diastolic blood pressure of more than 85 mm Hg; those on antihypertensives, those who had random blood glucose levels of more than 140 mg/dL or fasting blood glucose levels of 110 mg/ dL; those who were on oral hypoglycemics or injectable insulin, those who showed random triglyceride levels of more than 200 mg/dL or fasting levels of 150 mg/dL; high density lipoprotein cholesterol (HDL-C) levels of less than 40 randomly; or patients on lipid-lowering medications. Those who were defined as obese and without Mets were defined as MHO, whereas those who were defined as obese and with Mets were defined as MUHO. A total of 12 patients had hyperglycemia, and six of them were administered oral hypoglycemics. There were 85 patients who had high blood pressure, and 32 were treated with antihypertensive drugs. There were 83 patients with dyslipidemia, 19 of whom were treated with lipid-lowering medications. There was one patient with history of percutaneous coronary intervention, and two patients had history of angina. None of these patients had rapid eye movement sleep behavior disorder, restless legs syndrome, periodic limb movement disorder, narcolepsy, idiopathic hypersomnia.
Informed consent was obtained from all patients before enrollment in this study, which was performed in accordance with the principles of the Declaration of Helsinki and its later amendments. The study protocol was approved by the Institutional Ethics Committee of the Nakamura Clinic, Urasoe, Okinawa, Japan.
Measurement
All patients enrolled in this study underwent measurements of the waist circumstance, blood chemistry, electrocardiography (ECG), and pulmonary function test as a part of regular examination for patients on the day of their first visit to our clinic. We also measured blood pressure in the office after 5 minutes resting in a chair twice and made average of them. On the next visit, we measured blood pressure as the same way and averaged the two measurements.
Polysomnography (PSG)
As shown in our previous study [19], standard overnight PSG included continuous monitoring with central electroencephalography (EEG), electrooculography, submental and anterior tibial electromyography, and ECG using conventional leads. The airflow was monitored with oral and nasal thermistors, and the respiratory effort was measured by respiratory inductance plethysmography through transducers that were placed over the chest and the abdomen. Oxyhemoglobin saturation was continuously recorded using a pulse oximeter (3900P, Datex- Ohmeda Co., Louisville, CO, USA). All the parameters mentioned above were continuously recorded using REMbrandt ™ version 8.0 (EMbla, Broomfield, CO, USA) and RemLogic ™ version 3.2 (EMbla, Thornton, CO, USA). All recordings were scored directly on the screen by polysomnography certified by the Japanese Society of Sleep Research based on the 2014 guidelines from the AASM [9].
Statistical analyses
All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of the R commander designed to add statistical functions frequently used in biostatistics [20]. The Kruskal-Wallis test was used to compare characteristics and sleep parameters in individuals with non-obesity, MHO, and MUHO. Then, the post-hoc Steel- Dwass test was conducted. The Fisher’s exact test was used to compare the ratio of metabolic risk components and the number of metabolic risk components in these individuals. To calculate the sample size, we assumed α with 0.05, β0.2, effect size 0.25 and number of group 3. As a result, the sample size was 159. All continuous values were presented as mean ± standard deviation (SD). The p-values are two-sided, and values less than 0.05 were considered to indicate a significant difference.
Patient characteristics
Table 1 shows a summary of the characteristics of patients with non-obesity, MHO, and MUHO. No significant differences were observed between the three groups in terms of age. Significant differences in BMI, waist circumstance, neck circumstance, triglyceride, HDL-C, glucose, systolic blood pressure, and diastolic blood pressure were observed in the non-obese, MHO, and MUHO groups. The ratio of metabolic risk components and the number of metabolic risks components were also significantly among these groups.
| Total (n=180) | non-obesity (n=85) | MHO (n=53) | MUHO (n=42) | p-value | |
|---|---|---|---|---|---|
| Age (years) | 44.2 ± 11.6 | 42.5 ± 12.0 | 46.0 ± 12.0 | 45.5 ± 9.8 | 0.122 |
| BMI (kg/m2) | 26.2 ± 4.6 | 22.6 ± 1.8 | 29.0 ± 3.6 a | 29.9 ± 3.9 a | <0.001 |
| Waist circumstance (cm) | 92.6 ± 12.1 | 83.4 ± 6.8 | 99.7± 9.2 a | 102.3 ± 10.2 a | <0.001 |
| Neck circumstance (cm) | 43.5 ± 14.0 | 42.9 ± 16.0 | 44.5 ± 14.1 a | 43.4 ± 8.3 a | <0.001 |
| Triglyceride (mg/dL) | 197.3 ± 136.0 | 158.2 ± 122.6 | 177.2 ± 97.4 | 301.8 ± 151.4 a, b | <0.001 |
| HDL-C (mg/dL) | 53.7 ± 13.1 | 59.4 ± 14.3 | 51.5 ± 9.3 a | 44.9 ± 8.6 a, b | <0.001 |
| Glucose (mg/dL) | 96.4 ± 18.4 | 91.9 ± 14.8 | 97.2 ± 14.8 c | 104.6 ± 25.2 c | 0.005 |
| Systolic blood pressure (mmHg) | 125.3 ± 16.3 | 119.3 ± 16.3 | 128.1 ± 16.4 a | 134.0 ± 11.0 a, b | <0.001 |
| Diastolic blood pressure (mmHg) | 74.0 ± 12.7 | 68.7 ± 11.4 | 75.1 ± 12.8 c | 82.9 ± 9.5 a, b | <0.001 |
| Metabolic risk components | |||||
| Waist circumstance, n (%) | 132 (73.3%) | 37 (43.5%) | 53 (100%) a | 42 (100%) a | <0.001 |
| Dyslipidemia, n (%) | 83 (46.1%) | 26 (30.6%) | 15 (28.3%) | 42 (100%) a, b | <0.001 |
| High blood pressure, n (%) | 85 (47.2%) | 23 (27.1%) | 20 (37.7%) | 42 (100%) a, b | <0.001 |
| Hyperglycemia, n (%) | 12 (6.7%) | 3 (3.5%) | 1 (1.9%) | 8 (19.0%) a, b | 0.0021 |
| Number of metabolic risks components | |||||
| 0 | 47 (55.3%) | 16 (30.2%) a | 0 (0%) a, b | <0.001 | |
| 1 | 25 (29.4%) | 37 (69.8%) a | 0 (0%) a, b | <0.001 | |
| 2 | 11 (12.9%) | 0 (0%) | 36 (85.7%) a, b | <0.001 | |
| 3 | 2 (2.4%) | 0 (0%) | 6 (14.3%) c, b | 0.0021 | |
Note: MHO: Metabolically healthy obesity; MUHO: Metabolically unhealthy obesity; BMI: Body mass index; HDL-C: High density lipoprotein cholesterol; data are expressed as mean ± standard deviation, or number (percentage). ap<0.01 compared with non-obesity, bp<0.01 compared with MHO, cp<0.05 compared with non-obesity.
Table 1: Demographic and clinical data of all patients (n=180).
Comparison of sleep parameters in individuals among non-obese, MHO, and MUHO
As shown in Table 2, significant differences in Stage N1, Stage N3 were observed in the non-obese, MHO, and MUHO groups. As shown in Table 3, significant differences in sleep time with SpO2 <90%, lowest SpO2, AHI, and arousal index were observed in the non-obese, MHO, and MUHO groups. The arousal index showed a significant decrease between patients with MHO and patients with non-obesity in comparison to those with MUHO (MHO vs MUHO: 28.6 ± 15.5 vs. 40.2 ± 21.1, respectively, p<0.01, nonobesity vs MUHO: 24.7 ± 9.60 vs. 40.2 ± 21.1, respectively, p<0.01) (Figure 2). There was no significant difference in the arousal index in OSA patients between the non-obese and MHO groups (Figure 2). The AHI indicated a significant difference between patients with MHO and non-obese patients (non-obesity vs MHO: 22.5 ± 13.7 vs. 38.4 ± 22.8, respectively, p<0.01). In addition, a significant difference in AHI between MUHO and non-obese patients was observed (non-obesity vs MUHO: 22.5 ± 13.7 vs. 50.4 ± 28.6, respectively, p<0.01) (Figure 3). However, we could not know the effect of arousal index on cardiovascular outcomes in this study.
| Total (n=180) | non-obesity (n=85) | MHO (n=53) | MUHO (n=42) | p-value | |
|---|---|---|---|---|---|
| Total Sleep Time (min.) | 362.7 ± 64.6 | 363.6 ± 65.5 | 357.0 ± 60.3 | 368.0 ± 69.0 | 0.479 |
| Sleep latency (min.) | 7.0 ± 10.0 | 8.0 ± 11.2 | 6.9 ± 11.2 | 5.0 ± 4.5 | 0.521 |
| Sleep efficiency (%) | 81.7 ± 12.5 | 82.2 ± 12.3 | 81.1 ± 11.8 | 81.7 ± 13.9 | 0.766 |
| Wake (%) | 18.2 ± 12.5 | 17.7 ± 12.3 | 18.8 ± 11.9 | 18.4 ± 13.9 | 0.779 |
| Stage N1 (%) | 18.0 ± 9.8 | 16.2 ± 8.5 | 17.3 ± 9.9 | 22.6 ± 10.9 a, d | 0.0021 |
| Stage N2 (%) | 41.5 ± 12.4 | 43.0 ± 11.3 | 40.8 ± 13.4 | 39.5 ± 13.1 | 0.347 |
| Stage N3 (%) | 7.04 ± 6.57 | 8.08 ± 5.89 | 7.02 ± 7.67 | 4.96 ± 6.00 a | 0.0086 |
| Stage REM (%) | 15.2 ± 5.91 | 15.1 ± 6.17 | 16.0 ± 5.88 | 14.6 ± 5.42 | 0.603 |
Note: MHO: Metabolically healthy obesity; MUHO: Metabolically unhealthy obesity; REM: Rapid eye movement; data are expressed as mean ± standard deviation. a p< 0.01 compared with non-obesity, d p< 0.05 compared with MHO.
Table 2: Sleep parameters of all patients (n=180).
| Total (n=180) | non-obesity (n=85) | MHO (n=53) | MUHO (n=42) | p-value | |
|---|---|---|---|---|---|
| Sleep time with SpO2< 90 % | 7.62 ± 14.7 | 1.88 ± 3.80 | 9.77 ± 12.5 a | 16.5 ± 23.6 a | <0.001 |
| Lowest SpO2 (%) | 80.3 ± 9.34 | 84.4 ± 6.58 | 77.1 ± 9.13 a | 75.8 ± 10.9 a | <0.001 |
| AHI ( /h) | 33.7 ± 23.6 | 22.5 ± 13.7 | 38.4 ± 22.8 a | 50.4 ± 28.6 a | <0.001 |
| Arousal index ( /h) | 29.5 ± 15.9 | 24.7 ± 9.60 | 28.6 ± 15.5 | 40.2 ± 21.1a, b | <0.001 |
| PLMI ( /h) | 2.27 ± 7.57 | 1.74 ± 4.92 | 3.19 ± 11.1 | 2.16 ± 6.59 | 0.846 |
| PLMAI ( /h) | 0.234 ± 0.800 | 0.286 ± 1.03 | 0.165 ± 0.444 | 0.214 ± 0.590 | 0.996 |
Note: MHO: Metabolically healthy obesity; MUHO: Metabolically unhealthy obesity; REM: Rapid eye movement; AHI: apnea - hypopnea index; PLMI: Periodic limb movements index; PLMAI: periodic limb movements with arousal index; data are expressed as mean ± standard deviation. a p<0.01 compared with non-obesity, b p<0.01 compared with MHO.
Table 3: Sleep parameters of all patients (n=180).
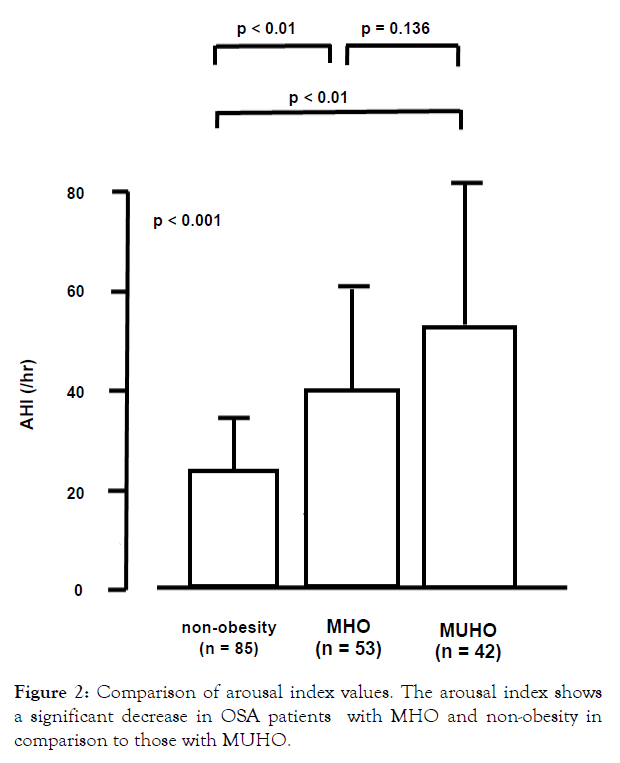
Figure 2: Comparison of arousal index values. The arousal index shows a significant decrease in OSA patients with MHO and non-obesity in comparison to those with MUHO.
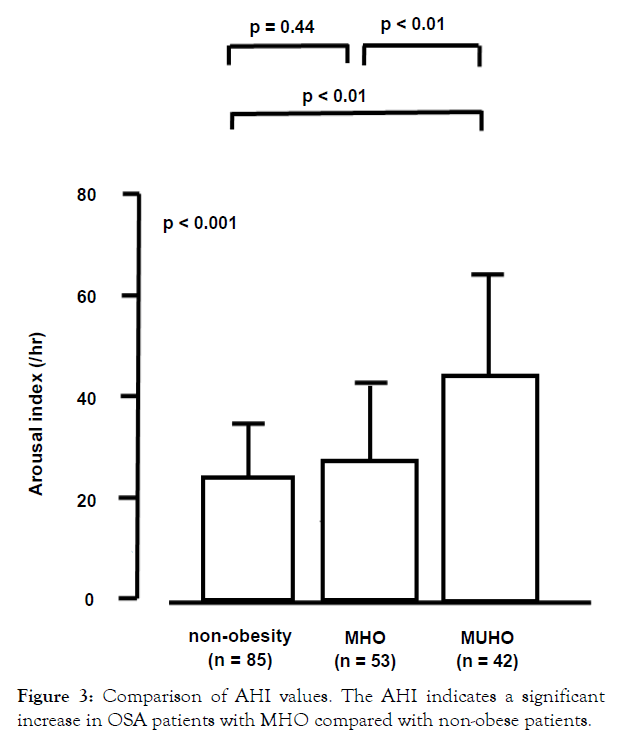
Figure 3: Comparison of AHI values. The AHI indicates a significant increase in OSA patients with MHO compared with non-obese patients.
This study had two major findings. Firstly, the arousal index was lower in OSA patients with MHO than those with MUHO. No significant difference in arousal index was observed between non-obese and MHO patients with OSA. To our knowledge, this is the first stydy to indicate the difference in the appearance of arousal index in OSA patients with MHO and MUHO. Secondly, AHI scores showed a significantly higher value in patients with MHO compared to those with non-obesity. While OSA patients with MHO are reported to be at a higher risk for cardiovascular comorbidity than those with non-obesity, the reports are conflicting [5-7].
Arousal index is reported to represent sympathetic nerve activity [10]. The relationship between sleep fragmentation and sympathetic activity has also been investigated in other studies [21]. It has been reported that patients with OSA have alteration in heart rate variability measures and sympathetic overactivity, with the arousal index being a more potent contributory factor for such activity [21]. In addition, MetS increases sympathetic activity [14]. Thus, the degree of sympathetic activation is much greater in patients with both obesity and MetS than in those with either condition. Consequently, an increase in arousal index in OSA patients with MUHO results in increased cardiovascular comorbidity compared to those with MHO. Another study has reported, using a sleep disorder questionnaire, the difference in quality of sleep between the MHO and MUHO individuals in a community-based cohort [22]. MHO individuals reported to have slightly better overall sleep quality [22]. In this study, we objectively indicated that there was no difference in sleep efficiency and wakefulness after sleep onset between OSA patients with MHO or MUHO. Arousals are suggested to lead to sleep fragmentation, which results in poor sleep quality [12,13]. Our results showed good sleep quality in MHO patients. Obesity without MetS may not worsen sleep quality compared to non-obese OSA patients.
One characteristic feature in MUHO patients in comparison with MHO patients is the increased accumulation of visceral adipose tissue and ectopic fat [1]. Sympathetic activation can be triggered by metabolic factors such as insulin resistance and adipokines secreted from visceral fat [14]. In this study, we suggested that the arousal index in patients with OSA and MUHO was higher than those with MHO, which indicates increased sympathetic activity in patients with MUHO.
No study on the difference in pathological state of OSA in patients with MHO and MUHO has been reported. A previous study reported association of neck circumstance, age, BMI, and lowest SpO2 with MetS in male OSA patients, but no association of AHI, and arousal index were reported [15]. However, their study did not demonstrate the effect of obesity without MetS or MHO in the pathology of OSA. In OSA patients with MetS, the deterioration of sleep quality due to increased arousal should be considered in comparison to those without MetS.
In the present study, 47% of OSA patients were non-obese, and 34% were MHO. A total of 64% OSA patients with obesity were categorized as MHO. OSA patients with MHO are relatively common. The prevalence of MHO was reported to be 8.8% in Japanese individuals and 38.3% in obese people who were less than 65 years old [23].
This study has several limitations. Firstly, the present data are from a single center and females, and accordingly, the present evidences may not be generalized. Secondly, there are currently no universally accepted criteria for identifying MHO, and the effect of MetS on the severity of AHI and arousal index may be different among each criterion. Thirdly, we categorized the random blood sugar levels as normal glycemia between 110 mg/dL and 140 mg/dL. Some of them may have impaired glucose tolerance, and we may underestimate the number of patients with hyperglycemia. Fourth, we could not assess the effect of antihypertensive drugs, drinking, smoking, and caffeine intake, which may affect the arousal index. Unmeasured confounders may have influenced the results. Further investigation is needed to the factor which may influence to the arousal index.
In OSA patients with MHO and MUHO, there was no significant difference in apnea severity, but the number of arousal indices in OSA patients with MHO was less than those with MUHO. The results demonstrate that the appearance of arousals during sleep may be due to the simple correlation with apnea appearance and metabolically unknown factors.
We would like to express our deepest gratitude to the excellent sleep technologists in our clinic for their technical assistance. We would also like to thank Editage (www.editage.com) for English language editing.
This study was performed without financial aid. Authors declare that they have no conflicts of interest. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Ethic Committee at the Nakamura Clinic, Urasoe, Okinawa in Japan. Informed consent was obtained from all individual participants included in the study.
Citation: Kato M, Yamaguchi Y (2021) Effect of Metabolically Healthy Obesity in Male Patients with Obstructive Sleep Apnea. J Sleep Disord Ther 10:325.
Received: 15-Jan-2021 Accepted: 01-Feb-2021 Published: 08-Feb-2021 , DOI: 10.35248/2167-0277.21.10.325
Copyright: © 2021 Kato M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.