International Journal of Physical Medicine & Rehabilitation
Open Access
ISSN: 2329-9096
ISSN: 2329-9096
Research Article - (2023)Volume 11, Issue 3
Background: Cetylated Fatty Acids (CFAs) have shown to reduce pain by decreasing the production of key mediators. They have been successful in improving many conditions including athletic pubalgia, shoulder tendinopathies, and osteoarthritis, but their effects on axial discogenic back pain have never been studied. This study aims to investigate if the short term supplementation of oral CFAs reduces pain and disability in patients with axial discogenic low back pain.
Methods: The study included 27 patients with an average age of 57 ± 16 years diagnosed with axial discogenic low back pain, based on axial symptoms of chronic low back pain for more than 3 months. The primary outcome of the study was the Oswestry Disability Index (ODI) score. The secondary outcomes were the Numeric Pain Rating Scale (NPRS) (best, worst, and current pain scores) and adverse events. Clinical evaluations were performed at baseline and after a 4-week supplementation period with oral CFAs.
Results: After four weeks of supplementation, our analysis determined a statistically significant reduction in ODI scores from 24.6% ± 16.0 to 16.2% ± 10.7 (p value=0.0022). 48% of patients were determined to be responders by fulfilling the calculated Minimal Clinically Important Difference (MCID) for ODI at 4 weeks. NPRS current, worst, and best scores all improved significantly (p-value<0.05) from baseline to 4 weeks. 11.1% of patients experienced adverse effects, none of which were life threatening.
Conclusion: The use of CFA supplementation reduced axial discogenic low back pain and disability in this prospective study. However, further research on the use of this treatment is warranted, including randomized controlled trials.
Low back pain; Cetylated fatty acid; Clinical outcomes; Disability
Low back pain is the leading contributor to disability and lost workdays in the United States [1]. There are many causes of low back pain, but the most frequent cause of nonspecific lumbar back pain is discogenic [2-4]. Axial discogenic back pain entails degeneration of the intervertebral disc without herniation [2]. While spinal surgery is effective for other causes of back pain such a radicular pain, spinal stenosis, spondylolisthesis, and other conditions, it is not frequently used as a treatment for axial discogenic back pain [2]. Axial discogenic pain is complex with potential contributions from the Intervertebral Disc (IVD), ligaments, facet joints, and surrounding musculature, making it very difficult to treat [1,2]. Due to the frequency of axial discogenic back pain and its impact on the population, determining appropriate treatment is essential. Currently, there is a lack of clarity on treatment recommendations for patients with axial discogenic back pain [5]. Conservative management including pharmaceutical therapy is often used as a first-line treatment [6-8]. Pharmaceutical therapy is often targeted at a reduction in inflammation. IVDs experience mechanical overload thought to cause increased infiltration of macrophages leading to production of pro-inflammatory mediators [9]. Therefore, NSAIDs are frequently utilized [1]. Anti-inflammatories have demonstrated significant results but come with notable side effects for extended use such as renal toxicity and GI side effects [10]. Opioids can also be used in very severe cases but are largely not recommended due to their risk of dependence and addiction [1]. Surgical interventions, including spinal fusion and total disc replacement, and regenerative medicine are options for patients who do not respond to conservative management [1,2]. However, surgical options have high risks of complication and regenerative medicine has only been effective in treating early IVD degeneration [1,2,11-13]. Hence, there is still a need for effective treatment options that can safely reduce inflammation and pain in patients.
In 2001, Cetylated Fatty Acids (CFA), which are fatty acids esterified with cetyl alcohol, were used to treat conditions associated with sports and arthritis [14,15]. Studies utilized a variety of administration techniques including topical CFA cream, capsule supplementation, and patch formulation [15-21]. They are believed to reduce pain by decreasing the secretion of leukotriene B4 from stimulated neutrophils, diminishing the release of IL-1 by monocytes, and ultimately reducing the production of IL-6, TNF, and MCP-1 [10,15-17,19,20,22-25]. In a variety of conditions, including athletic pubalgia, shoulder tendinopathies, osteoarthritis, and myofascial pain syndrome of the neck, fatty acids caused an improvement in muscle strength, pain, and range of motion [5,15-21]. However, CFAs have not been used in the treatment of axial discogenic low back pain. Therefore, we decided to carry out this study to evaluate the effects of the oral supplementation of Cetylated fatty acids (CFA) on patients with axial discogenic back pain.
The hypothesis of the study is that the oral supplementation of CFAs for 4 weeks will drastically reduce disability as measured by the ODI score, and pain as measured by the NPRS from axial discogenic low back pain. This was an IRB approved, prospective, single-center cohort study conducted at a single institution (ISRCTN16509365) and conducted in accordance with Good Clinic Practice guidelines and the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all patients prior to enrolling.
Study population
A total of 36 patients were recruited into the study. Patients over 18 years of age were enrolled if they had all the following inclusion criteria: axial symptoms of CLBP (>3 months of duration). The exclusion criteria included: patients currently on narcotic pain medication, patients who are pregnant or currently breastfeeding, patients with low back pain from traumatic injury, patients currently using a pain patch (e.g lidocaine), concurrent pathology that may contribute to the patient’s axial low back symptoms (e.g., spondylolysis, spondylolisthesis, facet arthropathy), severe lumbar disc degeneration, any peripheral neurological symptom attributed to the intervertebral disc pathology, a history of lumbar spine surgery, or a history of previous spine trauma. Since the above conditions usually result in lower back pain, placing them beyond the scope of this study enabled us to accurately observe the symptoms and progression of axial discogenic LBP. Patients were instructed to continue the treatments they have been utilizing without adding anything new except the CFAs.
Intervention
Subjects were given 3 bottles of Pharma Nutra Lipocet CFAs (Via Delle Lenze, 216/b-56122 Pisa). Ingredients of the CFAs included medium chain triglycerides, Cetylated fatty acids (refined olive oil, cetyl myristate, cetyl oleate) with excipients. Patients were instructed to take 2 capsules (300 mg CFAs each) twice a day for 4 consecutive weeks (1200 mg daily).
Patient evaluation
The primary outcome of the study was patient disability measured using the Oswestry Disability Index (ODI) scores. The secondary outcomes were the scores of the Numeric Pain Rating Scale (NPRS) and any adverse events. NPRS scores included pain at the worst, pain at the best, and current pain level. At the time of enrolment, ODI and NPRS scores were collected via a paper document. At the end of 4 weeks, patients were contacted via phone and/or email and were asked to complete a survey regarding their ODI scores, NPRS scores, and any adverse events.
Statistical analysis
The normality of the distributions of continuous variables was assessed using the Jarque-Bera test. Continuous variables were represented as mean ± standard deviation. A two-tailed paired t-test was performed to compare mean baseline ODI and NPRS scores to mean scores after 4 weeks. A non-parametric Wilcoxon-Mann-Whitney test was used for data that did not follow a normal distribution. The Minimally Clinically Important Difference (MCID) for the ODI at 4 weeks was calculated using a distribution-based method of dividing the standard deviation of the mean improvement from baseline, by two. A chi-squared test was performed to compare the demographics of responders and non-responders. A p-value below 0.05 was considered significant. The statistical analysis was carried out using Microsoft Excel Version 16.63.1.
Thirty-six patients were enrolled in the study (Figure 1). Two patients were lost to follow up, 2 patients were non-compliant with taking the medication, 1 patient failed to complete the surveys, and 1 patient stopped taking the medication due to concerns about mixing with other medications. A total of 27 patients were included in the final analysis. Participants’ ages ranged from 25 to 83 years (57 ± 16) with 52% identifying as female (Table 1). The average BMI of participants was 25.8. The baseline ODI scores significantly decreased from 24.6% ± 16.0 to 16.2% ± 10.7 after 4 weeks of oral CFA supplementation (p=0.0022) (Figure 2). NPRS worst scores decreased from pre-CFA supplementation (7.63 ± 1.71) to post (5.67 ± 2.13) supplementation (p=0.0006) (Table 2). There was also a statistically significant decline in NPRS current scores declining form pre (4.56 ± 2.45) to post (2.63 ± 1.96) (p=0.0015). Moreover, NPRS best scores decreased from pre-CFA supplementation (2.48 ± 1.91) to post (1.74 ± 1.79) supplementation (p=0.0343). Patients were separated into responders (48.15%) and non-responders (51.85%) (Figure 3) based on whether they fulfilled the MCID for ODI at 4 weeks. The baseline characteristics of responders and non-responders were compared. However, no significant differences were found between both groups. Adverse events were also gathered throughout the study for patients who contacted the research team and as a survey question at the end of the study. There was a total of 4 patients that presented were adverse events.
| Characteristic | Study population (n=27) |
|---|---|
| Age (mean ± sd) | 57 ± 16 |
| BMI (mean ± sd) | 25.8 ± 5.25 |
| Sex n (%) | 13 (48%) male: 14 (52%) female |
Note: BMI: Body Mass Index (units: kg/m2).
Table 1: Patient demographics.
| Clinical Evaluation | Baseline | Final | P-value |
|---|---|---|---|
| ODI score | 24.6% ± 16.0% | 16.2% ± 10.7% | 0.0022 |
| NPRS best | 2.48 ± 1.91 | 1.74 ± 1.79 | 0.0343 |
| NPRS worst | 7.63 ± 1.71 | 5.67 ± 2.13 | 0.000591 |
| NPRS current | 4.56 ± 2.45 | 2.63 ± 1.96 | 0.00153 |
Note: Final: 4 week follow up. Baseline and Final values stated as mean ± SD. P-value: baseline vs. final. A p-value of less than 0.05 was considered significant, NPRS: Numeric Pain Rating Scale.
Table 2: Passive range of motion.
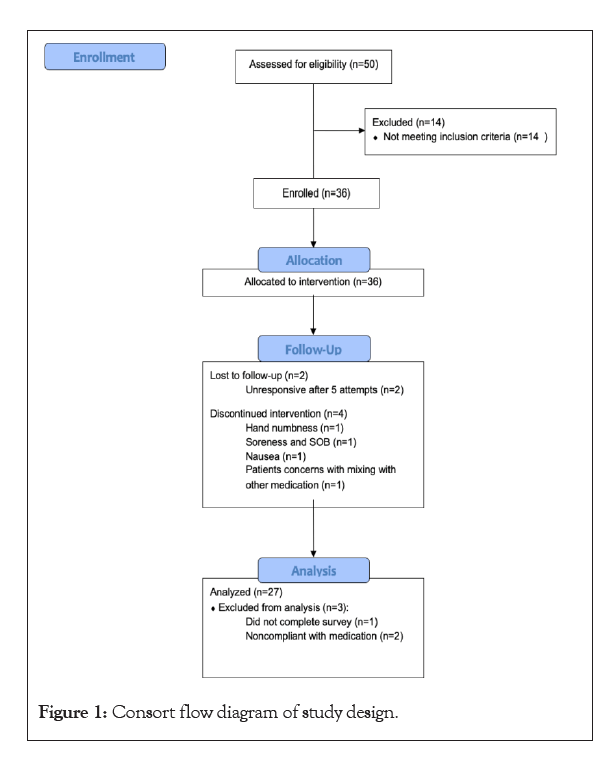
Figure 1: Consort flow diagram of study design.
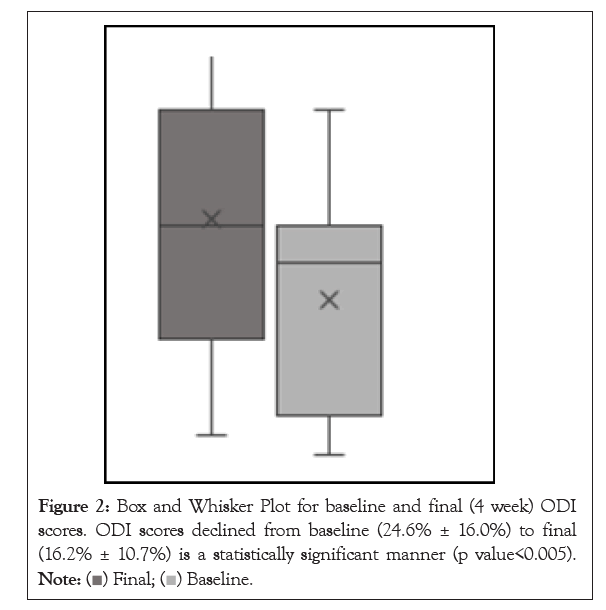
Figure 2: Box and Whisker Plot for baseline and final (4 week) ODI scores. ODI scores declined from baseline (24.6% ± 16.0%) to final (16.2% ± 10.7%) is a statistically significant manner (p value<0.005). 
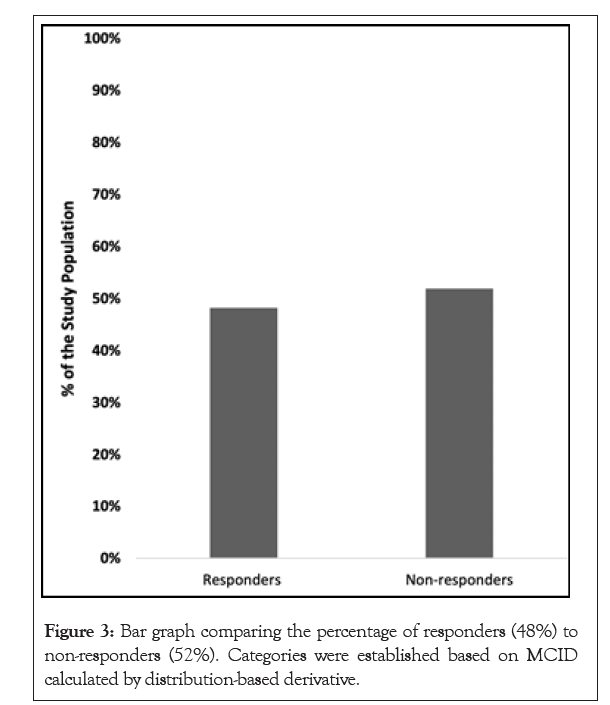
Figure 3: Bar graph comparing the percentage of responders (48%) to non-responders (52%). Categories were established based on MCID calculated by distribution-based derivative.
One patient had mild gastrointestinal upset but continued taking the medication. One patient discontinued the medication after 3 days due to nausea. One reported soreness and shortness of breath after 17 days of supplementation and discontinued its use. One patient reported feeling hand numbness after taking the study medication and discontinued its use after 6 days. Since these patients were unable to provide results after the target period of 4 weeks, they were excluded from the analysis.
To our knowledge, this is the only study utilizing CFAs to treat axial discogenic low back pain. This study found that oral supplementation of CFAs for 4 weeks, reduced patient disability and improved function. This was indicated by a statistically significant reduction in ODI scores. The severity of the pain also reduced, as shown by the statistically significant reduction of NPRS best, worst, and current scores. NPRS best, worst, and current scores were all taken as an effort to address the critique that NPRS does not account for the complexity and changing nature of chronic low back pain. While 48% of patients were responders using MCID for ODI scores, we must consider that axial discogenic low back pain is a complex condition, and this response rate is significant to an issue as difficult to treat. Based on these findings, CFAs are an effective treatment option for pain and disability management in axial discogenic low back pain, as compared to other substances that come with extensive side effects.
It is also important to frame the results of our study on CFAs in the scope of the other treatments available. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and opiates are the most common pharmaceutical options for treating axial discogenic low back pain, with NSAIDs currently used as the first-line analgesic option [6-8,26]. An extensive 2011 Cochrane review meta-analysis on NSAID use for low back pain concluded that NSAIDs have only shown a small impact for the treatment of chronic low back pain [6,26]. When compared to a placebo, the pool weighted mean difference was -12.40 (95% CI: -15.53 to -9.26) in favour of NSAIDs [6]. Overall, NSAIDs were slightly better than placebo at improving pain and disability from chronic low back pain. However, NSAIDs come with significant gastrointestinal side effects; 15%-30% of patients on chronic NSAIDs will develop erosions or ulcers according to endoscopy studies [6,27]. NSAIDs have also shown significant renal toxic and cardio toxic effects. However, their prevalence is lower than that of gastrointestinal side effects [27].
In comparison, opiates administered for the management of chronic low back pain, have shown to have an analgesic effect in the short term [28]. However, their ability to improve function and their effectiveness in the long term is unknown [28]. The average pain relief, encompassing chronic pain in general, was 30%; however, data on long term usage and chronic back pain specifically is still unknown [28]. Hence, the use of opioids for chronic pain must be highly regulated since they are the main contributors to the opioid epidemic. Opiates have high rates of development of abuse and/or dependence, and negative psychological changes including depression and anxiety, and hyperalgesia. Similar to NSAIDs and opiates, the usage of CFAs in this study, resulted in an improvement in pain and disability in the short term. The ODI scores and NPRS of all measures decreased in a statistically significant manner. Around half of the patients who received CFAs were considered as responders, which was a significant number of patients in comparison. Since our supplementation period was only 4 weeks, the comparison of the long-term effects and short-term effects of CFA supplementation is not possible. Moreover, since our study is not a randomized controlled trial, we are not able to make direct comparisons in success between NSAIDs, opiates, and CFAs. Patients in our study did not have the significant side effects of opiates and NSAIDs. In our study, around 10% of patients experienced adverse events, none of which were life threatening. One patient experienced shortness of breath. The patient pursued a cardiac stress test which was negative. We are unsure if this was a result of CFA supplementation or other conditions of the patient. Prior studies utilizing CFAs have supported limited side effects. In comparison with current pharmaceutical options, the side effects noted for CFAs are minor. Thus, CFAs show strong capabilities as a no harm first line treatment for patients with axial discogenic low back pain.
We are aware that this study has some limitations. First is the lack of a control group containing subjects receiving a placebo. Although comparison between CFA and placebo was not within the aim of this study, a placebo arm would have allowed us to obtain clearer results and should be pursued in future studies. Similarly, the study did not have blinding of physicians or patients. This has to potential to impact the results and should be considered in future studies. Moreover, this study was conducted at a single center which cannot be representative of the entire population. Inclusion of patients from various centers from across the country would prevent bias, reduce errors and improve the accuracy of the results. Finally, the length of follow up could be considered a limitation. Prior studies analysing the use of NSAIDs, utilized periods ranging from <2 weeks to >12 months [26]. While this range is variable, our supplementation for 4 weeks would be considered short-term.
A follow up at a later time point would help us to draw additional conclusions regarding the use of CFAs in the chronic pain and disability resulting from axial discogenic low back pain. In conclusion, the oral supplementation of CFA for a period of 4 consecutive weeks in patients with axial discogenic low back pain, indicated a reduction in disability and pain with minimal adverse effects. Oral CFA supplementation could be a promising solution to improve disability and pain in patients with axial discogenic low back pain as a first line treatment, though further studies need to be conducted.
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
Citation: Pelak A, Barve A, Carroll K, Madrazo-Ibarra A, Vad V (2023) Effect of Cetylated Fatty Acid Supplementation on Axial Discogenic Low Back Pain. Int J Phys Med Rehabil.11:662
Received: 26-Jan-2023, Manuscript No. JPMR-23-21566; Editor assigned: 30-Jan-2023, Pre QC No. JPMR-23-21566; Reviewed: 14-Feb-2023, QC No. JPMR-23-21566; Revised: 22-Feb-2023, Manuscript No. JPMR-23-21566; Accepted: 28-Feb-2023 Published: 28-Feb-2023 , DOI: 10.35248/2329-9096.23.11.663
Copyright: © 2023 Pelak A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.