
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Research Article - (2017) Volume 8, Issue 12
Background: There is a high prevalence of undiagnosed obstructive sleep apnoea (OSA) in patients with obesity undergoing bariatric surgery. We developed two novel scores in order to investigate the extent to which anthropometric and other objective measurements can be used to identify the presence of moderate-severe OSA (Apnoea/Hypopnoea Index (AHI) ≥ 15/h) in surgical patients with obesity.
Methods: We prospectively evaluated 1870 adult patients scheduled for elective laparoscopic bariatric surgery. Prior to surgery, body mass index (BMI), sex, neck circumference, STOP-Bang score, SpO2, and neck/trunk fat were recorded. Basic anthropometric measurements were obtained, and the A Body Shape Index (ABSI) was calculated using the Krakauer formula. Patients at high risk for OSA were referred for polysomnography. Auto-titrated positive airway pressure (APAP) therapy was initiated when AHI ≥ 15/h. The Dual-X Ray-Obstructive Sleep Apnoea (DXOSA) score included six items: STOP-Bang score, BMI, neck fat, trunk fat, baseline SpO2, and expiratory reserve volume (ERV). The Anthropometric-OSA (A-OSA) score included STOP-Bang score, BMI, NC, ABSI coupled with WC, baseline SpO2, and ERV. We then compared sensitivity, specificity, positive-predictive values, negativepredictive values, likelihood ratios, and post-test probabilities in these patients.
Results: Using a cut-off of 3, the DX-OSA and A-OSA scores exhibited similar sensitivity to STOP-Bang scores, but were associated with improved specificity, lower false positive rates, and increased probability for the diagnosis of moderate-severe OSA.
Conclusion: The A-OSA and DX-OSA scores may be useful in the identification of obese surgical patients requiring CPAP treatment for significant OSA, without the need for formal polysomnography.
Keywords: Obstructive sleep apnoea; Obese patient; Postoperative cardiac events
Obstructive sleep apnoea (OSA) is a common comorbidity in obese individuals [1]. Anaesthesiologists must be aware of the high prevalence of undiagnosed OSA in obese surgical patients (>24%) [2]. The postoperative complications such as coronary artery disease, heart failure, arrhythmias, and stroke are increased in patients with moderate to severe obstructive sleep apnoea (OSA) undergoing surgery [3,4]. For surgical patients with both obesity and OSA undergoing surgery, the perioperative period is known to be challenging, as the likelihood of post-surgical respiratory complications is also increased in patients with obesity [5,6].
The American Society of Anesthesiologists (ASA) and the Society of Anesthesia and Sleep Medicine recommend preoperative screening of surgical patients for OSA and treatment during the perioperative period in the event of significant OSA [7,8] and recent literature highlights importance of specifically designed periprocedural assessment and critical care strategies for obese patients to improve outcome of such patients in any clinical setting [9]. Furthermore, previous studies have indicated that preoperative continuous positive airway pressure (CPAP) improves exercise tolerance and reduces daytime sleepiness and negative cardiopulmonary physiological consequences (especially hypertension) in patients with OSA [10,11]. However, there remains a lack of consensus regarding the most appropriate methods of screening for and diagnosing OSA prior to surgery, and similarly inadequate details have been reported regarding the need for and timing of PAP therapy in such cases [2,12].
The aim of this prospective observational study was to identify the extent to which anthropometric and other objective measurements relevant to OSA and obesity can be used to predict clinically significant OSA, which we defined as an Apnoea/Hypopnoea Index (AHI) of ≥ 15/h. We further derived two novel scores and evaluated their ability to identify severely obese patients with moderate-to-severe OSA who require preoperative CPAP prior to bariatric surgery without application of formal polysomnography (PSG).
We prospectively evaluated 1870 consecutive adult patients who were scheduled for elective laparoscopic bariatric surgery at the Ponderas Academic Hospital Centre of Excellence for Bariatric and Metabolic Surgery in Bucharest, Romania, between January 2013 and June 2016. The study was approved by the Ponderas Academic Hospital research ethics committee (Ref. 01/01/2013), and written informed consent was obtained from all patients prior to their participation.
Patients with incomplete data and those diagnosed with OSA and receiving CPAP treatment at the time of the preoperative visit were excluded from further analysis. However, patients with weights higher than 204 kg who were unable to undergo iDXA analysis were retained.
All adult pre-surgical patients with American Society of Anesthesiologists (ASA) physical status I−IV, were assessed 3-4 weeks prior to surgery by a multidisciplinary team, which included an anaesthetist. In addition to the routine recording of body mass index (BMI), sex, presence of diabetes, neck circumference (NC), SpO2, and STOP-Bang questionnaire responses, we measured neck fat, lean neck tissue, trunk fat, and lean trunk tissue using dual X-ray absorptiometry (Lunar iDXA, GE Healthcare Madison, WI). The iDXA system offers a high-resolution densitometry technique that differentiates between bone and surrounding soft tissue and allows for the further discrimination of the latter into fat and fat-free (lean) components.
We also obtained anthropometric measurements from all patients. Body shape, as measured using A Body Shape Index (ABSI), was based on waist circumference adjusted for height and weight using the Krakauer [13] formula:
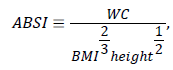
Where, WC represents waist circumference and BMI represents body mass index. We measured lung volumes via spirometry or lung plethysmography.
Patients who were identified at high risk for OSA based upon their responses to the STOP-Bang screening questionnaire or with excessive daytime sleepiness or high neck circumferences were recommended to undergo home sleep testing with polysomnography (PSG). The PSG was applied with a 12-channel portable PSG CID-LX type III device (CIDELEC, Sainte Gemmes/Loire, France). The polysomnographic recording montage for the CID-LX consisted of a nasal cannula (to measure nasal flow), a PneaVoX sensor, thoracic and abdominal inductive belts, a polygraph placed on the wrist, and a pulse oximetry. The PneaVoX technology allows for the recording of various physiological parameters with a single sensor: nose and mouth breathing, respiratory effort, snoring, etc.
The portable PSG device was adjusted to each patient by a PSG technician in the hospital; patients were taught how to use the device at home for overnight recording. PSG recordings were automatically analysed using CIDELEC software and were further reviewed by a sleep physician.
Stage and score of PSGs was performed accordingly to the 2007 American Academy of Sleep Medicine Manual (AASMM) for the Scoring of Sleep and Related Events [14]. In accordance with the AASMM, we defined apnoea as an at least 90% decrease in airflow from baseline, lasting at least 10 s. Hypopnoea was defined as an at least 50% reduction in airflow, lasting at least 10 s, and associated with an at least 3% decrease in arterial oxy-haemoglobin saturation or arousal [14]. AHI was defined as the average number of apnoeic and hypopnoeic episodes per hour, and the severity of OSA was graded based on AHI (None: 0-5, Mild: OSA 6-14, Moderate OSA: 15-30, Severe OSA: >30) [14]. We also recorded the minimum oxygen saturation during sleep and the oxygen desaturation index (ODI) defined as the mean number of episodes per hour, in which either one or both of the following occurred: 4% or greater desaturation and/or duration of more than 10 s [15].
In order to avoid delaying surgery, the anaesthesiologist made decisions regarding CPAP and oxygen therapy. CPAP therapy was indicated when AHI was ≥ 15/h. Patients received auto-titrating CPAP (APAP) devices such as the GoodKnight 418 (Mallinckrodt Respiratory Group, St Louis, MO) and S8 AutoSet Spirit™ II (ResMed, San Diego, CA) after the PSG and advised to bring their devices for perioperative use.
Auto-titrated CPAP (APAP) measures the flow of air breath-bybreath and adjusts the delivered pressure to the minimum level necessary for maintaining an unobstructed airway [9]. This technique allows initiation of treatment without in-laboratory CPAP titration [16].
Sample size estimation
The sample size estimation was based on an expected sensitivity of 0.80, specificity of 0.70, precision of 0.09, OSA prevalence of 69% (as reported for the STOP-Bang, Berlin Questionnaire, and ASA Checklist in the American Academy of Sleep Medicine Guidelines), and a type I error of 0.05 (Confidence level 95%). A precision of 0.09 for specificity resulted in an estimated sample size of 323. Therefore, we enrolled a total of 350 patients.
Statistical method
The data were collected in an electronic spreadsheet that enabled descriptive statistical analysis. The main investigators had access to the electronic spreadsheet. Missing data were excluded from analysis. Statistical analysis was performed with SPSS version 22 (Chicago, IL, USA), and the level of significance was set at p ≤ 0.05. We tested the normality of the data distribution using the Shapiro-Wilk test. Categorical data were reported as frequencies and percentages and subsequently compared using chi-square tests and odds ratios (ORs).
Logistic regression models were employed to identify predictors of OSA and the related probabilities. For each parameter of interest, we constructed receiver operating curves (ROC) to determine the area under the curve (AUC). The cut-off point for each score was assessed using the Youden index and Matthews’s correlation coefficient. After examining the AUCs and cut-off points for each variable in the whole cohort of patients (n=1870), we constructed two new scores, the Dual- X-Ray-Obstructive Sleep Apnoea (DX-OSA) Score (which included the STOP-Bang score as well as BMI, neck fat, trunk fat, baseline SpO2, and expiratory reserve volume [ERV] measurements); and the Anthropometric-OSA (A-OSA) score (which included the STOP-Bang score as well as BMI, NC, ABSI coupled with WC, baseline SpO2, and ERV measurements). If a patient had any parameter value greater or equal to the cut-off, we assigned a point value of 1. The score for each parameter ranged from 0-6 points (Table 1).
| DX-OSA score | Cut-off | Points | A-OSA score | Cut-off | Points |
|---|---|---|---|---|---|
| STOP-Bang score | ≥ 5 | 1 | STO-Bang score | ≥ 5 | 1 |
| BMI (kg m-2) | ≥ 45 | 1 | BMI | ≥ 45 | 1 |
| Neck fat (g) | ≥ 1330 | 1 | Neck circumference (cm) | ≥ 45 | 1 |
| Trunk fat (kg) | ≥ 40 | 1 | ABSI Waist Circumference (cm) |
≥ 0.085 ≥ 134 |
1 |
| Baseline SpO2 (%) | ≤ 94 | 1 | Baseline SpO2 (%) | ≤ 94 | 1 |
| Expiratory reserve volume (L) | ≤ 0.54 | 1 | Expiratory reserve volume (L) | ≤ 0.54 | 1 |
Table 1: DX-OSA and A-OSA scores.
As a preliminary part of the actual study we initially formulated the DX-OSA score, which had different parameters as the actual DX-OSA score .We sought the best combinations and the final decision was to clearly separate the iDXA measurements (neck fat, trunk fat) from clinical anthropometric measurements (NC, WC, ABSI) resulting the actual DX-OSA and A-OSA scores [17,18]. We then analysed the capabilities of the two novel scores to identify moderate-to-severe OSA in the subgroup of patients with complete data who had undergone PSG (n=350). For each of the two new scores, we used 2 × 2 contingency tables to determine the sensitivity, specificity, positivepredictive values, negative-predictive values, likelihood ratios, and post-test probabilities.
The Dual-X-Ray-Obstructive Sleep Apnoea (DX-OSA) Score included the STOP-Bang score as well as BMI, neck fat, trunk fat, baseline SpO2, and expiratory reserve volume [ERV] measurements. The Anthropometric-OSA (A-OSA) score included the STOP-Bang score as well as body mass index (BMI), neck circumference, A Body Shape Index score (ABSI) coupled with waist circumference, baseline SpO2, and ERV measurements. If a patient had any parameter value greater or equal to the cut-off, we assigned a point value of 1.
Table 2 presents the characteristics of included patients along with descriptive statistical information. A total of 1870 patients provided informed consent, though 1520 were excluded (10 with preoperative CPAP plus 1510 with incomplete data mostly PSG). In the whole group, 1318 patients had STOP-Bang scores ≥ 3, while 559 patients were referred for in-home PSG, and 350 scheduled and completed the protocol. The remaining patients failed to keep the scheduled polysomnography appointment. Of the 350 patients who completed the in-home PSG, 268 patients with AHI ≥ 15/h received APAP treatment. Twenty-two patients with weights higher than 204 kg who were unable to undergo iDXA analysis were retained and allowed to complete the in-home PSG (Figure 1). Using an AHI>5/h for the diagnosis of OSA, 57 (16.2%) of the 350 patients were diagnosed with mild OSA, 76 (21.7%) with moderate OSA (AHI>15) and 192 (54.8%) with severe OSA (AHI>30).
| n=350 (patients with PSG) | n=870 (All patients) | ||||
|---|---|---|---|---|---|
| No OSA | OSA | ||||
| Mild | Moderate | Severe | |||
| n=25 (7.1%) | n=57 (16.2%) | n=76 (21.7%) | n=192 (54.8%) | ||
| Age (yrs) | 44 (17) | 46 (16) | 46 (16) | 47 (18) | 41 (18) |
| BMI kg/m2 | 50.3 (10.3) | 49.7 (9.6) | 49.7 (9.3) | 49.4 (9.2) | 40.5 (10.4) |
| Height (cm) | 165 (17) | 175 (14) | 175 (15) | 176 (15) | 170 (14) |
| Weight (kg) | 137.8 (28.1) | 149 (36) | 150 (33) | 152 (38) | 115.8 (35) |
| Waist Circumference (cm) | 138 (23) | 142 (20) | 143 (19) | 144 (18) | 124 (24) |
| Neck Circumference (cm) | 45 (6.7) | 48 (6) | 49 (6.8) | 49.5 (6) | 42 (7) |
| AHI (events/h) | 2 (2) | 36 (46) | 45 (43) | 59 (35) | 33 (48) |
| SpO2 | 0.96 (0.03) | 0.95 (0.03) | 0.95 (0.02) | 0.95 (0.02) | 0.97 (0.03) |
| Fat Mass (kg) | 69.3 (24.3) | 71.7 (22.4) | 71.7 (21.7) | 71.5 (20.7) | 55.7 (23.9) |
| Trunk Fat (kg) | 39.4 (16.7) | 43.8 (15.5) | 44 (15.2) | 44.2 (14.8) | 31.9 (16.3) |
| Neck Fat (g) | 1510 (642) | 1704 (764) | 1731 (708) | 1712 (680) | 1252 (866) |
| Expiratory Reserve Volume (%) | 37.8 (37.7) | 36.3 (44) | 35.5 (45) | 37.2 (47) | 41 (44) |
| ABSI | 0.081 (0.008) | 0.081 (0.006) | 0.081 (0.006) | 0.081 (0.006) | 0.081 (0.008) |
| DXOSA | 3 (1) | 4 (2) | 4 (2) | 4 (2) | 4 (2) |
| AOSA | 3 (1) | 3 (1) | 3 (1) | 3 (1) | 4 (2) |
| STOP-Bang | 5 (3) | 6 (2) | 6 (2) | 6 (2) | 4 (1) |
| Sex (M/F%) | 40/60 | 66.1/33.8 | 69.4/30.6 | 76.5/23.4 | 36.5/63.4 |
| BMI: Body Mass Index; AHI: Apnoea Hypopnea Index; SpO2: Oxygen Saturation; ABSI: A Body Shape Index; DX-OSA: Dual-X Ray–Obstructive Sleep Apnoea; A-OSA: Anthropometric-OSA; PSG: Polysomnography; Categorical data are presented as frequency (%). | |||||
Table 2: Descriptive Statistics- Data presented as median and interquartile range (IQR).
Men in the study population were more likely to be diagnosed with OSA than women, with an odds ratio of 2.93 (p=0.009, 95% CI: 1.27-6.70). Furthermore, men continued to exhibit a higher odds ratio for diagnosis of OSA in the subgroup analysis when compared with women: men were 2.5 times more likely to have moderate OSA (p<0.0001, 95% CI: 1.5-4.1) and 3.3 times more likely to have severe OSA (p<0.0001 95% CI: 2.1-5.2) than women. Patients with OSA exhibited higher neck circumference and AHI values than those without OSA (p=0.005 and p<0.0001, respectively).
We chose the variables and cut-offs values for our new scores from the ROC curves (Table 1).
Neck fat as measured with iDXA was positively correlated with neck circumference (r=0.20, p<0.0001) while trunk fat was positively correlated with waist circumference (r=0.31, p<0.0001). We used a logistic regression model to determine whether our new scores could predict OSA and found that both scores were good predictors of OSA diagnosis (p<0.0001) (Figure 2). A-OSA and DX-OSA scores increase specificity for OSA diagnosis and decrease false positive rates. The probability for moderate-severe OSA is calculated as
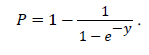
Tables 3 and 4 summarize the predictive values of A-OSA and DX-OSA scores compared with that of the STOP-Bang questionnaire for moderate OSA (AHI>15) and severe OSA (AHI>30), respectively. The evaluation of all scores is presented at a cut-off point of 3, and all comparable values obtained were statistically significant (p<0.0001). For the A-OSA score, the specificity for moderate OSA diagnosis increased by 29.2%, the false positive rate decreased by 29%, resulting in a 17.9% increase in the probability of accurate diagnosis of moderate OSA when compared to that of the STOP-Bang questionnaire.
| A-OSA | DX-OSA | STOP-Bang | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cut-off | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | 1-2 | 3 | 4 | 5 | 6 | 7-8 |
| Sensitivity | 1 | 0.95 | 0.75 | 0.44 | 0.18 | 0.03 | 0.99 | 0.97 | 0.88 | 0.64 | 0.30 | 0.07 | 0.98 | 0.98 | 0.93 | 0.80 | 0.60 | 0.06 |
| Specificity | 0.02 | 0.15 | 0.39 | 0.77 | 0.93 | 0.99 | 0.02 | 1.11 | 0.26 | 0.52 | 0.83 | 0.98 | 0.06 | 0.09 | 0.20 | 0.39 | 0.59 | 1 |
| FPR | 0.97 | 0.85 | 0.61 | 0.23 | 0.07 | 0.01 | 0.97 | 0.87 | 0.74 | 0.47 | 0.17 | 0.02 | 0.94 | 0.90 | 0.79 | 0.61 | 0.40 | 0 |
| LR+ | 1.02 | 1.11 | 1.22 | 1.88 | 2.4 | 3.06 | 1.02 | 1.09 | 1.19 | 1.34 | 1.77 | 3.21 | 1.05 | 1.09 | 1.18 | 1.32 | 1.51 | >10 |
| LR- | 0.15 | 0.36 | 0.65 | 0.73 | 0.89 | 0.97 | 0.31 | 0.27 | 0.45 | 0.69 | 0.84 | 0.94 | 0.24 | 0.19 | 0.31 | 0.50 | 0.66 | 0.94 |
| PPV | 0.77 | 0.78 | 0.80 | 0.86 | 0.89 | 0.91 | 0.77 | 0.78 | 0.80 | 0.81 | 0.85 | 0.91 | 0.77 | 0.78 | 0.79 | 0.81 | 0.83 | 1 |
| NPV | 0.66 | 0.46 | 0.32 | 0.30 | 0.26 | 0.24 | 0.50 | 0.53 | 0.40 | 0.30 | 0.27 | 0 | 0.55 | 0.61 | 0.50 | 0.38 | 0.32 | 0.24 |
| Odds Ratio | 6.67 | 3.11 | 1.88 | 2.56 | 2.69 | 3.1 | 3.32 | 4 | 2.63 | 1.94 | 2.10 | 3.4 | 4.28 | 5.68 | 3.86 | 2.65 | 2.30 | 1.32 |
| OSA Probability | 0.54 | 0.63 | 0.72 | 0.80 | 0.85 | 0.90 | 0.51 | 0.60 | 0.68 | 0.76 | 0.82 | 0.87 | 0.44 | 0.54 | 0.63 | 0.71 | 0.78 | 0.83 |
| AUC (95% CI) | 0.628 (0.561-0.696) | 0.620 (0.551-0.690) | 0.659 (0,593-0.725) | |||||||||||||||
| FPR: False Positive Rate; LR+: Positive Likelihood Ratio; LR: Negative Likelihood Ratio; PPV: Positive Predictive Value; NPV: Negative Predictive Value; OR: Odds Ratio; AUC: Area Under the Curve; *Positive and negative predictive values are strongly dependent on prevalence; for moderate OSA group the prevalence was 0.766. STOP-Bang is routinely administered to screen for OSA prior to bariatric surgery as part of hospital protocol. The predictive values were determined for each possible cut-off value. The evaluation of all scores is presented at a cut-off point of 3 unless stated otherwise. | ||||||||||||||||||
Table 3: Predictive values of A-OSA, DX-OSA, and STOP-Bang scores for moderate OSA (AHI>15/h)*.
| A-OSA | DX-OSA | STOP-Bang | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cut-off | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | 1-2 | 3 | 4 | 5 | 6 | 7-8 |
| Sensitivity | 0.99 | 0.96 | 0.80 | 0.50 | 0.19 | 0.03 | 0.99 | 0.97 | 0.90 | 0.67 | 0.34 | 0.06 | 0.98 | 0.98 | 0.96 | 0.84 | 0.66 | 0.08 |
| Specificity | 0.02 | 0.07 | 0.38 | 0.74 | 0.90 | 0.87 | 0.02 | 0.07 | 0.20 | 0.49 | 0.81 | 0.93 | 0.03 | 0.06 | 0.17 | 0.38 | 0.57 | 1.00 |
| FPR | 0.98 | 0.88 | 0.62 | 0.26 | 0.10 | 0.13 | 0.98 | 0.93 | 0.80 | 0.51 | 0.19 | 0.07 | 0.97 | 0.94 | 0.83 | 0.62 | 0.43 | 0.00 |
| LR+ | 1.02 | 1.10 | 1.28 | 1.91 | 1.90 | 1.44 | 1.01 | 1.05 | 1.11 | 1.31 | 1.78 | >10 | 1.02 | 1.05 | 1.16 | 1.36 | 1.55 | >10 |
| LR- | 0.00 | 0.30 | 0.55 | 0.68 | 0.90 | 0.99 | 0.00 | 0.34 | 0.56 | 0.67 | 0.82 | 1.00 | 0.41 | 0.25 | 0.21 | 0.42 | 0.59 | 0.92 |
| PPV | 0.55 | 0.58 | 0.60 | 0.70 | 0.70 | 0.64 | 0.55 | 0.56 | 0.57 | 0.61 | 0.68 | 0.52 | 0.55 | 0.56 | 0.58 | 0.63 | 0.65 | 1.00 |
| NPV | 1.00 | 0.73 | 0.60 | 0.54 | 0.47 | 0.63 | 0.75 | 0.70 | 0.59 | 0.55 | 0.50 | 0.45 | 0.67 | 0.76 | 0.79 | 0.71 | 0.58 | 0.77 |
| Odds Ratio | 0.00 | 3.61 | 2.32 | 2.80 | 2.11 | 1.40 | 3.69 | 3.07 | 1.98 | 1.94 | 2.18 | 0.89 | 2.48 | 4.25 | 5.44 | 4.28 | 2.64 | 1.89 |
| OSA Probability | 0.28 | 0.37 | 0.48 | 0.58 | 0.68 | 0.77 | 0.32 | 0.39 | 0.46 | 0.53 | 0.60 | 0.67 | 0.15 | 0.22 | 0.32 | 0.43 | 0.55 | 0.78 |
| AUC (95% CI) | 0.64 (0.58-0.70) | 0.60 (0.54-0.66) | 0.68 (0.63-0.74) | |||||||||||||||
| FPR: False Positive Rate; LR+: Positive Likelihood Ratio), LR: Negative Likelihood Ratio; PPV: Positive Predictive Value; NPV: Negative Predictive Value; OR: Odds Ratio; AUC: Area Under the Curve; *Positive and negative predictive values are strongly dependent on prevalence; for severe; OSA group the prevalence was 0.548; STOP- Bang is routinely administered to screen for OSA prior to bariatric surgery as part of hospital protocol. The predictive values were determined for each possible cut-off value. The evaluation of all scores is presented at a cut-off point of 3 unless stated otherwise. | ||||||||||||||||||
Table 4: Predictive values of A-OSA, DX-OSA, and STOP-Bang scores for moderate OSA (AHI>30)*.
For the DX-OSA score, the specificity for moderate OSA diagnosis increased by 15.8%, the false positive rate decreased by 16%, resulting in a 14.2% increase in the probability of accurate diagnosis of moderate OSA relative to that of the STOP-Bang questionnaire. For patients with severe OSA, both the A-OSA and DX-OSA scores maintained almost the same level of specificity, though increased sensitivity was observed when compared with that of the moderate group (80%-90% sensitivity vs. 75% and 88% sensitivity, respectively). For the A-OSA score, the specificity for severe OSA increased by 14%, while the false positive rate decreased by 32% relative to the STOP-Bang score. For the DX-OSA score, the specificity for severe OSA increased by 32%, while the false positive rate decreased by 14% relative to that of the STOP-Bang questionnaire. The overall effects were 24.9% (A-OSA) and 23.2% (DX-OSA) increases in the probability of accurate diagnosis of severe OSA.
For individuals with obesity who have elected to undergo bariatric surgery, a presumptive clinical diagnosis of OSA should be verified and its severity determined to establish the relative perioperative risk [6,19]. In the present study, we developed two novel, objective scores based on anthropometric data and assessed their ability to identify patients requiring CPAP treatment for OSA. Both the DX-OSA and A-OSA scores developed in the present study were associated with increased probability of accurate OSA diagnosis in moderate-severe and severe cases.
To date, the screening tools used to identify OSA in a pre-operative setting [8] that have been validated in surgical patients and found to have comparable accuracy include the Berlin Questionnaire [8,20] the American Society of Anesthesiologists Checklist [7,8,19] the STOP-Bang Questionnaire, [21-23] and Perioperative Sleep Apnea Prediction (P-SAP) score [24]. Their sensitivity varies from 50 to 90%, depending upon the severity of OSA targeted, though all exhibit suboptimal specificity (30-60%) [8,21,22,25].
The STOP-Bang Questionnaire is the fastest of these validated measures to implement [23]. However, given its moderate specificity, this questionnaire may produce a high number of false-positives, leading to unnecessary referrals for PSG [22].
For the diagnosis of OSA and initiation of CPAP, the American Thoracic Society [26] and American Academy of Sleep Medicine recommend overnight PSG in a sleep laboratory over a period of two nights [14]. However, relatively high cost, low availability in hospitals, and poor patient compliance reduce their applicability for verifying a diagnosis of OSA [27,28]. Furthermore the evidence to date does not support cancelling or delaying surgery in favour of OSA diagnosis via PSG [8,27].
Despite removing administrative barriers to obtain and interpret the results of the PSG in a timely fashion without delaying surgery, out of the 559 patients identified as having high-risk OSA, a high percentage of patients (38%) did not complete the PSG, and only 350 patients (62%) completed the assessment. In the whole group, 1318 patients had STOP-Bang scores ≥ 3. Thus, if a STOP-Bang cut-off of 3 had been used in the screening of patients for OSA, a higher number of patients would have been referred for PSG with higher false positive rates.
Additionally, our anaesthetists have observed that some STOP-Bang items (STO) are subjective and highly dependent upon patient reporting; hence, many patients may have provided unreliable answers to such items, resulting in non-referral. The perceived inconvenience of the PSG, a lack of understanding of the severe implications of untreated OSA associated with high cost, the unwillingness to use CPAP devices may have played a substantial role to the patients’ reluctance.
Although BMI alone is not a good predictor of sleep apnoea, BMI and OSA incidence are directly related [19,29]. The results of the present study align with those of previous reports, which have revealed that men exhibit a two-to threefold increased risk of OSA relative to women [1]. The differences in the distribution of adipose tissue in men, [19,30,31] who have a predominantly central fat deposition pattern around the neck, trunk, and abdominal viscera [31] are the fundamentals for the increased risk.
For a given BMI, relative risk for OSA is associated with body shape, which serves as a marker of abdominal fat deposition [13,32]. Krakauer proposed the ABSI, which is based on WC adjusted for height and weight, allowing for quantification of the pattern of fat distribution. An elevated ABSI appears to be a marker of the consistent risk for premature mortality in the general population [13]. Based on cut-offs from our patient data and the results of previous studies [17,18], we included WC and ABSI as items in the A-OSA score. We also included NC as a separate item at the cut-off found in our cohort, as previous researchers have observed this value to be more predictive than STOP-Bang score [33,34].
Simpson and colleagues first described sex differences in the associations between the severity of OSA [35] and measures of obesity in specific body regions using both iDXA and traditional anthropometry, observing a significant association between regional obesity and OSA severity. In the present study, we observed a strong association between OSA severity and both novel scores, as well as a direct correlation between iDXA (neck and trunk fat) and anthropometric measurements (NC, ABSI, and WC).
We formulated the A-OSA score based only on clinical measurements due to the practical drawbacks of iDXA measurements: [17,18] There are physical limits to body weight (204 kg), length, thickness, and width that can be determined with the type of iDXA machine, and such tools may not be readily available for all patients [36,37]. Twenty-two of the patients in this study had body weights >204 kg; they underwent PSG and were considered at high risk on clinical grounds.
Obesity affects ERV due to premature closure of the small peripheral airways [38,39]. In turn, reductions in end-expiratory lung volumes, which are accentuated in sleep, may lead to a reduction in tracheal traction on the pharynx, increasing its collapsibility [2,5,34]. The high tissue O2 consumption rates and reduced ERV in patients with obesity, especially when in the recumbent posture, cause more rapid depletion of lung O2 stores during apnoea, resulting in more severe arterial O2 desaturation for any given apnoeic length [34,40]. Patients with severe OSA experience higher levels of hypoxemia during wakefulness, during both non-REM sleep and REM stages of sleep, and during obstructive events [40]. Paradoxically, the severity of OSA was not quantified using indices of hypoxemia during sleep, though overnight hypoxemia is considered to underlie the cardiovascular impact of OSA [40]. Therefore, we considered ERV, baseline SPO2 measurement, and hypoxemia the best indicators of OSA severity where intervention with PAP is crucial and included them in both the DX-OSA and A-OSA scores.
We evaluated the predictive performance of DX-OSA and A-OSA in diagnosing OSA in patients with morbid obesity electing to undergo bariatric surgery. Both scores (with a cut-off of 3) exhibited improved specificity and decreased false positive rates relative STOP-Bang scores (3-5) for moderate-severe OSA.
The DX-OSA and A-OSA scores consist of objective measurements related to morbid obesity such as BMI, neck fat, trunk fat, neck circumference, ABSI, ERV, and baseline SpO2, with the exception of the subjective STOP-Bang score, which is used in the initial screening for OSA. Therefore, the scores may be useful in identifying patients with severe obesity who require preoperative CPAP treatment for OSA but less useful in identifying OSA in average or overweight patients.
The present study has some limitations. Although we included a large number of patients, this was a single-centre observational study, the results of which require further validation. The DX-OSA score was the first score formulated in two previous variants [17,18] but due to the weight limitation and its availability, our tendency is to reduce its use in favour of the A-OSA. We included the STOP-Bang in both new scores, which may have biased our objective approach. Nevertheless, this is the first study to identify a combination of objective clinical items that by themselves can provide a post-test probability >80% for the diagnosis of moderate-severe OSA amongst individuals with obesity prior to bariatric surgery.
The DX-OSA and/or A-OSA scores exhibit potential for routine use in the assessment of patients with obesity who have elected to undergo surgery due to their high probability of accurately diagnosing moderate-severe OSA that requires immediate APAP treatment. The implementation of these scores may combat inefficient allocation of resources, limited access to or compliance of patients with PSG, and prevent delay of surgery for patients prior to risk assessment.
The results of the present study indicate that the clinically objective DX-OSA and A-OSA scores more specifically identified the presence of significant OSA in patients who had undergone traditional subjective screening, suggesting the potential for these scores in reducing falsepositives, unnecessary referrals for PSG, and overcrowding of postoperative care units. Ultimately, prospectively randomized trials are needed to test the validity and cost-effectiveness of the DX-OSA and A-OSA in a pre-operative setting.
We thank to Ms. Andreea Constantinescu (Assistant Secretary in Ponderas Hospital, Bucharest, Romania) who helped collect the data. Logistic support was provided from institutional and/or departmental sources in Ponderas Hospital. Finally, we would like to thank Editage (www.editage.com) for English language editing.