
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Review Article - (2021)Volume 12, Issue 9
Chronic or long-term insomnia is a common disorder. It can also make it hard to stay asleep. It affects a wide spectrum of societies almost all over the world. It saps an individual’s energy in such a way one still feels tired during day time. The condition consequently affects mood, life quality, health, and performance of normal daily activities. Acute or short-term sleeplessness that lasts days or few weeks may be attributed to stressful life events including socioeconomic burdens. In this review work, we discuss the drug treatment of this illness that includes antihistamines, melatonin, benzodiazepines, and the Z-drug (zopiclone). We compare both the advantages and disadvantages of these agents with special emphasis on the benzodiazepine (diazepam DZP) and the Z-drug Zopiclone (ZPC). As an alternative to benzodiazepines, the pharmacology of zopiclone including side effects, intoxication, and how to treat this will be discussed here in this work. Currently available hypnotic agents have no selective actions thus have a wide spectrum of drawbacks such as tolerance, addiction, and thus withdrawal effects. The main purpose of this work is to instruct junior doctors, general practitioners, and other health caregivers on how and when to prescribe these agents to individuals that suffer from insomnia when other means like sleep hygienic and psychological means are of no help or as an adjunct to these.
Insomnia; Anxiety; Hypnotic; Melatonin; Zopiclone; Diazepam; Antihistamine
ZPC: Zopiclone; BZD: Benzodiazepine; DZP: Diazepam; GABA: Gamma-Aminobutyric Acid: t/2: Elimination Half-Life
Prelude and epidemiology
Insomnia or sleeplessness may be short-term or last for days and weeks. If chronic (lasting more than a month), it can be a serious health problem, in which individuals experience difficulty falling asleep, staying asleep as they want, or wake up too early and not be able to get back to sleep. As a result, these individuals get sleepiness during the daytime. Thus, they may suffer from irritability, low energy, and depressed mood. All these effects result in concentration problems even in normal daily activities such as learning and ordinary work [1]. Insomnia could be primary where genetic causes play an important role in etiology, which also varies between males and females [2]. It is worthy of mention that insomnia is a major symptom in the diagnostic spectrum of anxiety disorders. Different psychologic and many psychiatric disorders may also emerge as sleeplessness as an early symptom. This is common in major depression, bipolar affective disorder, and schizophrenia. Secondary causes of insomnia are many, the most important of these include chronic pain, heart failure (resultant of orthopnea), menopause (hormonal misbalance in women), thyrotoxicosis, heartburn (hiatus hernia, reflux esophagitis) and certain drugs/agents such as cerebral stimulants (nicotine, caffeine) and alcohol (especially as a withdrawal symptom) [3-6]. Sleep apnea and night shift working are also among well-known causes of secondary insomnia [7]. Bad nutritional habits such as big meals before sleep are also among the causes of insomnia [8]. Extra calories especially late in the evening will be deposited as fatty acids in triglycerides which increase body weight and lead to obesity, which may be another potential cause of insomnia. A natural byproduct of fatty acid oxidation to produce energy (biosynthesis of ATP) is free radicals. These reactive species are unstable molecules and often have extra electrons which normally are delivered to molecular oxygen in the electron transport chain. Thus consumption of regular amounts of antioxidants in form of vitamins (C, A, and E) and other micronutrients is important to support the body’s natural processes to scavenge these reactive species. Oxidative stress is both a causative factor and a result of insomnia [9-11]. Insomnia is a common problem in almost all societies of the globe, it affects middle-aged people above 65 years, females more than men [12,13]. This is attributed to the change in sex hormones both in males and females as they get older [14,15].
It is believed that between 10%-30% of adults can get insomnia at any given point in time; moreover half of the people in any given society can have insomnia in a given year [16], meaning that insomnia is a potential problem that can jeopardize prosperity and well-being of humans in all societies [16].
Diagnostic criteria of primary insomnia according to DSM-5
The major criteria are:
Difficulty initiating sleep
Difficulty to maintain sleep with frequent awakenings and/or difficulty to sleep after awakenings.
Early-morning awakening with inability to return to sleep again.
In addition to these, several minor criteria distinguish insomnia, these include
• The sleep difficulty occurs at least 3 nights per week.
• The difficulty should be present for at least 3 months.
• The difficulty occurs despite adequate opportunity for sleep.
• The difficulty should not be secondary to other somatic diseases or sleep-wake disorders such as narcolepsy (disturbance of sleep-wake cycles), breathing-related insomnia, circadian rhythm sleep disorder (disturbance of sleep timing), parasomnia (abnormal movements, behaviors, emotions, perceptions, and dreams that occur while falling asleep, sleeping between sleep stages, or during arousal from sleep.
• The ailment should not be attributable to physiological effects of substance misuse e.g. drugs of abuse or alcohol.
• When coexisting mental disorder and/or somatic medical conditions cannot adequately alone explain the reason for existing insomnia.
Pathophysiology and mechanism of insomnia
Regarding the pathophysiology of insomnia, according to the available findings, two models have been proposed to elucidate the etiology of this morbidity. These are the cognitive and physiological models. Taken all together, findings of these two models suggest dysregulations of the arousal system, cognitive system, and hypothalamic-pituitary axis in the bodies of these individuals. All these dysregulation events hand in hand contribute to insomnia [17,18]. The confusion here is, whether the hyperarousal is resultant of insomnia or a cause of it. Another important finding in these individuals is the altered GABA (Gamma-Aminobutyric Acid) levels, this non-proteinous neurotransmitter has an inhibitory effect on the arousal system, but this has not been confirmed yet by solid pieces of evidence.After approval by the Ethics Committee of North-West and Central Switzerland (EKNZ 2014-176, provided by Chairperson Prof. A. P. Perruchoud on 22. June 2014), data were obtained between January 2012 and December 2017 in a single, non-university Swiss central hospital for interventional angiology. Data were analyzed retrospectively. The requirement for written informed consent for this quality-process control survey was waived by the EKNZ. In 2012 and 2013 CAS was conducted without RA (control group). Since January 2014, we have been routinely conducting CAS with RA. The interventional angiology team (two persons) and the CAS techniques remained unchanged during the entire observation period. The anesthesiology team consisted of 20 experienced anesthesiologists. Their anesthesiology strategies remained unchanged during the observation period.
Aims and objectives
To assess the postoperative analgesic effect of wound irrigation with 0.25% bupivacaine through surgical wound after MRM.
Relevance
The present study was to assess the role of wound irrigation with bupivacaine through wound irrigation catheter in alleviating early post-operative pain after the MRM. This study would provide good understanding of the sufferings of a patient undergoing a major surgery and provide an effective pain alleviation method for it. Hence this study can be used in future application of the said method in post MRM patients.
Study design: A Longitudinal observational study.
Study setting: MES medical college, department of anaesthesiology.
Study population: Patients undergoing modified radical mastectomy in MES medical college, Perinthalmanna.
Sample size: The data from MRD in MES medical college showed that there were cases of MRM in previous year. So we enrolled 30 patients in our study. A purposive sample of 30 patients were studied.
Sampling procedure: Convenient sampling procedure was utilized.
Study duration: The data was collected from 1st January 2017 to 31st July 2018, a total duration of one and a half years.
Inclusion criteria: Patients posted for MRM satisfying American Society of Anaesthesiologists (ASA) physical status I, II and III.
Exclusion criteria: Patients with history of adverse drug reaction to local anaesthetics; clinically significant hepatic, neurologic and psychiatric disease and patients with history of chronic analgesic drug usage were excluded.
Data collection: This observational study was carried out in patients after obtaining written informed consent and applying inclusion and exclusion criteria. All patients underwent a prescribed standard anaesthetic protocol. Informed consent form, proforma.
Patients underwent general anaesthesia with balanced anaesthesia endotracheal intubation. Before closure of the wound, a 20 G scalp vein set was used to prepare for continuous irrigation catheter. Using sterile technique, length of the incision was measured, and multiple puncture was given starting from distal end of scalp vein set; length of which will be equal to the incision length. Distal end was cut and closed. This catheter was placed subcutaneously, and wound was closed. 10 ml 0.25% bupivacaine was given and patient was reversed. Later patient was shifted to postoperative unit. Continuous wound irrigation was given using 0.25% bupivacaine at 0.04 ml/kg/hour for 24 hours. Pain score was noted sixth hourly in a Visual Analogue Scale (VAS). If the VAS exceeded '4' at any point of time, rescue analgesia with IV tramadol 1 mg/kg was administered. The number of demands and the total cumulative analgesic requirement was noted for 24 hour. Surgical site related untoward effects like haematoma, infection and wound dehiscence was observed clinically till the patient is discharged (Figure 1).
Figure 1: Scalp vein set.
Statistical analysis: Data was coded and entered in MS excel and analysis was done using epi-info (version 7). Descriptive analysis was done. Proportions were expressed in percentage. Mean and standard deviation calculated for continuous variables. Chisquare/ fischer exact test was done to look for associations between categorical variables.
Ethical consideration:
• Written informed consent form will be obtained from the care givers before the study.
• Institutional scientific and ethical committee clearance obtained.Zopiclone (ZPC) belongs to the cyclopyrrolone family of hypnotic anxiolytics (subclass within z-drugs). The agent is known under the trade name (Imovane), it has also other names (Zimovane, Dopareel, and others) in different countries. The agent was developed in 1986 by Rhone-Poulenc SA (part of Sanofi-Aventis). The chemical formula of the agent is (C17H17CIN6O3), the molecular mass is (388.81 g/mole), the PubChem CID (5735), and the IUPAC name ((RS)-6-(5- chloropyridin-2-yl)-7-oxo-6, 7-dihydro-5H-pyrrolo [3, 4-b] pyrazin-5-yl 4-methyl piperazine-1-carboxylate). It is not a benzodiazepine agent, although it has almost similar pharmacological properties to those of benzodiazepine drugs. It increases the normal transmission of neurotransmitter Gamma- Aminobutyric Acid (GABA) in the CNS and thus modulates benzodiazepine receptors in a similar way to that of benzodiazepine agents. Its sedative action is attributed to the fact that it causes tranquilization via depression of the CNS. The agent has another active stereoisomer (eszopiclone). ZPC is classified as Z-Drug; it is classes of psychoactive drugs that are very benzodiazepine-like, although are not benzodiazepines [23].
Routes of administration
The agent is available as oral tablets of (3.75 mg, 5 mg, 7.5 mg, and 10 mg) in different countries. The agent is poorly soluble in water, for parenteral uses it exists as acetonitrile soluble of 100 μg/ml ampules.
Clinical indications of zopiclone
The agent is indicated to treat insomnia where induction of sleeping is the problem and/or sleep maintenance is the main complaint. Long-term use even for clinical purposes is not recommended, because ZPC is an addictive agent,thus tolerance and dependence are possible in uncontrolled use.
Contraindications of zopiclone
Because of its CNS depressive and hypnotic action, the drug should not be prescribed for vehicle drivers, because of the increased risk of car accidents associated with the use of this agent [24]. Machine workers should not use ZPC as this cyclopyrrolone agent can carry its hangover effects to the day after (next day) and cause psychomotor incoordination [25, 26]. The drug should not be prescribed to patients suffering from muscle weakness (myasthenia gravis), those of poor pulmonary function (as the drug is mainly eliminated via the lungs), and those with any untreated thyroid diseases [27].
Interactions of zopiclone
Zopiclone can interact with certain agents/drugs and increase or decrease the therapeutic actions of this cyclopyrrolone agent. The interaction of course has a lot to do with the absorption of the ZPC and thus its elimination half-life (t/2). The possible major interactions ZPC can give with different agents are summarized in Table 1 [28]. Concerning the interactions of "eszopiclone", the stereoisomer of ZPC are far more tremendous, these are almost with 330 drugs/agents. These include major, moderate and minor interactions, to mention and discuss all these falls outside the scope of this review work, to see these in detail check (drugs.com).
| Drug/agent | Type of interaction |
|---|---|
| Caffeine | Caffeine might slightly antagonize action of ZPC. |
| Alcohol | It has an additive effect on ZPC action, enhances adverse effects including those of overdose potential of ZPC. Alcohol should be avoided when ZPC is used. |
| Trimipramine | The bioavailability of this tricyclic antidepressant, is decreased when coadministered with zopiclone. This should be considered when treating depressive patients (48 wiki). |
| (TCA) | |
| Antibiotics | Erythromycin: increases absorption rate of ZPC and prolongs its elimination t/2. |
| Rifampicin: causes significant reduction in ZPC half-life and thus reduction in ZPC hypnotic effects. | |
| Antifungals | Itraconazole: has similar effect of erythromycin on zopiclone, it increases the t/2 of ZPC. |
| Ketoconazole: Interferes with metabolism of ZPC. Ketoconazole uses the same metabolic pathway of ZPC, that is cytochrome P-450 3A4 and 2C8 pathway, thus weakens therapeutic effects of ZPC. | |
| Nonantibiotic antimicrobial | Sulfaphenazole: Interferes with metabolism of ZPC. This sulfa agent uses the same metabolic pathway of ZPC, that is cytochrome P-450 3A4 and 2C8 pathway, thus weakens therapeutic effects of ZPC. |
| Antiepileptics | Phenytoin and carbamazepine: may exert similar interactions to those of rifampicin in such a way they reduce the bioavailability t/2 of ZPC, thus reducing hypnotic effects of the agent. |
| Nefazodone | Atypical antidepressant (SARI agent): impairs metabolism, increases serum ZPC levels, cause pronounced hangover sedation the day after. |
Table 1: Major drug interactions can be committed by zopiclone with different drugs/agentsSide effects of ZPC can summarized as following Risks of sudden death.
Gastrointestinal: nausea, vomiting, dry mouth (anticholinergic effects).
Neurological symptoms: dizziness, drowsiness (daytime), confusion, incoordination, lightheadedness, amnesia (rarely).
Psychiatric: depression, delusions, hallucinations, nightmares.
Paradoxical effects: such as sleep-waking, restlessness, agitation, irritability, aggression, anger, inappropriate behavior, and others as is the case with many other sedative and hypnotic agents.
Tolerance, recreational use of zopiclone
As is the case for benzodiazepines, Z-agents including ZPC should not be used for more than two successive weeks. Abusing this drug can expose patients to the risk of drug dependence. As has been stated elsewhere in this review ZPC is more fat-soluble than being a hydrophilic agent. The drug exerts effects both therapeutic and adverse effects via acting on different sites of GABAA receptors. This means that the drug easily traverses neuronal membranes including nuclear membranes and acts on GABAA genes and influences the translation of these receptor proteins. This process is also possible with other agents that act on the central nervous system such as analgesics, hypnotics, neuroleptics, and other psychedelic drugs. ZPC thus has misuse potential because of dose escalation to exert the same previous hypnotic effects. Accordingly, drug dependence becomes inevitable if the agent is used for periods more than what is prescribed. If this happens as is the case with other recreational agents, the individual must get professional help to stop this. The worst is, to induce euphoria with both sedation and hypnosis, the agent is often combined with alcohol to achieve these dual desires, a practice that widens the panorama of unwanted effects.
Withdrawal symptoms of zopiclone
This can be encountered as it is the case with other hypnotic/ sedative drugs after long-term use yet of normal doses for therapeutic use even after gradual tapering strategy. As it is accepted for benzodiazepines, ZPC prescription should not exceed 7-10 days (maximum 2 weeks). The reason for this is the existence of a potential risk of addiction, tolerance, and physical dependence as has already been stated [28-31]. For ZPC two types of misuse are possible, these are: recreational when the agent is taken in higher doses to achieve the desired sedative effects or when used continuously for longer terms against professional medical advice [32,33].
Symptoms of addiction and withdrawal include dysphoria, hopelessness, depression, cognitive dysfunction, irritability, nervousness, and social problems such as worrying, isolation, and sexual anhedonia [34].
In a similar manner to benzodiazepines (BZDs), ZPC binds to the same sites of GABAA- receptors. An action similar to that of BZDs causes an enhancement in the action of GABA and exerts its pharmacological affects both therapeutic and adverse effects. Desmethylzopiclone is an active metabolite of ZPC that acts mainly as an anxiolytic. The metabolite was found to have partial agonist properties when binds to α1, α2, α3, and α5 GABAA benzodiazepine receptor complex. According to Petroski et al, on the corresponding hand, ZPC was found to exert a sort of slight selectivity on α1 and α2 subunits, although it is accepted that the agent exhibits unselective full agonist action upon its binding to α1, α2, α3, and α5 GABAA receptors [35,36].
Pharmacokinetics and metabolism
The 1st pass after oral administration is fast with the bioavailability of 75%-80%. The duration to reach peak serum concentration is about 1-2 hours. Although the agents’ slightly more lipophilicity than being hydrophilic, fatty meals preceding the oral administration of the agent do not influence absorption ZPC. High fatty meals reduce the period in which the agent reaches peak plasma level. The binding of ZPC to serum proteins is weak (45%-80%), the mean of this being 52%-59%. Via systemic circulation, it extensively and rapidly reaches the brain, and after being metabolized, the agent is excreted in the urine, saliva, and breast milk of lactating women. In the liver, ZPC is extensively metabolized, and almost 30% of the administered dose is metabolized into two distinct metabolites, the 1st is active (N-desmethylzopiclone) and the 2nd is inactive (zopiclone-N-oxide). The enzymatic systems of the liver that have a major role in the metabolism of this agent are CYP3A4 and CYP2E1. The remarkable phenomenon here is that about 50% of the total administered dose is decarboxylated and excreted via the respiratory system. It is worthy of mention that ZPC is extensively metabolized before being excreted, almost 7-10% of the agent is reabsorbed from urine to be furtherly metabolized in the hepatic system. The elimination t/2 (half-life) of the agent is about 5 hours on average [37]. ZPC designated for therapeutic use is indeed a mixture of two isomers (enantiomers) dextrorotatory (D-isomer) and levorotary (L-isomer). The terminal elimination t/2 value of the D-isomer is higher than that of the L-isomer. That is to say, D-enantiomers of Ndesmethylzopiclone and zopiclone-N-oxide are higher than those of corresponding L-enantiomers of the agent (when it comes to elimination t/2). The metabolism is influenced by aging, hepati, and renal functions [38,39].
Zopiclone is sometimes used as a method to commit suicide [40,41]. Although when taken alone is usually nonfatal. The death potential increases when the agent is coadministered with alcohol, opioids, or other central nervous system depressants and piperazine (anthelmintic and acetylcholine blocker at the myoneural junction) [42-44]. Overdose of ZPC can also be fatal with the coexistence of other morbidities such as hepatic and/or respiratory disorders (as the agent is metabolized by the hepatic cytochrome enzymes and eliminated mainly by the respiratory system). Clinically, ZPC intoxication may be manifested as excessive sedation, mydriatic pupils, a depressed respiratory function which may progress to stupor, coma, and death in severe intoxications [42].
Usually done include blood tests, serum electrolytes, glucose, tests of renal function (blood ammonium), blood gas analysis (O2 saturation and pH), hepatic functions (C-reactive protein). Determination of serum levels of ZPC, the normal therapeutic levels of which being less than 100 μg/l, in cases of severe intoxication this value may raise to 250 μg/l [43-45]. This step is important to rule out intoxication with other psychedelic drugs especially when mixed intoxication is expected with agents such as benzodiazepines, barbiturates, cannabinoids, opiates, methadone, cocaine, buprenorphine, propoxyphene, ethanol, LSD, etc. For this reason, a urine examination is also requested to rule out intoxication with such agents. Electroencephalography (EEG) is performed; this might be informative to show a sustained α-rhythm during EEG recording and diffuse superimposed β-rhythm (fast activity). The increase of β- EEG activity if shown is a prominent feature of ZPC intoxication [46]. This tool is far better diagnostic than standard immunoassay investigations which are usually available in most well-equipped emergency rooms of different centers [47]. Brain CT and MRI might be required to rule out brain pathologies and intracranial physical damages and encephalitis, and for these two latterly mentioned reasons examination of CSF might also be requested.
After stopping the offending drug, treatment of toxicity is mainly supportive. The patients should be lodged in a quiet room; vital signs should be checked in intervals according to the severity of the case. The patient should have an open airway and the intravenous fluids he/she needs should be calculated properly. Flumazenil (flumazepil), trade name (Anexate) is a GABAA receptor antagonist and may be administered as intravenous injections to counteract the action of ZPC [48]. Indeed, it is an antidote and an antagonist to benzodiazepines (particularly in cases of overdose). Flumazenil was also found to exert the same action (antidote and antagonist) against ZPC [49]. Flumazenil acts on GABAA receptors and counteracts ZPC action as a competitive inhibitor, it displaces ZPC from its binding site on benzodiazepine receptor [50]. This antagonist action denotes the similarity in the actions of benzodiazepines and the Z-drug ZPC. The accepted therapeutic daily dose is 1.5 mg for several days (a week or so) to recovery, dependent on the clinical situation and vital signs of the patient.
Chronic insomnia is a common problem in societies all over the world. Healthy adults need at least 8 hours of sleep per 24 hours. This number of hours may vary according to the age of individuals and the nature of their professions. One should understand the reason behind this morbidity before commencing the treatment. Sleep centers in many countries can offer management without the need to prescribe hypnotic agents. Moreover, there exist many drug lines of treatment to be offered to individuals who complain of insomnia when this is necessary. Because insomnia can be a primary problem, it can cause irritability, anxiety, and depression. And vice versa neuropsychiatric disorders such as anxiety neurosis, depression, post-traumatic stress, Parkinson's, and Alzheimer's diseases are among the most common reasons for insomnia. Somatic diseases such as chronic pain of any etiology, cancer, heart diseases, asthma, reflux esophagitis, hyperthyroidism, and complications of diabetes are also among many causes of sleeplessness. The list is very long indeed and would be inconvenient in this context to mention all here.
Drug treatment includes many lines (classes) which include antihistamines, melatonin, antidepressants, antipsychotics, benzodiazepines, and Z-drugs. Apart from benzodiazepines, these agents traditionally are not prescribed to treat primary insomnia, either because of their insufficient potency or because of the broad-spectrum side effects they exert on individuals on long period use. In this work, we elucidate the impact of the cyclopyrrolone agent zopiclone (a Z-drug) and compare it to the benzodiazepine agent diazepam, with a special emphasis on the efficacy and side effects profiles. Diazepam (DZP) is an absolute fat-soluble agent thus can readily cross the blood-brain barrier, cell membranes, and consequently membranes of different organelles of both neuronal and somatic cells.
As regards ZPC, it is also almost insoluble in water [51]. The logP value of ZPC is 0.8, this positive number denotes that the agent is more lipophilic than being hydrophilic, but not as that fat-soluble as diazepam, for chemical structures of these agents (Figure 1). It is worthy of mention that both ZPC and DZP exert their therapeutic effects via attaching to GABAA receptors. They also share the same antidote (flumazenil) to treat the cases of intoxication they might cause. Concerning the antihistamines such as alimemazine (vallergan) and promethazine (phenergan). These two agents are phenothiazine agents, and they were not dedicated for use as hypnotic (relaxant) agents. They were designed as their class name indicates to be used as antihistamines to different classes of allergic situations such as allergic rhinitis, sneezing, hives, and animal allergies, and other allergic conditions [52]. Structurally they mimic chlorpromazine, (a 1st generation antipsychotic agent) see Figure 1. The subclass of antihistamines that precisely exert this therapeutic action is called H1 receptor antagonists. H1-antihistamines were later found to be useful to treat insomnia (as they cause hypnosis as a secondary effect), motion sickness (or vertigo). Indeed, all these actions (of pharmacodynamic category) are side effects of these agents [53]. The most known agents of this group used for this purpose are diphenhydramine and doxylamine (1st generation antihistamines). These agents can also cross the blood-brain barrier and have a wide spectrum of side effect profiles. Antihistamines do not contribute to release of free radicals, and thus do not cause oxidative stress. In that sense it is a good treatment alternative for insomnia [54]. Their only disadvantage is the broad spectrum of their side effect profiles, especially the older generations of these agents. Newer drugs of this class do not cause the same degree of hypnosis, therefore not prescribed therapeutically for this purpose.
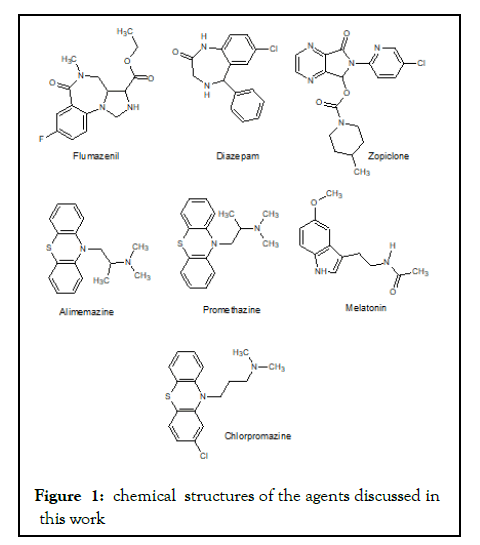
Figure 1:chemical structures of the agents discussed in this work
Melatonin, the hormone that is released normally by the pineal body at night, controls what is known as circadian rhythms including the sleep-wake cycle of humans [55]. It is used as a supplement to the diet to treat short-term insomnias, although the evidence of its use is not solid [56,57]. Despite its minimal side effects in low doses for short periods, these can almost affect all systems of the human body [58,59]. The agent is contraindicated in pregnancy, breastfeeding, and in those with abnormal liver function tests [58-60].
Ramelteon has more or less the same effects as melatonin. It is worthy of mention that melatonin when used as a hypnotic is not always effective. This is because short half-life of the agent and the small amounts of the agent that is employed for therapeutic uses (2-3 mg/daily). To induce hypnosis one might need to use higher doses which can be hazardous [61]. Melatonin can cause the reverse effects namely rebound insomnia upon discontinuation, a fact that indicates that we know very little about the pathophysiology of insomnia and its pharmacotherapy and pharmacology of melatonin. Melatonin’s primary metabolite has an antioxidant function in the human body, a property that might also lie behind its hypnotic action [62-64].
Antipsychotic agents, especially the 1st generation agents have sedative and hypnotic actions. This is a drawback of these agents that promote psychoactive drug designers to prepare better agents that do not exhibit this side effect. In a dose-dependent manner, certain agents of 2nd generation side effects also show hypnotic effects e.g. quetiapine [65]. Fortunately, quetiapine exerts this property (hypnosis) in low doses, because in higher doses this agent can release free radicals that may make insomnia worse. Other antipsychotic agents also share this property such as clozapine [66]. It is not known precisely how these agents exert their antipsychotic or hypnotic effects [67].
Benzodiazepines, such as diazepam have been a good choice to induce hypnosis and thus to treat insomnia. This agent indeed, when used for short periods (maximum 2 weeks) is efficient to treat certain types of acute insomnia. Prolonged use of benzodiazepines to treat chronic insomnia causes tolerance, addiction, and devastating withdrawal symptoms when abruptly stopped. Moreover, benzodiazepines readily cross biomembranes and the blood-brain barrier; this is attributed to their absolute lipophilicity, see Figure 1. Diazepam should not be prescribed for long-term uses in pregnant women as it can cause floppy infant syndrome, not in lactating mothers because it can be secreted via breast milk [68,69]. Benzodiazepines can affect all cells of the body via influencing the function of mitochondria and thus negatively affect oxidative phosphorylation that is the biosynthesis of adenosine triphosphate (ATP) when used in higher doses for long periods [70,71]. Zopiclone might be a better alternative or at least the other alternative as it does not cause such harmful side effects. Although it is more lipophilic than being a hydrophilic agent, still not absolutely fat-soluble like diazepam, look at the chemical structure in Figure 1. Therefore its side effect profile is not expected to be that broad as that of diazepam. Still, it should not be prescribed for periods longer than 2 successive weeks because it can also cause tolerance, addiction as well as withdrawal symptoms when abruptly stopped [72,73]. But it is probably among the best agents we have to combat insomnia that affects broad population spectrum almost all countries of the world both the developed western societies as well as many countries of the developing world [74].
Our knowledge about the pathophysiology and the biochemical changes in the brain that is associated with insomnia is not sufficient to design selective agents to treat insomnia. Therefore, the agents we use to treat this ailment are not optimal and have a broad spectrum of side effects. Moreover, some of these agents exert almost similar effects at neuronal levels and share the same antidote as is the case with diazepam and zopiclone.
Benzodiazepines, Z-drugs (zopiclone), are the best agents we have to treat insomnia and to a lesser extent quetiapine (2nd generation antipsychotic). Benzodiazepines and z-agents exert the best therapeutic effects despite their serious drawbacks (when used for longer periods). We have to acknowledge that these two agents when used therapeutically for periods, not more than 2 weeks, do not cause serious effects such as neuroleptic malignant syndrome as is the case with antipsychotics of both 1st and 2nd generations. Antipsychotics and antidepressants, although limited evidence for their use as hypnotics to treat insomnia disorders, may improve sleep while they efficiently treat the comorbid disorders that lie behind insomnia. We must admit that these agents have also their side effects although with different profiles than those of BZDs and Z-drugs. The clinician has to choose the proper agent for each case accordingly. Clinicians and patients should share the vision of what is to be prescribed according to the experiences of clinicians and the compliance of patients.
Efforts should be directed towards preparing better agents to treat this syndrome. These agents must be selective in their actions and cause minimum possible side effects.
There is no conflict of interest
Citation: Oruch R, Pryme IF, Fasmer OB, Lund A (2021) Drug Treatment of Insomnia: Impact of Zopiclone. J Anesth Clin Res. 12:1029.
Received: 01-Oct-2021 Accepted: 15-Oct-2021 Published: 22-Oct-2021 , DOI: 10.35248/2155-6148.21.12.1029
Copyright: © 2021 Oruch R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.