Internal Medicine
Open Access
ISSN: 2165-8048
ISSN: 2165-8048
Research Article - (2019)Volume 9, Issue 4
Few studies regarding arthritic diseases have been performed to verify the presence of the neurodegeneration. Given the increased oxidative stress and extra-articular effects of the rheumatoid arthritis, the gastrointestinal studies should be further investigated aiming a better understanding of the systemic effects the disease on enteric nervous system. To determine whether the rheumatoid arthritis affects the nitrergic density and somatic area of the nNOSimmunoreactive (IR) myenteric neurons, as well as the morphometric areas of CGRP and VIP-IR varicosities of the ileum of arthritic rats. Twenty 58-day-old male Holtzmann rats were distributed in two groups: control and arthritic. The arthritic group received a single injection of the Freund’s Complete Adjuvant in order to induce arthritis model. The whole-mount preparations of ileum were processed for immunohistochemistry to VIP, CGRP and nNOS. Quantification was used for the nitrergic neurons and morphometric analyses were performed for the three markers. The arthritic disease induced a reduction 6% in ileal area compared to control group. No significant differences were observed in nitrergic density comparing both groups. However, arthritic group yielded a reduction of the nitrergic neuronal somatic area and VIP-IR varicosity areas. However, an increase of varicosity CGRP-IR areas was also observed. Despite arthritis resulted in no alterations in the number of nitrergic neurons, the retraction of ileal area and reduction of nitrergic somatic and VIP-IR varicosity areas may suggest a negative impact the disease on the ENS.
Rheumatoid arthritis; Enteric nervous system; Freund’s adjuvante; Rats; Nitrergic neurons
Rheumatoid arthritis (RA) is a progressive, chronic inflammatory, autoimmune and multi-systemic disease that affects several tissues, but mainly the joints, and reaches approximately 1% of the world’s population. The development of rheumatoid arthritis is associated with auto-immune and environmental factors, although its etiology is still unknown. Furthermore, the RA is characterized by the infiltration and activation of inflammatory cells in the tissues and fluids of the synovial joints [1-4]. The complex pathogenesis of rheumatoid arthritis involves T cells that stimulate monocytes, macrophages and synovial fibroblasts, which lead to the production of proinflammatory cytokines, prostaglandins and chemokines as well as the stimulation of B cells. Pro-inflammatory mediators promote and perpetuate chronic immune-mediated inflammation of soft tissue, resulting in joint destruction [2,5-8].
The inflammatory process of RA is mediated by cytokines (e.g. IL-1, TNF-α, IL-1, IL-6, IL-17, etc) and reactive oxygen species (ROS) that modulates the activity of inflammatory cells [3-10]. The ROS is produced by activated phagocytes, with a protective function against pathogenic invaders, although these cells can promote tissue through intensifying the inflammation process11. Furthermore, the synthesis ROS also occurs due to the cycles of hypoxia-reperfusion during movement of the inflamed joints [4,7,9,11-13].
The pathogenesis of RA has barely been explored in detail or in studies of the gastrointestinal wall, affecting the Enteric Nervous System (ENS). Enteric neurons have been widely investigated using experimental models that induce oxidative stress (e.g. aging, diabetes, cancer, parasitic diseases and physical activity). These studies evaluate whether the intestinal dysfunctions are associated with morphological and quantitative changes of neurons and expression of neurotransmitters by the ENS [14-20].
Considering the complex division of the Autonomic Nervous System, the ENS displays an independent function, which is able to perform the functions of the digestive system, without relying on central nervous commands. The ENS is organized in plexuses, ganglionated and aganglionated, and enteric neurons release various neurotransmitters. Besides its neural network, the ENS also has extrinsic neuronal terminations of the sympathetic and parasympathetic nervous system, connected by afferent and efferent neuronal fibers to the Central Nervous System. The ENS may control and coordinate intestinal motility, exocrine and endocrine secretions, absorption, microcirculation and the local immune system, and modulate the inflammatory response of the gastrointestinal tract [21-23].
Enteropathies are commonly associated with loss of enteric neurons and its subpopulations such as neuronal nitric oxide synthase (nNOS). Both ganglionated plexuses of the SNE express different neuropeptides such as calcitonin gene-related peptide (CGRP) and vasoactive intestinal polypeptide (VIP). VIP ergic neuropeptide promotes smooth muscle relaxation and stimulates the secretion of fluids and electrolytes and CGRP is a vasodilator neuropeptide that enhances the intestinal blood flow through stimulation of smooth muscle relaxation [22,24]. Both CGRP and VIP are considered an antioxidant and antiinflammatory neuropeptides [24].
Given that poor correlation between the extra-articular effects of RA on ENS and susceptibility of enteric neurons to the RAinduced oxidative stress, the aim of this study was to investigate whether the RA affects the ENS evaluating quantitative and morphometric parameters of vasoactive intestinal polypeptide (VIP) and neuronal nitric oxide synthase (nNOS) and calcitonin gene-related peptide (CGRP).
Experimental groups
All animals were obtained from the Central Animal Facility of the State University of Maringá and the experiments were approved by the Committee of Ethics in Animal Experimentation of the State University of Maringa.
Twenty 58-day-old male Holtzmann rats (Rattus norvegicus) were obtained and distributed into two groups (n=10 rats per group): control (C) and arthritic (ART). Both groups were submitted to a 60-days experimental period. The animals were kept in polypropylene cages (40 × 33 × 17 cm) for 60 days under standard laboratory conditions (cycle of 12 hr light/12 h dark) and controlled temperature (24°C ± 2°C). Water and standard rat chow (Nuvital; Nuvilab, Colombo, PR, Brazil) were available ad libitum.
Adjuvant-induced arthritis was obtained by a single intradermal injection with 100 μL of a suspension of Freund’s Complete Adjuvant (FCA; heat-inactivated Mycobacterium tuberculosis) suspended in mineral oil at a concentration of 0.5% (w/v) into the left hind paw [25].
Material collection and fixation
After 60 days of experiment, animals were anesthetized with Thiopental (40 mg kg/kg body weight; Abbott Laboratories, Chicago, IL, USA) and the ileum was collected. After tissue collection, intestinal samples were rinsed in phosphate-buffered saline (PBS 0.1 M, pH 7.4; 10 mmol/L Na2HPO4 and 150 mmol/L NaCl), followed by incubation in Zamboni’s fixative for 18 h at 4°C and then, washed in 80% alcohol. Whole-mounts were sequentially dehydrated in 95% and 100% alcohol, diaphanized in xylene and then successively rehydrated in 100%, 90%, 80% and 50% alcohol. Afterwards, ileal samples were cut along the mesenteric border and rinsed in PBS for 30 min and then, stored in PBS with 0.08% sodium azide at 4°C. Subsequently, intestinal whole-mounts were dissected under a Stemi DV4 stereomicroscope (Zeiss, Jena, Germany) containing outer muscle layers and the myenteric plexus. Ten ileal segments were dissected and processed for the subsequent immunohistochemistry technique to the nNOS, VIP and CGRP.
Immunostaining for nNOS, VIP and CGRP
The whole-mounts preparations were initially rinsed in PBS solution of 0.5% Triton X-100 three times. Afterwards, intestinal tissues were incubated in a solution containing PBS, 0.5% Triton X-100 and 1% BSA for 1 h. After blocking, the intestinal tissues were incubated in specific primary antibody for the n NOS (48 hr), VIP and CGRP (24 hr) (Table 1) at room temperature (RT). The ileal tissues were rinsed in PBS three times and then, incubated with the secondary antibody (Table 1) for two hours at RT. After incubation, tissues were rinsed in PBS three times and mounted on slides using buffered glycerol (9:1). Negative control was performed with the omission of the primary antibody.
| Immunohistochemical techniques | |
|---|---|
| Dilution | |
| Primary antibody | |
| Monoclonal Mouse anti-nNOS; sc-5302 | 0.38888889 |
| Polyclonal Rabbit anti-Vasoactive intestinal peptide (VIP); sc-20727 | 0.25 |
| Polyclonal Rabbit anti-Calcitonin gene-related peptide (CGRP); sc-28920 | 0.18055556 |
| Secondary antibody | |
| Alexa fluor 488 (Donkey anti-mouse); A-21202 | 0.31944444 |
Table 1: Primary e secondary antibodies used in the immunohistochemical techniques.
Image acquisition
All photomicrographs (30 images per animal) for nNOS, VIP and CGRP were obtained using an Olympus BX40 optical fluorescence microscope (Olympus Co., Tokyo, Japan) connected to a high-resolution 5.0 Mega Pixel Moticam® 2500 camera (Motic China Group Co., Shanghai, China). Photomicrographs were recorded using Motic Images Plus 2.0ML software (Motic China Group Co.).
Quantitative analysis for nNOS-immunoreactive (nNOSIR) myenteric neurons
The quantification of nNOS-IR myenteric neurons was performed using the images obtained from sampling the intermediate region (60°-120°and 240°-300°) of the ileum circumference of each animal, considering 0° as the mesenteric border [26]. The results were expressed as the number of neurons in 5.8 mm2 ileum. The neuronal density correction of the arthritic group was calculated using the measurements of intestinal length and width in comparison to control group. Morphometric analysis for nNOS-IR myenteric neurons The morphometric measurement of the somatic areas of nNOSIR myenteric neurons was performed using the earlier images. The area (μm2) of 100 neuronal cell bodies per animal was measured through Image-Pro Plus with a total 500 neuronal body areas per group.
VIP and CGRP-IR myenteric fibers
For the measurement of the area of the VIP and CGRP-IR varicosities, the Image-Pro-Plus was used and the areas (μm2) of 400 varicosities of each animal were measured, reaching a total of 2000 varicosities per group for each technique.
Statistical analysis
Results were subjected to statistical analyses through the programs Statistica and GraphPad Prism, expressed as media ± standard error. Morphometric data were analyzed by the Mann- Whitney test for non-parametric parameters. Values of P<0.05 were considered statistically significant.
Development of RA
Arthritic animals developed the characteristic signs of the disease one week after injection. The arthritic animals exhibited edema paws with consequent locomotor difficulties. Furthermore, a reduction of water and feed intake was observed. The area of the small intestine for ART group was smaller than C group (Table 2).
| Groups | Length (cm) | Width (cm) | Area (cm2) |
|---|---|---|---|
| C | 101.5 ± 6.4 | 1.56 ± 0.26 | 158.4 ± 12.9 |
| ART | 99.20 ± 4.8 | 1.50 ± 0.0 | 148.8 ± 3.2* |
Data are expressed as mean ± standard error. *P<0.05 compared to C group.
Table 2: Length, width and area of ileal samples from the control (C) and arthritic (ART) groups. Data are expressed as mean ± standard error. *P<0.05 compared to C group.
However, no significant differences were observed evaluating the length and width of the ileal samples between both groups (Table 2). The neuronal density was corrected in 6% for the ART group.
nNOS-IR myenteric neurons
No significant differences (P>0.05) were observed for the neuronal density comparing both studied groups (C: 159.2 ± 6.8; ART: 201.5 ± 25.2 neurons/5.8 mm2).
For the nNOS-IR neurons, a reduction of the body cell area (14.59%) was observed comparing C group with the ART group (P<0.001). Such alteration of the somatic neuronal body area can also be observed through the frequency of cell body size distribution and mean neuronal area (Figure 1A and 1B). Regarding the immune staining ’ s for the nitrergic neurons, primary, secondary and tertiary plexuses were visualized in our enteric plexus. Most nNOS-IR neurons were present in the periphery of the ganglion, but the nitrergic neurons for some ganglia were seen in central position (Figure 2A and 2B).
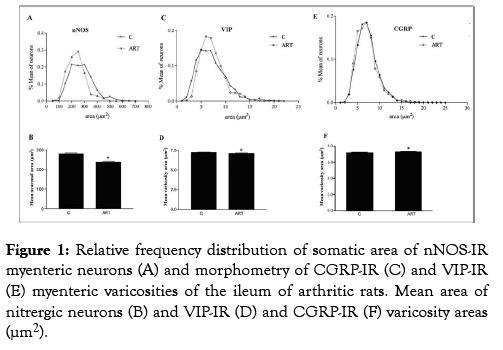
Figure 1: Relative frequency distribution of somatic area of nNOS-IR myenteric neurons (A) and morphometry of CGRP-IR (C) and VIP-IR (E) myenteric varicosities of the ileum of arthritic rats. Mean area of itrergic neurons (B) and VIP-IR (D) and CGRP-IR (F) varicosity areas (μm2).
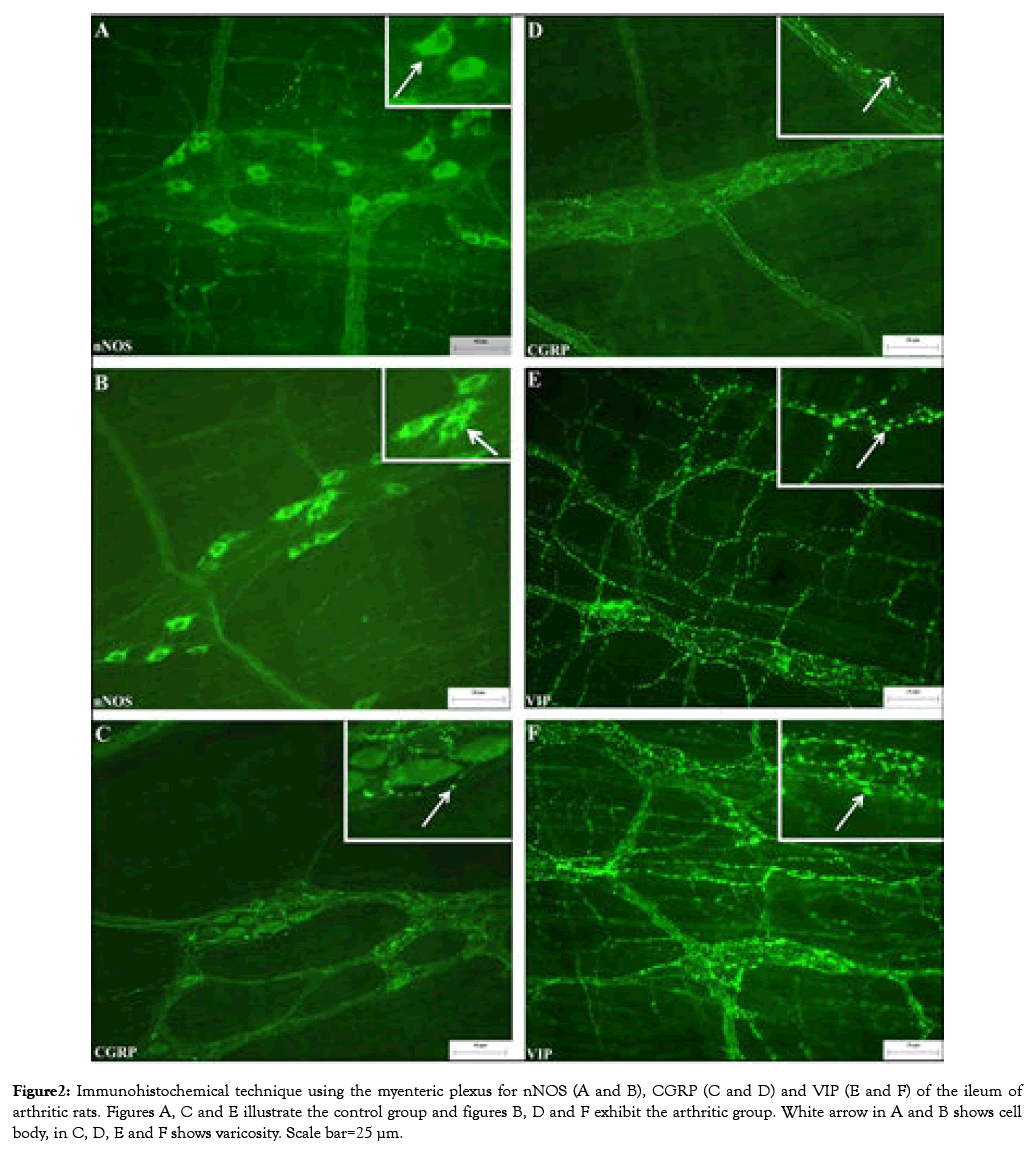
Figure 2: Immunohistochemical technique using the myenteric plexus for nNOS (A and B), CGRP (C and D) and VIP (E and F) of the ileum of arthritic rats. Figures A, C and E illustrate the control group and figures B, D and F exhibit the arthritic group. White arrow in A and B shows cell body, in C, D, E and F shows varicosity. Scale bar=25 μm.
VIP-IR and CGRP-IR neuronal fibers
For the total whole-mounts immune stained to CGRP, the presence of CGRP-IR fibers in the primary, secondary and tertiary plexuses of the my enteric region was observed. The obtained mean for the CGRP-IR varicosity area was higher for the ART group (P<0.001; Figure 1F) compared to C group. This difference can be observed by the frequency distribution in Figure 1E. Regarding the VIP-IR neuronal fibers, a high density of fibers was observed in all animals. However, the VIP-IR varicosity area in ART group was smaller compared to C group (P<0.01; Figure 1C). Representative photomicrographs of the immune staining’s for CGRP and VIP are shown in Figure 2.
In this study, all features of development of RA were observed for the arthritic group: reduction of weight body, formation of paw edema and extra-articular effects. The slight retraction of ileal area of the arthritic rats suggests an atrophic effect of the disease on small intestine, likewise Souza et al. observed such effects in the jejunum and ileum [26]. Atrophy of the intestinal area have been reported in other experimental models (e.g. diabetes mellitus, aging and cancer) [14,27-29].
Despite no changes in nitrergic neuronal density were observed by the disease, a reduction of neuronal size of nNOS-IR neurons. Neuronal plasticity phenomena occur due to adaptation to changes of environmental factors or bowel inflammatory diseases. In the ENS, neuronal NOS-derived NO acts as neurotransmitter, which mediates the relaxing components of bowel peristalsis [29]. These phenotypic changes may indicate a compensatory mechanism to either GI dysfunctions or neurons loss in order to balance its bowel activity closer to ideal [30]. However, in our study, the reduction of somatic area of the nNOS-IR myenteric neurons suggests a negative effect induced by the AR since no alterations were observed in the nitrergic density.
The hypertrophy of the neuronal size is commonly associated with an increased enzymatic synthesis as a compensatory mechanism against the neuronal density loss, whereas the neuronal atrophy may indicate negative effects by the reduction of production of the neurotransmitter and oxidative injury induced by the disease on the ENS, resulting in a decrease of gastrointestinal function [28,30]. In our study, no nitrergic changes were observed but associated with reduction of the neuronal body area of the nNOS-IR neurons, which may also suggest an arthritis-induced harmful effect. Souza et al. reported that RA did not result in quantitative alterations on enteric nervous system, only morphometric changes in myenteric population of neurons from the ileal and jejunal tissues of Holtzmann rats induced by the FCA using an arthritis model of 30 and 60 days of experimental period, our results demonstrated only alterations in the morphometry of the nitrergic neurons [26].
The ganglionated plexuses of the ENS exhibit the expression of different neuropeptides such as the VIP and CGRP, which stimulate smooth muscle relaxation [22,23]. As described earlier, both varicosity areas for CGRP and VIP were reduced, which may indicate a decrease in the production and release of these neuropeptides, thereby affecting the normal functioning of the GI tract24. The VIP neuropeptide has anti-inflammatory, antioxidant and anti-apoptotic effects, mainly in inflammatory and autoimmune diseases since it is capable of regulating various nuclear transduction and transcription pathways. Furthermore, the VIP is involved in mechanisms of neuroplasticity and neuroprotection, since it is considered a neurotrophic factor that stimulates the synthesis of other growth factors and inhibits cellular apoptosis induced by oxidative stress [31,32]. The CGRP is a vasodilator neuropeptide that acts in the increase of the intestinal blood flow, stimulate the smooth muscle relaxation, downregulate the inflammatory responses and participate in the pathways of pain transmission. Furthermore, CGRP has antioxidant and anti-inflammatory effects [22,33,34]. In this study, the reduction of VIP-IR varicosity areas may indicate the presence of inflammatory process in the ileum induced by the AR, which may lead to a decrease of production and release of this neuropeptide [24,30]. Furthermore, NO induces the release of VIP in the myenteric plexus and both morphometric reductions may indicate correlations with decrease in the production of the neuronal NO and VIP [35,36].
Since VIP displays crucial roles against the inflammation and oxidative stress in the GI system, its reduction in the production and release by the varicosities suggests a negative impact of the disease. However, the increased CGRP-IR varicosity areas suggest a compensatory mechanism against the other negative impacts above described, mostly due to antioxidant and antiinflammatory properties of this neuropeptide. Varicosity hypertrophy indicates higher production and release of the CGRP which would ameliorate the intestinal function affected by the disease [24,36].
For these reasons, these morphometric changes in the nitrergic somatic areas and the varicosities in the myenteric plexus revealed neuroplasticity and further studies should be done to better investigate interferences of the RA in the ENS functioning [36].
Therefore, the retraction of intestinal area and decrease of somatic area of the nitrergic neurons and VIP-IR varicosity areas may indicate the RA-induced deleterious effects, affecting the normal functioning the GI system. However, further studies should be investigated whether the disease induces changes in the levels of oxidative stress, protein expressions, evaluated by molecular techniques, and morphology of GI wall.
Citation: Ashour AS, Virginia K (2019) Does Rheumatoid Arthritis Affects the Nitrergic Density and Somatic Area of the nNOS-Immunoreactive (IR) Myenteric Neurons, as well as the Morphometric Areas of CGRP and VIP-IR Varicosities of the Ileum of Arthritic Rats? Intern Med 9:311. doi: 10.35248/2165-8048.19.9.311
Received: 14-Sep-2019 Accepted: 27-Sep-2019 Published: 07-Oct-2019 , DOI: 10.35248/2165-8048.19.9.311
Copyright: © 2019 Ashour AS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.