Journal of Oceanography and Marine Research
Open Access
ISSN: 2572-3103
ISSN: 2572-3103
Research Article - (2019)
Seaweeds are aquatic angiosperms, which are widespread in many coastal settings. Due to their various type of application, for instance, therapeutic agents, they are considered as one of the key environmental resources. In order to find out the diversity, abundance and pattern of species distribution of algae, a study was carried out in the three selected south coastal areas in the sand plain (Ahagama, Talpe and Koggala) in Sri Lanka. Quadrats method and three-line transects (horizontal & vertical) with 15 m length laid perpendicular from shore were adopted to collect data. Diversity was determined by Shannon-Wiener diversity index and quality of seawater (water temperature, pH, and salinity and dissolved oxygen) was also determined. Statistical analysis revealed significant difference in distribution and diversity between Sargassum and Ulva species in three study area. “Shannon-Wiener” diversity index value indicated a close relationship of the diversity in Koggala and Ahangama when compared to Talpe. It was also obvious that a positive correlation (P<0.05) existed between Sargassum ilicifolum and Ulva lactuca. This study provides clear evidence that patterns and habitats of Sargassum ilicifolum and Ulva lactuca differ along the south coastline in Sri Lanka.
Distribution; Quadrat; Seaweeds; Sargassum sp; Ulva sp
Seaweeds are macroscopic, multicellular, benthic marine algae, and recognized as important marine natural resources. There are three major classes namely, Chlorophyceae, Rhodophyceae and Phaeophyceae [1]. However, their distribution is limited from the lower intertidal to the shallow subtidal zones of the marine environment. From them, an array of substances has been extracted and their applications are involved as stabilizers and stiffeners in food industry, precursors for cosmetics and biotechnology [2-4]. Recent studies conducted using seaweeds or their compounds have explored new avenues in medical advancement; bioactive compounds [5,6], as a CO2 sink [7] and as bio-fuel [8]. Over the last few decades, marine natural products have been used as a leading compound for the discovery of drug in every part in the world [9]. In addition, they have been widely used as model organism to study biogeographic patterns and for testing of various ecological theories. Some of the seaweeds have been explored under rocks and in the intertidal habitat [10-12]. Presence of mudflats, estuaries, and coral reefs along the lagoons and rocky beaches provides an ideal habitat for sustainable growth of seaweeds in Sri Lanka [13].
The primary living cells on earth emerged from the sea and life has developed from the growth of mono-cellular algae [14]. Moreover, it’s estimated about 90% of marine plant species are algae which contribute 40% global synthesis [15]. The Sri Lankan coastal water is rich in marine flora and variety of seaweeds has been identified [16]. The marine algae had been reported as early as the 19th century [17,18]. Further there are 440 taxa of marine algae belonging to 148 genera. Amongst the Sri Lankan seaweeds, Sargassum species are found in most of the coastal areas [19]. Furthermore, species such as Laminaria, Fucus, Ascophyllum and Tubinaria are also found in the Sri Lankan waters. Since 1960 to 1970 alginic acid has been extracted from seaweeds [20], and the cultivation of seaweeds too has been introduced [21]. A further study on the various aspects of macro algae is needed to diversify and find the richness of the species.
The main focus of this study is on the diversity and distribution of seaweeds along the south-coast of Sri Lanka. However, information regarding the identities and diversity of the economically important seaweeds species are lacking in Sri Lanka. Such information can provide a baseline for future complex ecological studies in this area and further to manage and explore seaweeds.
Description of the study area
The study site selected for the sampling is the south coastal area with the geographical platform of Ahangama (N 05° 58.006' E 080° 22.482'), Koggala (N 05° 59.390' E 080° 33.121') and Talpe (N 05° 59.792' E 080° 16.898') in Sri Lanka and research was conducted in 2015. Accordingly, nine stations were selected to perform sampling of seaweeds from this geographical setting. The sites varied in distance from one another ranging from 7.2 km to 4.0 km (Figure 1).
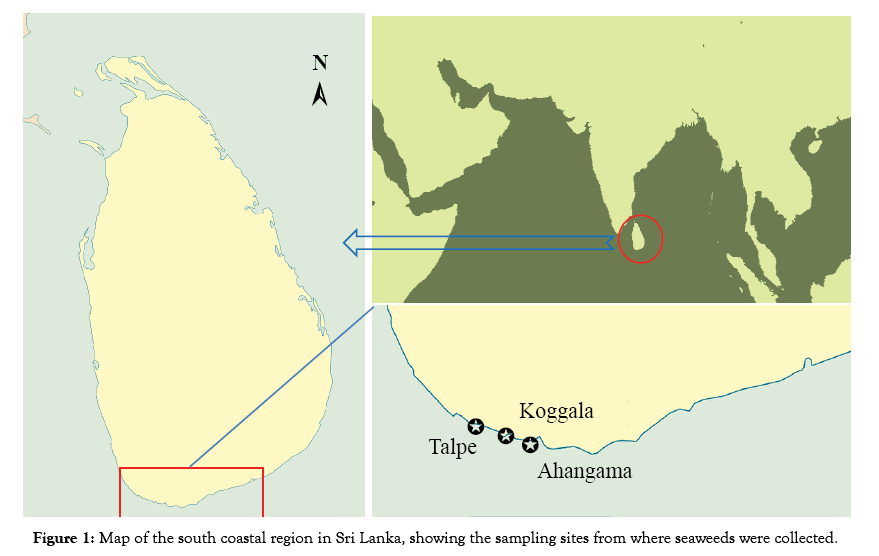
Figure 1: Map of the south coastal region in Sri Lanka, showing the sampling sites from where seaweeds were collected.
Collection of data and samples
The zonation profile of the study area and distribution pattern of the macrophytes was observed physically by placing three transects perpendicular to the shore [22]. All the seaweed samples were taken by snorkeling from shallow waters (0-2 m in depth) along the subtidal area. At each site, different types of macrophyte specimens were collected manually by hand and using a knife during the low tide. All the samples were stored in pre-labeled plastic bags while macro algae were collected in plastic pots containing 5% neutral formalin. These specimens were identified using the identification guide [20] and voucher specimens and herbarium sheets were prepared. Furthermore, the seaweeds were authenticated at the “National Herbarium of the Peradeniya Botanical Garden in Sri Lanka” and specimens were deposited for future references.
Sampling site and quadrats method
To estimate the cover of each seaweed species in each location, we used representative sites and random quadrats of 90 × 90 cm2 and subdivided into 81 square units of 10 × 10 cm2 with thick nylon line. The presence or absence of the species were recorded for each unit. Transect lines marked in 1m increments were placed at random at different sites. They were used as guides for placing the subdivided quadrats contiguously along the transect. These transects were aligned perpendicular to the coastline. Each extended outwards from the rocky shore to the sand bottom at a distance varying from 9-15m, depending on the inclination of the shore. Snorkeling divers were used to assess the vertical distribution and abundance of the seaweed species. 8100 cm2 of quadrat (90 × 90 cm2) was used to estimate the percentage of cover by the dominant benthic seaweeds.
When sampling, the time and depth at each quadrat were recorded and the depth was corrected according to the tide level. Three depth ranges were sampled at all sites. Abundance of each algae group was expressed as number of individuals of that group/m2 as a percentage of the total number of algae individuals of all species / m2 (Figure 2).
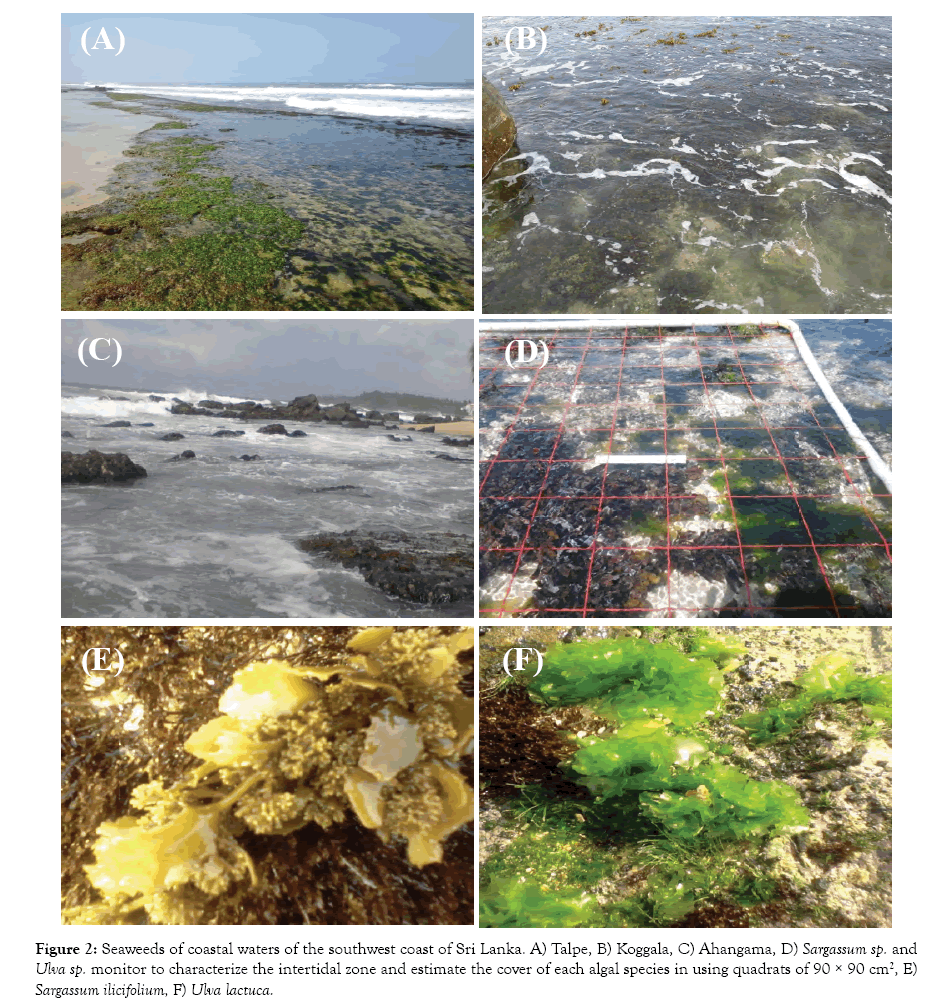
Figure 2: Seaweeds of coastal waters of the southwest coast of Sri Lanka. A) Talpe, B) Koggala, C) Ahangama, D) Sargassum sp. and Ulva sp. monitor to characterize the intertidal zone and estimate the cover of each algal species in using quadrats of 90 × 90 cm2, E) Sargassum ilicifolium, F) Ulva lactuca.
Statistical analysis
Graph Pad Prism Version 4.03 for Windows (Graph Pad Software, San Diego, CA, USA) and SPSS were used for the statistical analysis. All data were obtained in order to show the seaweed distribution and their relative abundance (%). The percentage of taxa was estimated for the communities at each depth site. To test the differences in species and depths, 09 quadrats were randomly sub-sampled from the data sets and divided in three depth intervals. At each site, overall mean percentage covers of each taxon were calculated using one-way and two-way analysis of variance. Multiple comparisons between the significant levels of interactions of the variables were done by Tukey’s method and values were expressed as the Mean ± SE and P<0.05 considered significant.
Shannon-Wiener diversity index values indicated that the diversity of Koggala was closely related to the diversity of Ahangama whereas the diversity of Talpe was not closely related to the diversity of Ahangama and Koggala coastal areas (Figure 3).
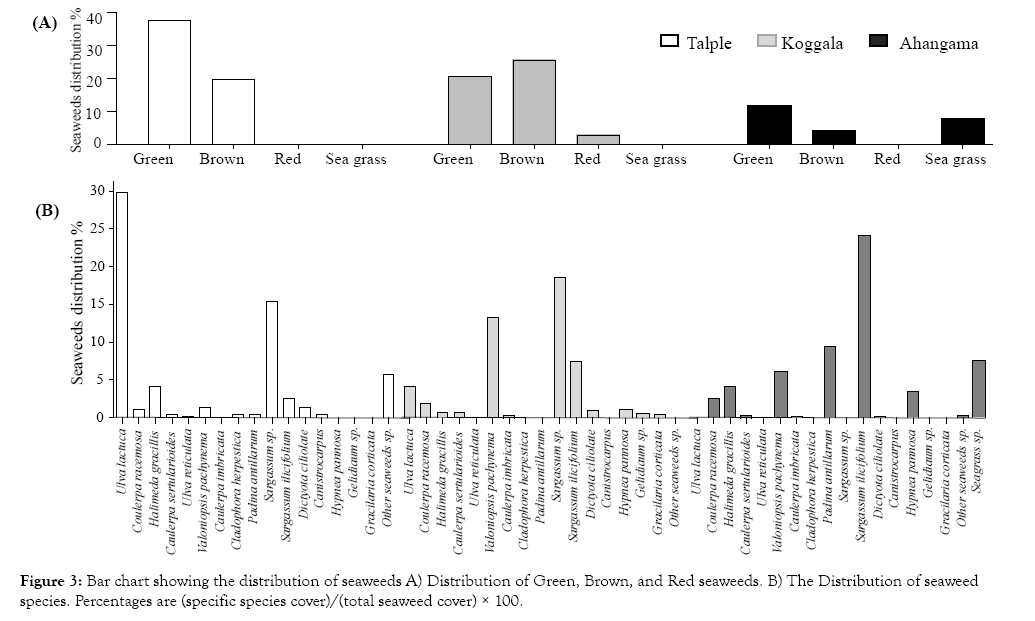
Figure 3: Bar chart showing the distribution of seaweeds A) Distribution of Green, Brown, and Red seaweeds. B) The Distribution of seaweed species. Percentages are (specific species cover)/(total seaweed cover) × 100.
A statistically significant difference was found between seaweed distributions in Talpe, Koggala and Ahangama coastal areas (Table 1).
| Algae Species Name | Talpe | Koggala | Ahangama |
|---|---|---|---|
| Ulva lactuca | + | + | - |
| Caulerpa racemosa | + | + | + |
| Halimeda gracillis | + | + | + |
| Caulerpa sertularioides | + | + | + |
| Ulva reticulata | + | - | - |
| Valoniopsis pachynema | + | + | + |
| Caulerpa imbricata | + | + | + |
| Cladophora herpestica | + | - | - |
| Padina antillarum | + | - | + |
| Sargassum sp. | + | + | - |
| Sargassum ilicifolum | + | + | + |
| Dictyota ciliolate | + | + | + |
| Canistrocarpus sp. | + | - | - |
| Hypnea pannosa | - | + | + |
| Gelidiaum sp. | - | + | - |
| Gracilaria corticata | - | + | - |
+ Present; - Absent
Table 1: Species list of seaweeds at Talpe, Koggala and Ahangama coastal in Sri Lanka.
A statistically significant relationship was found between abundance of Sargassum species and Ulva species. Sargassum ilicifolium dominates the lower intertidal zone when compared with other seaweeds species. Ulva lactuca occurs closer to the shore. There was significant difference with temperature, salinity and pH, dissolved oxygen and wave within the chosen study site (Figure 4).
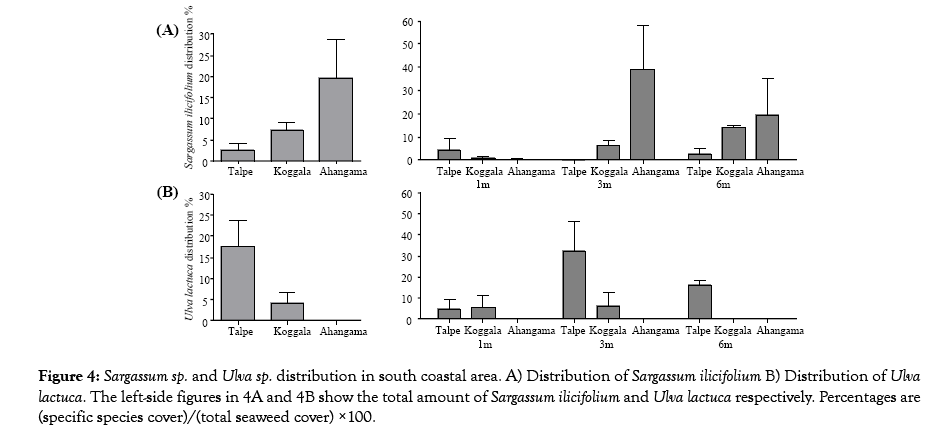
Figure 4: Sargassum sp. and Ulva sp. distribution in south coastal area. A) Distribution of Sargassum ilicifolium B) Distribution of Ulva lactuca. The left-side figures in 4A and 4B show the total amount of Sargassum ilicifolium and Ulva lactuca respectively. Percentages are (specific species cover)/(total seaweed cover) ×100.
Out of eight species, Ulva lactuca was the most common and widely spread in Talpe coastal area, where as Sargassum ilicifolum is widely spread in Ahangama coast. A statistical relationship (P<0.05) was found to exist between relative abundance of Sargassum ilicifolum and Ulva lactuca. Sargassum ilicifolum appears in Koggla coastal area water with high dissolved oxygen content. Ulva lactuca species appears in water with low dissolved oxygen content and a quiet environment when compared with Sargassum species distribution (Figure 5).
Figure 5: Normal Q-Q Plot and Detrended Normal Q-Q Plot of Average percentage of seaweed distribution. A) Normal Q-Q Plot of Average percentage of Sargassum ilicifolium, B) Normal Q-Q Plot of Average percentage of Ulva lactuca, C) Detrended Normal Q-Q Plot of Sargassum ilicifolium distribution, D) Detrended Normal Q-Q Plot of Ulva lactuca distribution, E) Box Plot of Sargassum ilicifolium distribution, F) Box Plot of Ulva lactuca distribution.
Two algae species, Sargassum ilicifolium and Ulva lactuca were selected to characterize the intertidal zone and explore the macrophyte patterns in the shallow subtidal regions. The brown seaweed of Sargassum ilicifolum and green seaweed of Ulva lactuca be the important groups in abundance. They ranged respectively from 13-66% and 27-68% of total algae. Considering from the ecological and economic view, Ulva lactuca could be an important green seaweed in the Talpe area. Besides, the importance of these aquatic inter-tidal macrophytes for fishery resources and overall ecosystem processes should not be over looked in this coastal area.
Horizontal distribution of the benthos
We recognized the existence of benthic communities occupying the same habitat but they were in different depth zones. The algal community seem to be dominated by turf algae (mostly green seaweeds) at shallowest depths and the brown seaweed Sargassum ilicifolum were found at deeper levels. Different patterns were found at different sites, demonstrating that different spatially discrete factors may be acting on each rocky shore. Brown seaweeds Sargassum ilicifolum have more cover (19.59 ± 9.129%) than other seaweeds at Ahangama. In this area Ulva lactuca species are not present. This Sargassum ilicifolum species has been shown to dominate the brown seaweeds of many regions in Sri Lanka. At the Talpe sites a green seaweed Ulva lactuca (17.67 ± 5.930%) community dominated and Sargassum ilicifolum community coverage (2.497 ± 1.679%) was small in this area. Koggala sites were normally covered by Sargassum ilicifolum (7.106 ± 2.078%) and Ulva lactuca (3.992 ± 2.644%).
Vertical distribution of the benthos
Our analysis indicates that the distribution of taxa showed a clear vertical differentiation. Vertical distribution of species on rocky shores varies due to many factors such as hydrodynamics [23], turbidity [24], temperature [25], wave exposure [26-28], slope [29], light intensity [30], grazing activities [31-33], and predation [34]. Ahangama area has been colonized by Sargassum ilicifolium which is distributed in lower intertidal zone. The site in Ahangama located at a narrow depth range of (0.5 –1.5 m) housed sea grasses and brown algae (Sargassum ilicifolium, Padina antillarum and Dictyota ciliolate), red algae (Hypnea pannosa), green algae (Halimeda gracilis, Valoniopsis pachynema, Caulerpa sertularioides, Coulerpa racemosa, Caulerpa imbricate). The intertidal zone is the area of the marine shoreline that affects the amount of incident light and so the development of an algal community [35]. The algae bed slope was gentle and shallow; plenty of light was available, so growth rates of macro-algae were high [36]. At Koggala the depth and high turbidity reduce light intensity and so reduce growth of algal species.
The brown algae (Sargassum sp., Padina antillarum, Sargassum ilicifolium, Canistrocarpus and Dictyota ciliolate), green algae (Halimeda gracilis, Coulerpa racemosa, Valoniopsis pachynema, Caulerpa sertularioides, Ulva lactuca, Ulva reticulate, Cladophora herpestica, Caulerpa imbricate) are favoured as substrates and are among the dominant algae species along the Talpe coastal area. The rough sea obstructed sampling at Koggala and extension of rocky shore only up to 10 m limit the collection of samples at Talpe.
Human activities are intense in coastal areas and marine communities are subjected to pressure from a multitude of stressors, resulting in large scale changes in the abundance and distribution of species [37,38]. Ahangama algae bed is situated very close by the fisheries harbor and anglers can utilize this area for their fishing activities every day. The brown algae (Sargassum sp., Sargassum ilicifolium and Dictyota ciliolate) red algae (Gracilaria corticata, Gelidiaum sp., Hypnea pannosa) and green algae (Halimeda gracilis, Valoniopsis pachynema, Caulerpa sertularioides, Ulva lactuca, Caulerpa imbricate) are favoured as substrates and are among the dominant algae along the Koggla coastal area. The brown algae Sargassum ilicifolium and green algae Ulva lactuca were found as substrates since they are among the dominant algae forms along the Ahnagama and Talpe coastal area respectively.
This study provides evidence that the most abundantly distributed seaweed species in this area are brown and green seaweeds. However, brown seaweed namely; Sargassum species are found to be the commonest in the area most exposed to sea waves with high dissolved oxygen contents, whereas green seaweed namely; Ulva species dominates in the shallow depths. Further we observed changing patterns of distribution in communities of seaweeds along the depth gradient; in fact, the highest diversity is obvious close to the coast. Density of Sargassum ilicifolum species was higher in Ahangama compared with Talpe and Koggala. It was also obvious that a positive correlation (P<0.05) existed between Sargassum ilicifolum and Ulva lactuca. It is pronounced that there is a marked difference of respective seaweed communities in Ahangama and Talape. However, further investigations are essential to establish assertive correlations among seaweed community, structure and distribution in these locations.
The data and materials are contained within the paper. Samples and data collections were obtained from the south coastal algae bed in Sri Lanka.
The author(s) declare that they have no competing interests.
This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
The authors wish to thanks Faculty of Fisheries & Marine Science, Ocean University of Sri Lanka for providing us with a pleasant environment to complete this research project. Support given by Mr. KA Wijesekera by editing the manuscripts is also highly appreciated.
Citation: Premarathna AD, Kumara AMCP, Jayasooriya AP, Jayanetti DE, Adhikari RB, Sarvananda L, et al. (2020) Distribution and Diversity of Seaweed Species in South Coastal Waters in Sri Lanka. J Oceanogr Mar Res 8:196. doi: 10.35248/2572-3103.20.8.196
Received: 18-Nov-2019 Accepted: 06-Jan-2020 Published: 13-Jan-2020
Copyright: © 2019 Premarathna AD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.