Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2020)Volume 9, Issue 5
A study was conducted to determine the effects of boiling time, rhizome form and drying methods on the biochemical quality characteristics of turmeric. The experiment consisted of four levels of boiling times; two levels of rhizome forms and two levels of drying methods laid out in a 4 × 2 × 2 factorial arrangement using Completely Randomized Design. Boling of unsliced rhizomes for 15 minutes followed by tunnel drying yielded higher oleoresin, curcumin and essential oil content. Boiling for 60 minutes then slicing paired with sun drying resulted in a reduction of oleoresin, curcumin, and essential oil content. Generally, all three factors display significant role in influencing different quality attributes of turmeric rhizomes. Among the various boiling time, rhizome form and drying techniques were studied, unsliced rhizome boiled for 15 minutes followed by poly tunnel solar drying was found superior based on the biochemical analysis and concluded for future study on turmeric processing.
Boiling time; Curing methods; Processing method; Quality; Turmeric
Currently, turmeric has increased participation in food products, mainly as dye in pasta, sauces (curry), cheeses, eggs and snacks like potato chips, and is also used in margarine and meat with antioxidant purposes [1]. The biologically active principles, the curcuminoids, can be used as anti-inflammatory, hypocholestraemic, choleratic, antimicrobial, insect repellent, anti-rheumatic, anti-fibrotic, antivenomous, antiviral, antidiabetic, anti-hepatotoxic, anti-cancerous and antihelminthic [2].
The postharvest processing of turmeric involves many units operations such as washing, cleaning, curing or blanching, drying, polishing, size reduction and packaging. The duration when boiling is stopped significantly affect the colour and aroma of the final product. Over-cooking spoils the colour of the final product while under-cooking renders the dried product brittle [3]. The period at which boiling is stopped largely affects the final colour and aroma of the final product. Wet blanching methods, viz. hot water or steam blanching have drawbacks such as leaching of nutrients and color [4] and quality deterioration [5]. Some studies reported that high temperatures, such asexperienced in blanching cause thermal degradation of curcumin [6], while other studies have shown that blanching protects the bioactive ingredients from the effects of drying [7].
Traditionally sun drying of turmeric is extremely weatherdependent and requires unduly long processing times (20 to 30 days) and to some extent leads to prone to infestation, which is not acceptable for industry [8]. The traditional open sun-drying widely practiced by rural farmers has inherent limitations; of high crop losses due to inadequate drying, fungi attacks, insects, birds, rodent encroachment and unpredictable weather effects [9]. Traditional drying method could result in the loss of volatile oil (up to 25%) by evaporation, and in the destruction of some of the light-sensitive oil constituents [10]. Drying has been documented to cause substantial reduction in essential oil content due to the temperature of drying, in Basil [11].
Post-harvest processing of turmeric was not much explored to get quality product. The main contributing factors for this low quality product had been absence of appropriate processing method to get the optimum extraction yields and other quality parameters. In our country the most practice of boiling and drying method practiced by farmers were among the important steps that lead to low quality turmeric, hinders its export potentials, and need further study and recommendations. For these reasons, this study was undertaken to determine the effect of boiling time, rhizome form and drying methods on the turmeric biochemical quality.
Description of the study area
The experiment was conducted at Teppi Agricultural Research Centre. It is located 611 Km away from Addis Ababa at Latitude of 7°10' 54.5'' N and Longitude 35° 25.04' 28.2'' E and altitude of 1200 m.a.s.l. The site receives mean annual rainfall of 1688 mm with maximum and minimum temperatures of 29.5°C and 15.3°C, respectively [12]. The soil of experimental site is reddish brown sandy clay loam classified as nitisol with pH range of 5.60 to 6.0 [13].
Experimental materials
Turmeric rhizome of Dame Variety was collected on January 2018 from Teppi National Spice Research Centre (TNSRC) seed multiplication plot. A total of 216 kg of finger rhizome were used for this experiment. About 4.5 kg of turmeric finger rhizome was used in one running for each experimental unit.
Experimental design and analysis
The experiment consisted of three factors, namely boiling time, drying method and rhizome form. The boiling time had four levels i.e., 15, 30, 45 and 60 minutes. The drying method consisted of two type i.e., direct sun drying and poly tunnel solar drying. The rhizome forms were two i.e., sliced and unsliced. This way the experiment consisted of a 4 × 2 × 2 arrangement resulting a total of 48 with replications laid out in a Completely Randomized Design (CRD) Factorial design. Each treatment was done in triplicates. Analysis of variance was performed using the ANOVA procedure of SAS Statistical Software 9.2 version. Mean comparison was undertaken with LSD at 5%, when significant treatment effects were observed.
Experimental procedure
Boiling time: Curing of rhizomes is attained by boiling at 98°C using a 15 litter capacity stainless steel water heater. The electrical heater had maximum of 220 volt, 5000 watt and up to 1100°C temperature range. The temperature of the hot water is controlled thermostatically. The ratio of sample mass to water volume was 1:2. About 10 litters of water was filled in heater and boiled to the desired temperature of 98°C. Then each finger of 4.5 kg was put into the boiling water and left for the required time, i.e., 15, 30, 45 and 60 minutes. When the specified time of boiling was reached the samples were took out to stop further boiling. The experiment was done in triplicate. The boiled rhizomes after cooling were divided into two groups before drying. One group is directly sent to the dryers to be dried in direct sun light (sun drying) and/or poly tunnel solar drier. The second group was sliced manually perpendicularly at a thickness of 10 mm. The sliced rhizome were further divided into two to be dried in direct sun light (sun drying) and in the poly tunnel solar dryer. The hot water treatment was set based on method reported by Zerihun Fenta et al. [14] and the slicing treatment was according to Prasad et al. [15] (Figure 1).
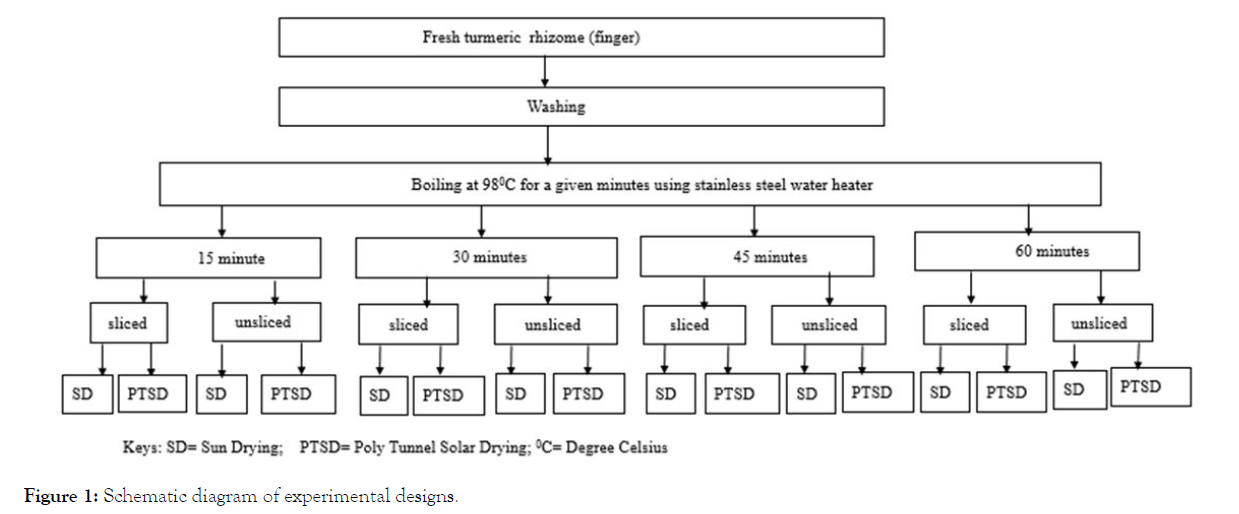
Figure 1: Schematic diagram of experimental designs.
Drying:
Sun drying: The processed turmeric rhizome were spread into two metallic stand with two tray made up of mesh wire each having 1.40 m height above the ground and partitioned by 70 cm × 50 cm tray length and width. The samples were spread at 5-7 cm thickness and covered by plastic sheet to protect from moisture absorbance during overnight.
Poly tunnel solar dryer set up: For this experimental study, Poly Tunnel Solar Dryer (PTSD) with 50 m2 base area was used. The poly tunnel solar dryer relies on the sun as a source of energy as does sun drying. Tunnel shaped semi cylindrical solar drier had size of 10 m × 5 m × 2.7 m of length, width and height, respectively. The roof and drying chamber were constructed with the hoop structure covered with UV-stabilized transparent polyethylene sheet (0.2 mm thickness). In the front side of the poly tunnel solar dryer, a door of 1.2 m × 2 m covered by UVstabilized transparent polyethylene sheet was provided to access the into the poly tunnel solar dryer. Two windows 0.9 m × 0.6 m were provided to remove the moist air. Inside the drier the floor was constructed with cement and two metallic stands with two main trays having a total of 32 drying partition/trays containing mesh trays of 70 cm × 50 cm tray mesh in thin layers of 5-7 cm.
Temperature and relative humidity measurements: Ambient and poly tunnel solar dryer temperatures and relative humidity were measured using a digital thermo-hygrometer (model HTC-1) on a one hour interval from 08:00 am to 5:00 pm. One thermo-hygrometer was placed inside at the midpoint of the PTSD just below the raised trays to monitor the conditions of the air. The other thermo-hygrometer was placed outside the drier for measuring the outside conditions.
Data collected
Total ash content: The total ash content was determined by using muffle furnace (Carbolite 5336RB, Model RWF12/13 (type 50-60); England) apparatus. Weight of empty crucible was taken as (W) and then 2 gram of sample was added to crucible and weighed as (W1). Sample with crucible was then heated over or burns on flame until no fumes are longer produced. Then the muffle furnace containing crucible sample heating started gradually till a temperature 600°C was reached. The furnace was then allowed to stay at this temperature for 6 hours [16]. The crucible with ash sample was taken out and weighed as (W2). Then percentage of total ash content was calculated.
Total ash (TA)% = W2-W/ W1-W × 100 (1)
W =Weight of empty crucible; W1=Weight of crucible and sample; W2=Weight of crucible and ash sample left.
Oleoresin content: A hot continuous extraction (Soxhlet) method was used to extract oleoresin. As cited by Parthasarathy et al. [17] oleoresin was determined by the solvent extraction method using acetone (95%) as organic solvent for 4 to 5 hours. About 10 g of powdered turmeric embedded in filter paper were placed in glass columns blocked with non-absorbent cotton below which a volumetric flask (500 mL) was kept to collect the extract. A thin layer of cotton over turmeric powder was placed. About 250 mL of acetone were used for each sample extraction. The extraction process was carried out at a temperature of 56°C for 4 hours. Solvent removal from the miscella was done by pressure rotary vacuum evaporator at 40°C and 90 RPM. Rotary evaporator was used for distilling off the solvent generally under vacuum. When the last traces of acetone were evaporated, the flask was placed in a hot air oven at 110 ± 2°C until two consecutive weightings taken at 11/2-hours intervals did not differ by more than 1 mg [18]. Finally, the flask was cooled in desiccators, and then weighed and quantified as percent weightweight basis based on the formula described by American Spice Trade Association [19].
Oleoresin (%) = Weight of oil (g) / Weight of sample (g) × 100 (2)
Determination of essential oil content: Turmeric the essential oil content was obtained by hydro distillation with Clevenger apparatus held to a round bottom flask. The sample was ground to pass No 20 (850 μ) sieve size [20]. About hundred gram of dried turmeric powder was transferred quantitatively in 2000 mL flask. About 1000 mL of water was added to flask fitted with an electrical heating mantle and Clevenger apparatus with condenser. The stand road and stand base were connected to heating mantle by clump holder. A continuous flow of water was maintained through hose tube and the flask was heated to boiling to 100°C and maintained a reflux rate of 1 to 2 drops per second. Distillation was continued for 5 hrs [21]. At a top of layer all oil and vapour mixture was got condensed and collected by using separator funnel having volume count up to 15 mL reading and quantified as volume-weight basis. Average value of volatile oil content, on moisture free basis, expressed in the unit of mL/100 g of sample was recorded
Essential oil = Volume of oil (mL) / Weight of sample (g) × 100 (3)
Determination of curcumin
In the present investigation curcumin content in rhizome of Curcuma longa was determined by solvent extraction followed by spectrophotometer method according to modified method of Joshi [22].
Preparation of calibration curve for curcumin: Standard curve was obtained using the standard solution in the range of 1.6 μg/mL to 8 μg/mL. Accurately 100 mg of standard curcumin and 50 mL of ethanol were added in a 100-ml beaker followed by stirring vigorously. The mixture was made up to volume with ethanol. A 200, 400, 600, 800 and 1000 μL of above solution was taken and diluted to 10 mL with ethanol to prepare 1.6, 3.4, 4.8, 6.4 and 8 ppm of curcumin. The calibration curve was plotted as concentration against absorbance serial diluted solution. Accordingly, 1.6, 3.4, 4.8, 6.4 and 8 μg/mL standard of curcumin exhibits absorbance of 0.1886, 0.4391, 0.6626, 0.8698 and 1.0209, respectively. The absorbance of these solutions were recorded in 1 cm cells at the wavelength of maximum absorption at about 425 nm with uv-vis Spectrophotometer [23].
Preparation of test Solution: Test solution was prepared by adding about 100 mg of crude turmeric rhizome powder in 50 mL of alcohol (95%) contained in 100 mL volumetric flask. The solution was sonicated for about 10 minutes and the volume was made up to 100 mL by alcohol (95%). The solution was filtered and 2 mL of this solution was diluted up to 25 mL by alcohol (95%). Absorbance of the resultant solution was taken by Spectrophotometer VIS 630 Jasco, S/n (A112761148), Japan) at 425 nm.
The percentage of curcumin content was found out from calibration curve of standard curcumin shown in Figure 2.
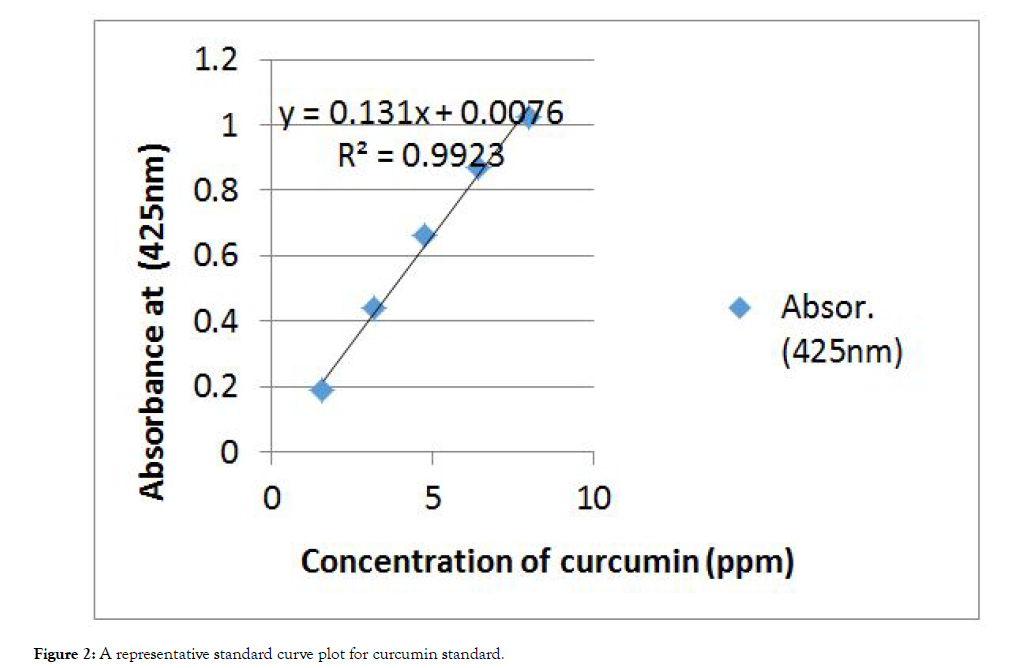
Figure 2: A representative standard curve plot for curcumin standard.
Effect of interaction among boiling time, rhizome form a nd drying method on biochemical quality attributes of t urmeric
Oleoresin content: Table 1 presents chemical quality attributes data of turmeric as affected by interaction of all three factors and showed significant differences at (P<0.05) for all chemical quality parameters. The highest 17.57% oleoresin content was recorded for tunnel dried unsliced samples which were boiled for only 15 minutes. The lowest (13.73%) oleoresin was determined for samples subjected to the longest (60 minutes) boiling time of sliced tunnel dried samples. These interactions appeared to have strong influence of boiling time. However, many of the treatment combinations exhibited medium value oleoresin content of between 15.00 to 17.00% tunnel dried, 16.53% showing significant differences (P<0.05) among them. The highest (17.57%) had about 21.85% and 11.04% more yield oleoresin advantage over the overall mean (15.63%) and the least mean value 13.73% respectively. This may be due to the effect of boiling time increment in water; the effect of direct sun light and high temperature during drying and form of rhizome causes the break up the oleoresin cells and facilitates the rapid release of oleoresin containing curcuminoids to the surrounding during processing. Similar to this result Suresh et al. [24] stated the significant loss 27-53% in spice active principles when subjected to heat processing and yielded 18.8% and 17.3% oleoresin when boiled for 10 and 20 minutes, respectively. The same trend was reported by Zerihun Fenta et al. [14] the highest mean oleoresin yield (19.27%) was recorded from mother rhizomes boiled at 100°C temperature for 30 minutes, showing the effect of longer processing time on the quality. In other study [25] reported maximum oleoresin content of 17.65% from finger rhizome dried on mesh wire with drying thickness of 6 cm.
| Boiling time | Drying method | Rhizome form | Oleoresin (%) | Essential oil (mL/100 g) |
|---|---|---|---|---|
| 15 minutes | Sun Drying | Unsliced rhizome | 15.87c ± 0.03 | 3.45def ±0.40 |
| Sliced rhizome | 15.44cd ± 0.08 | 3.40efg ±0.13 | ||
| Poly tunnel solar drying | Unsliced rhizome | 17.57a ± 0.25 | 3.93a ± 0.14 | |
| Sliced rhizome | 16.50b ± 0.30 | 3.75b ± 0.05 | ||
| 30 minutes | Sun Drying | Unsliced rhizome | 16.53b ± 0.12 | 3.50de ± 0.09 |
| Sliced rhizome | 15.26d ± 0.25 | 3.28gh ± 0.10 | ||
| Poly tunnel solar drying | Unsliced rhizome | 16.62b ± 0.62 | 3.93a ± 0.19 | |
| Sliced rhizome | 16.66b ± 0.25 | 3.66bc ± 0.06 | ||
| 45 minutes | Sun Drying | Unsliced rhizome | 15.16de ± 0.26 | 3.30fgh ± 0.10 |
| Sliced rhizome | 14.76e ± 0.12 | 3.26gh ± 0.06 | ||
| Poly tunnel solar drying | Unsliced rhizome | 16.76b ± 0.15 | 3.53cde ± 0.09 | |
| Sliced rhizome | 15.53cd ± 0.21 | 3.60bcd ± 0.05 | ||
| 60 minutes | Sun Drying | Unsliced rhizome | 14.74e ± 0..31 | 3.17h ± 0.06 |
| Sliced rhizome | 14.42f ± 0..20 | 2.82i ± 0.13 | ||
| Poly tunnel solar drying | Unsliced rhizome | 14.80e ± 0.26 | 3.31fgh ± 0.07 | |
| Sliced rhizome | 13.73g ± 0.12 | 3.30fgh ± 0.08 | ||
| LSD (5%) | 0.45 | 0.16 | ||
| CV (%) | 1.73 | 2.86 | ||
Mean values down the columns with the same letters are not significantly different at 5% level significance. CV=Coefficient of variance; LSD= Least significance difference
Table 1: Effect of interaction among boiling time, rhizome form and drying method on oleoresin and essential oil of turmeric.
Essential oil
Data of essential oil as influenced by the interactions of the three factors is shown in Table 1. The highest yield of 3.93 mL/100 g essential oil was obtained from unsliced samples dried in tunnel with boiling time of 15 and 30 minutes. The next higher values were 3.75, 3.66 and 3.60 mL/100 g with respective boiling time of 15, 30 and 45 minutes of sliced samples dried in the tunnel. Thus, combinations of shorter boiling time of unsliced rhizome and tunnel drying resulted in higher essential oil, whereas longer boiling times combined with sun drying of sliced rhizome led to more loss of essential oil content. From this it can be seen that boiling time had less role to play in the interaction effect whereas tunnel drying favor or maintain essential oil extraction. On the other hand the lowest essential oil (2.82 mL/100 g) was recorded for sample boiled for a time of 60 minutes and then sliced sun dried samples. Generally from above results about 28.24% more yield difference existed between the highest and lowest essential oil content due to the interaction effect of processing. Similarly, this result was in agreement with Pruthi et al. [26] and Jose et al. [10] who reported traditional drying method could result in the loss of volatile oil of up to 25% by evaporation, and in the destruction of some of the light-sensitive oil constituents.
In this study combination of sun drying and slicing had resulted in low records of the oil regardless of the boiling time. This yield was determined by the volume to mass ratio showed value among those found in literature, relatively up to 5% [27] and range between 0.97 to 7.55 mL/100 g according to Souza and Gloria [28].
Curcumin content (%)
The result in Table 2 had shown significant (P<0.01) difference on curcumin content due to the interaction effect among boiling time, rhizome form and drying method on curcumin content. All factors had balanced influence in affecting the results. The top three values (4..46, 4.34 and 4.06%) with significant differences among them were recorded for unsliced samples dried in the tunnel after boiling times of 15, 30,and 45 minutes respectively. The lowest percentage of Curcuma (3.53%) was recorded for sliced sun dried sample which was boiled for the longest time, 60 minutes. The degradation of curcuminoids of finger rhizomes could possibly be due to extended curing time, sliced samples dried in the open sun light which caused loss of curcumin constituents. Unslicing and polytunnel drying of rhizomes help to maintain curcumin yield for boiling times of 15 to 45 minutes. This result is in line with the work of Shinde et al. [29], who found decreasing value of curcumin content when the boiling time increased and reported that boiling turmeric for 15, 20, 25 and 30 minutes yielded curcumin contents of 4.23, 4.21, 3.91 and 2.29%, respectively.
| Boiling time | Drying method | Rhizome form | Total ash (%) | Curcumin (%) |
|---|---|---|---|---|
| 15 minutes | Sun Drying | Unsliced rhizome | 8.86a ± 0.08 | 3.81ef ± 0.08 |
| Sliced rhizome | 8.70bc ± 0.10 | 3.86de ± 0.05 | ||
| Poly tunnel solar drying | Unsliced rhizome | 8.83ab ± 0.10 | 4.46a ± 0.06 | |
| Sliced rhizome | 8.77abc ± 0.03 | 3.87de ± 0.03 | ||
| 30 minutes | Sun Drying | Unsliced rhizome | 8.70bc ± 0.09 | 3.81ef ± 0.07 |
| Sliced rhizome | 8.62cde ± 0.07 | 3.78efg ± 0.03 | ||
| Poly tunnel solar drying | Unsliced rhizome | 8.76abc ± 0.03 | 4.34b ± 0.06 | |
| Sliced rhizome | 8.68bc ± 0.16 | 3.93d ± 0.12 | ||
| 45 minutes | Sun Drying | Unsliced rhizome | 8.46ef ± 0.15 | 3.70gh ± 0.02 |
| Sliced rhizome | 8.65cd ± 0.05 | 3.83def ± 0.03 | ||
| Poly tunnel solar drying | Unsliced rhizome | 8.30gh ± 0.10 | 4.06c± 0.06 | |
| Sliced rhizome | 8.26h ± 0.12 | 3.86de ± 0.06 | ||
| 60 minutes | Sun Drying | Unsliced rhizome | 8.45fg ± 0.09 | 3.75fgh ± 0.05 |
| Sliced rhizome | 8.68bc ± 0.16 | 3.53i ± 0.06 | ||
| Poly tunnel solar drying | Unsliced rhizome | 8.68bc ± 0.08 | 3.80ef ± 0.05 | |
| Sliced rhizome | 8.50def ± 0.00 | 3.66h ± 0.08 | ||
| LSD (5%) | 0.16 | 0.10 | ||
| CV (%) | 1.14 | 1.55 | ||
Mean values down the columns with the same letters are not significantly different at 5% level significance. CV=Coefficient of variance; LSD= Least significance difference.
Table 2: Effect of interaction among boiling time, rhizome form and drying method on total ash and curcumin content of turmeric.
Curcumin content in rhizomes and fingers changed depending upon the curing methods adopted [30]. Noticeable difference with the yields of curcuminoids between the 15 minute (17.41%) and the 30 minute (13.74%) blanched treated samples reported as reported by Green et al. [31]. This result was close to the report of Sahu [32], who stated the values of curcumin content for convective dried sliced turmeric samples between 2.74 to 2.99 percent. Some studies reported that high temperatures, such as experienced in blanching, causes thermal degradation of curcumin [33], implies a direct effect of prolonged boiling, sun drying and sliced rhizome resulted in more curcumin loss. The study of Kadam et al. [34] stated that as the thickness of sample is reduced the curcumin content goes on decreasing for different drying temperatures.
Total ash (%)
Ash content gives us the picture of total amount of inorganic substances, including trace and major mineralsThe ash content has been affected significantly by the interactions of the three factors, appeared to have no noticeable trend and ranged between 8.26% to 8.86%. The minimum variation due to interaction effect implies the amount of inorganic residue left over during curing and when heat is applied. In this case it determines the mineral makeup of the turmeric, nutritional value and quality. The amounts of total ash content in the rhizomes are more affected due to increased boiling duration and sliced form of rhizomes used. The present result, which signifies the quality and purity of the turmeric powder, is in accordance with Kadam et al. who reported an average ash content of 8.39% represented the purity of the samples. Total ash content of 13.08% from finger rhizomes treated at 80°C for 30 min and the least 9.95% ash for rhizome boiled at 100°C for 75 minutes was reported by Zerihun Fenta et al. [14] also implies the same trend due to processing effect.
The results of the study revealed that almost all chemical quality attributes (oleoresin, essential oil, total ash and curcumin) were significantly (P<0.05) affected by curing and drying methods. It was found that the three factors interacted with one another influencing the quality characteristics of the turmeric. Generally, the shortest boiling time, unsliced rhizomes and poly tunnel drying appeared to give sample with better performance in the biochemical quality attributes expected for the rhizomes of any other combination of factors.
Sun drying resulted in a significant loss in all biochemical quality than a poly tunnel solar drier one. Quality of turmeric was obtained more from unsliced rhizome than from sliced one. Thus, majority of chemical quality characteristics were not much degraded or lost in case of unsliced rhizome as it was for sliced samples. From this study, it can be concluded that shorter boiling time of 15 minutes, unsliced rhizome form and use of the poly tunnel solar dryer give product of highly acceptable quality characteristics.
There are no conflicts of interests.
The author would like to thank the Ethiopian Institute of Agricultural Research for funding the research.
Citation: Hirko B, Abera S, Mitiku H (2020) Effect of Curing and Drying Methods on the Biochemical Quality of Turmeric (Curcuma longa L.) Rhizome Grown in South Western Ethiopia. Med Aromat Plants (Los Angeles) 9: 357. doi: 10.35248/2167-0412.20.9.357
Received: 29-Jul-2020 Accepted: 17-Aug-2020 Published: 24-Aug-2020 , DOI: 10.35248/2167-0412.20.9.357
Copyright: © 2020 Hirko B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.