
Immunotherapy: Open Access
Open Access
ISSN: 2471-9552

ISSN: 2471-9552
Research Article - (2023)Volume 9, Issue 3
Background: The novel coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 that belongs to betacoronaviruseis, and is an enveloped, single and positive-stranded RNA virus. In this study, we describe the production and characterization of four monoclonal antibodies with neutralization activities.
Methods: Thirteen monoclonal antibodies that identified positive were characterized by neutralization assays, binding affinities identification, and the indirect sandwich Enzyme-Linked Immunosorbent Assay (ELISA) to evaluate the immunocompetence. The genes of neutralizing antibodies were sequenced and analyzed.
Results: All monoclonal antibodies recognized two different epitopes respectively, including the receptor binding domain and human fragment crystallizable, and the binding affinities are also different. Four of nine monoclonal antibodies that can recognize the epitopes of the receptor binding domain are conformation neutralizing antibodies that have high binding affinity and neutralization ability. Based on the results sequencing, the monoclonal antibodies are unique antibodies with specificity.
Conclusion: We characterized thirteen monoclonal antibodies, four of which are neutralizing antibodies and identified unique antibodies with specificity. The results highlight the promise of antibody-based therapeutics and provide the structural basis of rational vaccine design.
Neutralizing antibody; SARS-CoV-2; Vaccine
In December 2019, a new infectious respiratory illness and pneumonia emerged in Wuhan, Hubei province, China, and spreaded national and international rapidly which pose a global health emergency [1-3]. The emerging pathogen belongs to betacoronavirus genus and has currently been named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), which has a phylogenetic similarity to SARS-CoV [3-5]. Globally, as of 14 October 2021, there have been 239,007,759 confirmed cases of COVID-19, including 4,871,841 deaths, reported to the World Health Organization (WHO) [6]. Considering the readily transmission from human to human, the WHO declared the SARS-CoV-2 epidemic a public health emergency of international concern on January 30, 2020 [6,7].
SARS-CoV-2 is an enveloped, single and positive-stranded RNA virus, which belongs to lineage B betacoronaviruse, and the other two coronaviruses cause deadly pneumonia in humans: Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV), Middle-East Respiratory Syndrome Coronavirus (MERS-CoV) [8-11]. For the coronaviruses, its genome RNA encodes a nonstructural replicase polyprotein and structural proteins, including Spike (S), Envelope (E), Membrane (M) and Nucleocapsid (N) proteins [12,13]. Studies revealed that 2019-nCoV makes use of a densely glycosylated Spike (S) protein to gain entry into host cells which also plays an essential role in viral attachment, fusion, and transmission. The Spike glycoprotein (S) homotrimer on the COVID-19 virus surface engages Angiotensin-Converting Enzyme 2 (ACE2) as the entry receptor to fuse the viral membrane with the host cell membrane for virus entry [6,14,15]. The S protein is a trimeric class I fusion protein and comprises two functional subunits responsible for binding to the host cell receptor (S1 subunit) and fusion of the viral and cellular membranes (S2 subunit) [8]. The Receptor Binding Domain (RBD) locates on the S1 region [16].
For controlling viral infection, inducing virus Neutralizing Antibodies (nAbs) by vaccines or infected virus is crucial. Currently there are four specific nAbs developed, including Monoclonal Antibodies (mAbs), their Functional Antigen-binding Fragment (Fab), the Single-chain Variable Region Fragment (scFv), or single-domain antibodies [Nanobodies (Nbs)] [17]. Quite a lot studies from SARS-CoV and MERS-CoV have demonstrated that most of nAbs target the fragments (S1-NTD, RBD, S2) in S proteins [17,18]. It has been shown that the recently published nano-bodies were bound to those unique epitopes on the S protein RBD to block the interactions of ACE2 or non-neutralizing antibody [19]. This implies further evidence in support of the possibility for the neutralizing antibody drug to block the binding site of the endogenous antibodies [20,21]. The antibody neutralizing potency is determined by competition with ACE2 receptor for RBD binding. RBD-specific antibodies have greater potency to neutralize infection with divergent virus strains, suggesting that the RBD of SARS-CoV-2 can also serve as an important target for the development of potent and specific nAbs and many SARS-CoV-2 Neutralizing Antibodies (nAbs) were developed with efficient therapeutic potential and the drug for the therapy against COVID-19 [20,22]. For SARS-CoV-2, only neutralizing antibodies directly against the pathogenic sequence in the S protein sequence can neutralize the virulence of the virus and prevent the virus from infecting the body.
To date, only a few studies about anti-novel coronavirus antibodies with high binding activity and/or high neutralization activity have been reported [21,23]. Therefore, the preparation of monoclonal antibodies with neutralizing activity is of great value for the development of ELISA kit that could rapidly identify and quantify SARS-CoV-2, the kit is crucial for clinical detection of SARS-CoV-2 infections and quality control during the process of vaccine production. Moreover, based on the pathogenic mechanism of this virus, it can provide targeted prophylaxis and early treatment for the epidemic or even large-scale enclosure of these virus infections in the future.
Here we report the isolation and characterization of 13 RBD-specific monoclonal antibodies derived from hybridoma cell lines from 3 mice infected with RBD-SD1-hFc antigen. We identified antibodies that potently neutralize SARS-CoV-2 which may be candidates for development of clinical interventions against SARS-CoV-2.
Study approval
Experimental mice were handled by licensed laboratory animal practitioners in all procedures, and were injected intraperitoneally with nembutal sodium for euthanasia at the end of the experiments.
Hybridomas
Three BALB/c mice were immunized using the method in the literature [24]. The serum of immunized mice was collected and detected by an indirect sandwich Enzyme-Linked Immunosorbent Assay (ELISA) to preliminarily evaluate the titer of immunogen in serum. The titers of the mouse No. 1 presented relatively higher; the results suggested that has strong immune response. Therefore, No. 1 was selected for fusion and subsequent production of hybridoma cells. In the process, diluted supernatants of culture were screened using indirect ELISA for reactivity.
Indirect ELISA: 2 μg/ml of RBD-SD1-hFc (CN 202011454175.1) (200 ng per well) was coated onto 96-well microtiter plates and stored overnight at 4°C or 2 h at room temperature. Plates were washed (300 μl per well) with Phosphate Buffered Saline with 0.05%Tween20 (PBS-T) for twice, and then blocked with 2% Bovine Serum Albumin (BSA) in PBS-T for 1.5h (300 μl per well) at room temperature. Mouse serum and the supernatants was diluted and added to plates (100 μl per well) for 1.5 h at room temperature after washing. The wells were subsequently incubated with Horseradish Peroxidase-conjugated (HRP-conjugated) goat-anti-mouse IgG (100 μl per well) for 1 h at a ratio of 1:2000. The absorption of the supernatants was read at 450 nm. The serum from immunized mice was used as a positive control; the supernatants of cultures with no cell growth as well as the serum from normal mice were the negative controls.
Antigen recognition domain of monoclonal antibody by indirect ELISA
The antigens including RBD-SD1-hFc, RBD-hFc, RBD-HAS were diluted to final concentrations of 2 μg/ml, then coated onto 96-well plates and incubated at 4°C overnight. Either serially diluted the supernatants of culture contained mAbs were added the plates and incubated at 37°C for 1 h. Wells were then incubated with goat-anti-mouse IgG labeled with HRP and TMB substrate and Optical Density (OD) was measured by a spectrophotometer at 450nm.
Binding activity
For binding ELISA, 96-well ELISA plates were coated with 5 μg/ml of antigens (RBD-SD1-hFc) overnight at 4°C. Plates were blocked with assay diluent (PBS-T containing 2% (w/v) BSA) at room temperature for 1.5 h. Purified monoclonal antibody samples were diluted to 1 μg/ml, then serially diluted tenfold in assay diluent (PBS-T containing 0.1% (w/v) BSA). Samples were added to the antigen-coated plates (100 μl per well) and incubated for 1.5 h at room temperature. HRP-conjugated goat anti-mouse IgG antibody was diluted (PBS-T containing 0.5% (w/v) BSA) to 1:2,000 and incubated at room temperature for 1 h. These plates were developed with Tetramethylbenzidine substrate (TMB) and the reaction was stopped with 1 M H2SO4. The OD at 450 nm was determined. The dilution was the negative controls.
Neutralization activity
Neutralization assays with SARS-CoV-2 pseudotyped viruses were performed in HEK293-ACE2 cells (Beijing Biodragon Immunotechnologies Co., Ltd) as described previously [25].
Serial dilutions of mAbs were mixed separately with a certain amount of pseudotyped viruses, incubated at 37°C for 1 h. The pre-cultured HEK293-ACE2 cells were incubated 2 × 105/mL, subsequently, seeded(100 μl/well) at in 96-well culture plates, was incubated with the experimental group, blank control group, negative control group at 37°C. After incubation 20~28 h, viral supernatants were sucked away (150 μl/well), luciferase detection reagent were seeded (100 μl/well) for reaction 2 minute. After the cells are fully cracked, the supernatant is sucked out, and added to the 96-well chemiluminescence detection plates (150 μl/well), and the luminous value were determined using chemiluminescence Analyzer.
Cells mixed with virus were used as a positive control; cells unexposed to the virus were the negative controls. The fluorescence inhibition rate was calculated as follows:
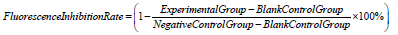
Half-maximal Inhibited Concentration (IC50) was determined for neutralization titer of the monoclonal antibody against PsV SARS-CoV-2 using GraphPad Prism 6 (GraphPad Software Inc.).
Identification of antigen-recognizing
Subsequently, we selected the top four neutralizing antibodies to measure the recognition ability of SARS-CoV-2 VLPs (including HuB01, 501Y.V2, Delta, Omicron) by indirect ELISA.
By indirect ELISA method, the 96-well microtiter plates were coated with four VLPs (100 ng per well) overnight at 4°C [24]. After drying, the plates were blocked with 2% BSA (300 μl/well) for 2 h, and incubated with antibodies (100 μl at 0.3 μg/ml) for 1 h at room temperature. HRP-conjugated goat anti-mouse IgG (1:4,000 in assay diluent) was added to the dried plates and incubated for 1 h at room temperature. The plates were washed with PBS 5 times prior to the addition of the chromogenic substrate solution (100 μl/well). The reaction was stopped by the addition of the termination solution (100 μl/well) after 10-minute incubation in the dark, and the absorbance was measured at 450 nm.
Gene sequencing
For hybridoma cell lines (6D11, 7C4, 7H9 and 8D1) were harvested, and total mRNA was extracted. Using the first strand of reverse transcribed cDNA as a template, heavy chain and light chain genes were amplified by PCR with variable region universal primers. The PCR products were recovered to obtain the target DNA products, and then the target DNA products were linked and transformed. The positive clones were obtained by PCR amplification and electrophoresis identification, and determined their DNA sequences. The DNA sequences were translated into amino acid sequences that were aligned and analyzed subsequently.
Production of hybridomas and generation of mAbs
A certain amount of mAbs against COVID-19 were developed from mouse hybridoma cells following the standard method, thirteen of which were identified as positive clones by indirect ELISA (Figure 1). As shown in Figure 1, with three different batches of antigen proteins, thirteen antibodies have high OD values, suggesting that exhibit the binding abilities to RBD-SD1-hFc, but not sure to identify which one of RBD or SD1 or hFc. In order to confirm the antigen recognition domain, the mAbs were subjected to further characterizations.
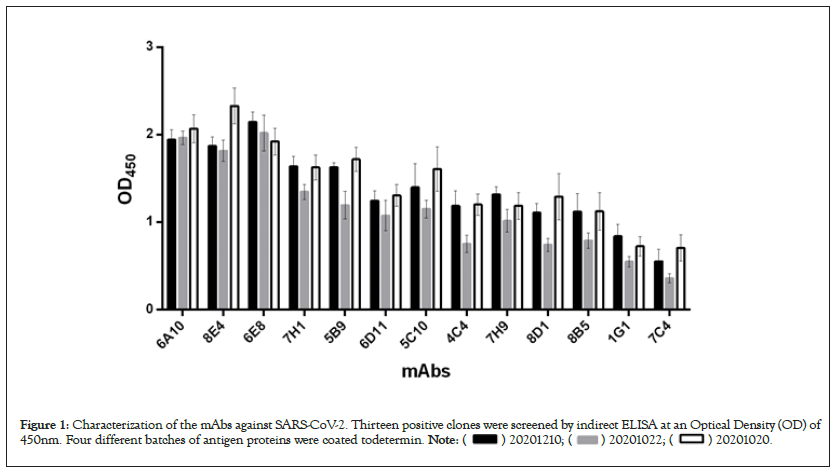
Figure 1: Characterization of the mAbs against SARS-CoV-2. Thirteen positive clones were screened by indirect ELISA at an Optical Density (OD) of
450nm. Four different batches of antigen proteins were coated todetermin. 
Identifying the epitopes of mAbs
Both thirteen antibodies exhibit the binding abilities to RBD-SD1-hFc, suggesting that the epitopes of these antibodies locate on any site of RBD-SD1-hFc. Next, to determine whether both thirteen antibodies target the same epitope, we measured each antibody's binding abilities for binding to the different antigens, including RBD-SD1-hFc, RED-hFc, RBD-HSA. Specifically, each antigen was immobilized on a 96 well plate and interacted with each antibody by indirect ELISA. Binding capacity of each antibody was measured as optical density (OD) at 450nm (Figure 2). As shown in Figure 2, the evaluated antibodies demonstrated different identify domain against antigens. These four antibodies (5C10, 6A10, 6E8, 8E4) have relatively lower optical density that failed binding to RBD-HSA, while having high OD value with other antigens, suggesting that the epitope is hFc. However, other antibodies with relatively higher OD values (6D11, 7C4, 7H9, 8D1, 7H1, 5B9, 8B5, 4C4, 1G1) exhibit the binding abilities to RBD-SD1-hFc, RBD-hFc and RBD-HSA, indicating that their epitope is RBD.
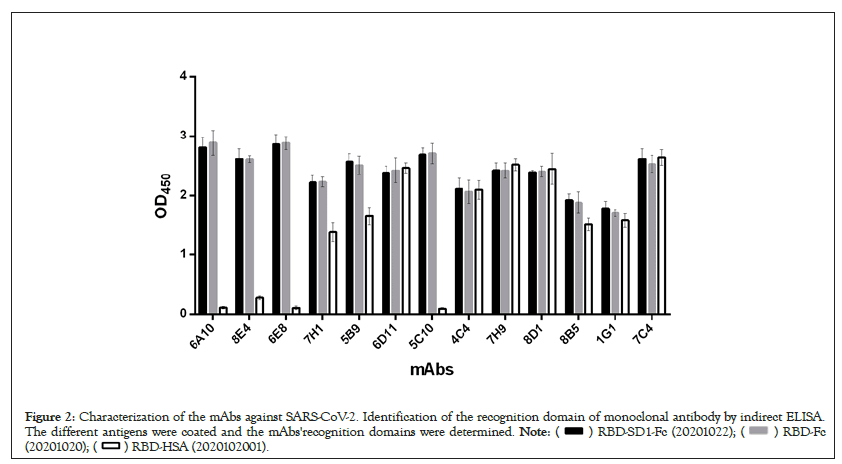
Figure 2: Characterization of the mAbs against SARS-CoV-2. Identification of the recognition domain of monoclonal antibody by indirect ELISA.
The different antigens were coated and the mAbs'recognition domains were determined. 
Binding and neutralizing properties of mAbs
We then further measured the binding affinity using indirect ELISA. The reactivity of these mAbs towards RBD-SD1-hFc, was tested at different concentrations using the indirect ELISA method. The binding strength of each mAb to RBD-SD1-hFc was shown in Figure 3. These thirteen antibodies displayed various binding abilities to COVID-19 virus RBD, with the concentrations of the mAbs ranging from 101 to 10-4 μg/ml. 6A10, 6E8, 7H1 presented relatively higher binding affinity, the signals for both mAbs were positive when the concentrations of the mAbs reached 0.0001 μg/ml, while 1G1, 4C4, 8B5 displayed relatively weaker binding affinity, with the concentrations of the mAbs reaching 0.1 μg/ml. When the concentrations of the mAbs reached 0.01 μg/ml and 0.001 μg/ml, 8D1 and the signals for both mAbs of 5B9, 5C10, 6D11, 7C4, 7H9, 8E4 have binding affinity, respectively.
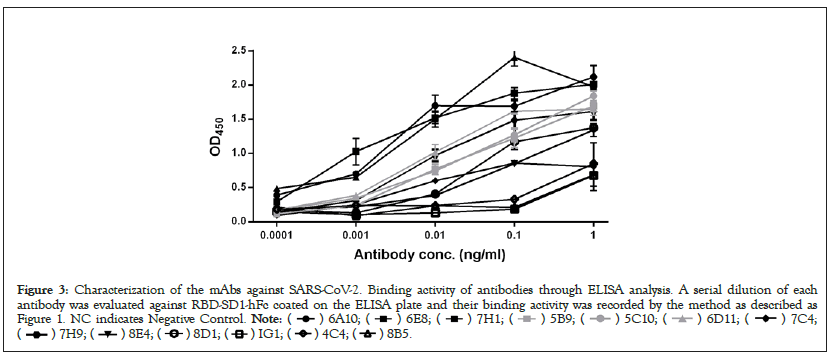
Figure 3: Characterization of the mAbs against SARS-CoV-2. Binding activity of antibodies through ELISA analysis. A serial dilution of each
antibody was evaluated against RBD-SD1-hFc coated on the ELISA plate and their binding activity was recorded by the method as described as
Figure 1. NC indicates Negative Control. 
We next studied the neutralizing activities against COVID-19 virus. Only these four of the nine antibodies (6D11, 7C4, 7H9, 8D1) exhibit neutralizing activities, with IC50 values ranging from 33.6 ng/ml to 446.4 ng/ml (Figure 4). 6D11 was the most potent antibody, followed by 7H9, 8D1 and 7C4. These data demonstrated that these four mAbs had a certain degree of inhibition reactions, and thus neutralization activities, however, the other ones don’t.
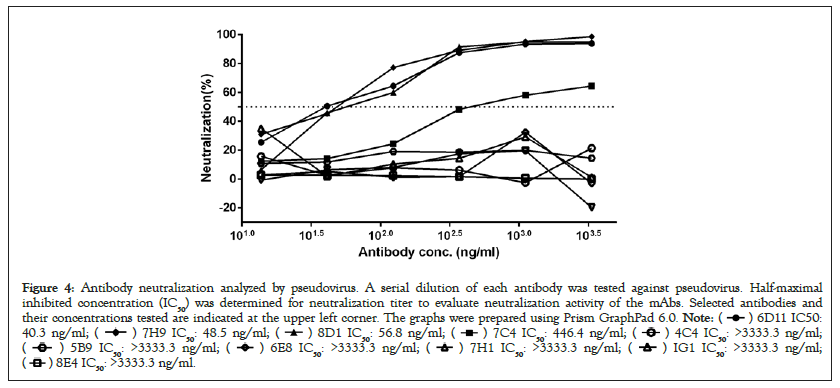
Figure 4: Antibody neutralization analyzed by pseudovirus. A serial dilution of each antibody was tested against pseudovirus. Half-maximal
inhibited concentration (IC50) was determined for neutralization titer to evaluate neutralization activity of the mAbs. Selected antibodies and
their concentrations tested are indicated at the upper left corner. The graphs were prepared using Prism GraphPad 6.0. 
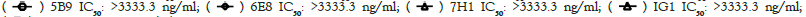
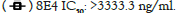
Identification of antigen-recognizing
The top four neutralizing antibodies (6D11, 7C4, 7H9, 8D1) have strong reaction with three VLPs (HuB01, 501Y.V2, Delta), and had a signal of >1; whereas the OD450 values of other reactions were <1 (Figure 5). These results suggested that the four mAbs had recognized most types of COVID-19 virus, but weaker for Omicron.
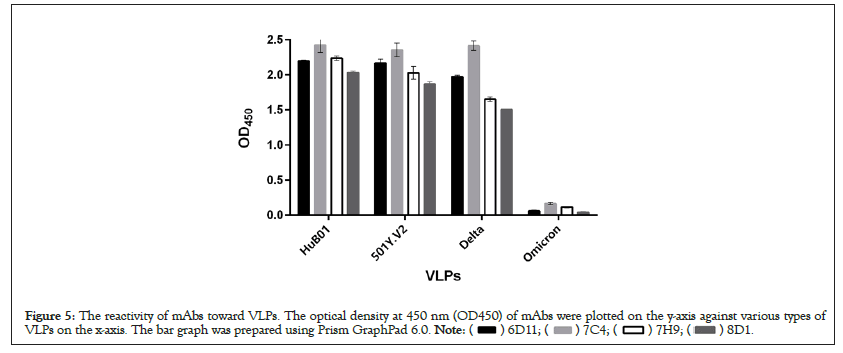
Figure 5: The reactivity of mAbs toward VLPs. The optical density at 450 nm (OD450) of mAbs were plotted on the y-axis against various types of
VLPs on the x-axis. The bar graph was prepared using Prism GraphPad 6.0. 
Gene sequencing
To identify the mAbs obtained, the four mAbs (6D11, 7C4, 7H9, 8D1) were sequenced (Figure 6). Amino acid sequence alignments of the light- and heavy-chains of these mAbs vary in the amino acid sequences, suggesting the obtained mAbs are unique specificity antibodies.
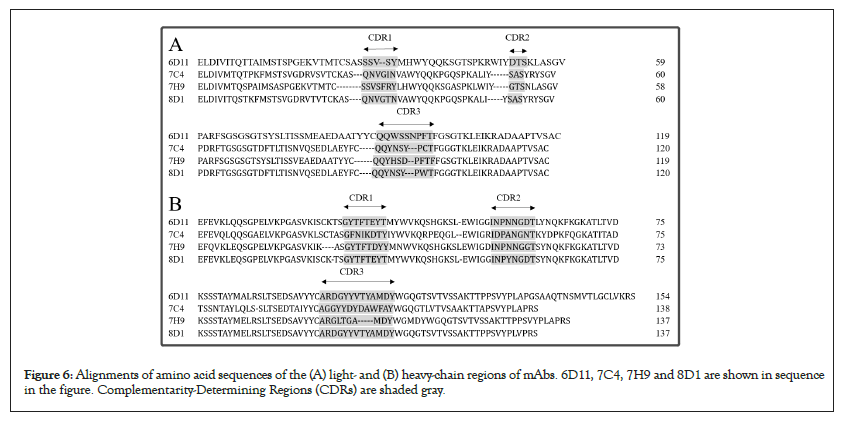
Figure 6: Alignments of amino acid sequences of the (A) light- and (B) heavy-chain regions of mAbs. 6D11, 7C4, 7H9 and 8D1 are shown in sequence in the figure. Complementarity-Determining Regions (CDRs) are shaded gray.
In this study, thirteen mAbs were screened from a certain amount of mAbs expressed by hybridoma cells stably expressing, four of which (6D11, 7C4, 7H9, 8D1) were type-specific neutralizing antibodies against COVID-19 virus. A few features of mAbs, including positive identification, antigenic epitopes they recognized, binding ability, neutralization affinity, and sequences were characterized. The molecular features of the neutralizing antibodies targeting epitopes are helpful for the development of small molecule or peptide drugs/inhibitors [15]. Both thirteen antibodies exhibited the binding abilities to RBD-SD1-hFc, and further were confirmed that the epitopes of these four antibodies (5C10, 6A10, 6E8, 8E4) are hFc and others are RBD.
Furthermore, the binding affinities of thirteen antibodies are different. 6A10, 6E8, 7H1 presented relatively higher binding affinity than others. However, binding affinity alone does not predict neutralizing activity. Among the antibodies tested, only four neutralizing antibodies were found that have neutralization activities. 8D1 was the most potent antibody, followed by 7H9, 6D11 and 7C4. Combined with the results of the competitive binding assay, it is suggested that different mAbs may exert neutralizing activities through distinct mechanisms.
The four strongest neutralizing antibodies against RBD can recognize most COVID-19 virus, but Omicron is weak. Studies have shown that mutations in Omicron will make therapeutic antibodies ineffective, because these mutations are on the binding site of the antibody. Therefore, these antibodies can indirectly recognize Omicron from COVID-19 virus.
The studies are reported that the S-RBD-binding antibodies show a normal distribution of CDRH3 length from 5 to 30 amino acids whether CDRH3 length has direct correlation to neutralizing activities of SARS-CoV-2 antibodies is still unknown [26]. The CDRH3 lengths of 8D1, 7H9, 6D11 and 7C4 are ranging from 5 to 30 amino acids. The gray-shaded Complementarity-Determining Regions (CDRs) vary in the amino acid sequences, suggesting the obtained mAbs are unique antibodies with specificity.
As the COVID-19 outbreak continues to spread, characterization of the epitopes on COVID-19 virus RBD protein is essential, which will provide valuable information to develop vaccines. This study has also provided us a guideline for the rational design of high-neutralization activity monoclonal antibodies that is a specific neutralizing antibody that recognizes the COVID-19 virus. The mAbs specifically recognizes the epitopes of RBD, while comparing with the monoclonal antibody targeting S1 non RBD. Moreover, based on the pathogenic mechanism of this virus, it provides a more extensive application value for screening, diagnosing, preventing and treating. Broad-spectrum inhibitors targeting the HR region are expected to be used to treat current and future SARS associated coronavirus infections. In conclusion, the neutralizing antibodies identified here are promising candidates for prophylactic and therapeutic treatment against COVID-19 virus.
The experimental animals (BALB/c mice) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China), and fed in the animal facility of Beijing Biocytogen Co., Ltd.. The manipulation and vaccination on the animals were strictly referred to the guideline and compliant with the regulation, which was provided by Beijing Biocytogen Co., Ltd.. Prior to the implementation, the experiment schemes and protocols were reviewed by Beijing Municipal Science and Technology Commission Administration Office of Laboratory Animals, and approved by Beijing Municipal Science and Technology Commission Laboratory Animal Management Ethics Committee (no reference number was given). During the experiments, all animals were well-fed and monitored twice per day.
Not applicable.
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
All authors were employees of Beijing Health Guard Biotechnology Co., Ltd. when this study was conducted, and potentially own stock or hold stock options in the Company. YZ, YL, ZY, HZ, ES, YL, YW, MZ, XC, JL and SW hold a relevant patent.
Beijing Health Guard Biotechnology Co., Ltd. was the primary source of funding for this study. Beijing Health Guard Biotechnology Co., Ltd. designed this study, collected and analyzed the data, and wrote the manuscript. The study has gotten application patent (Pat. No:202111165770.8).
YZ and LL contributed equally to this work; YL and HZ designed the study; YZ, YL, ZY, AZ, ES, YL, YW, MZ, XC and SW conducted the experiments; LL wrote the manuscript. All authors reviewed and approved the final manuscript.
We sincerely thank all reviewers for their constructive comments and informative suggestions.
All authors were employees of Beijing Health Guard Biotechnology Co., Ltd. when this study was conducted.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Zhang Y, Li L, Li Y, Yang Z, Zhang A, Shen E, et al. (2023) Development and Characterization of Neutralizing Antibodies against SARS-CoV-2. Immunotherapy (Los Angel). 9:231.
Received: 14-Jul-2023, Manuscript No. IMT-23-25655; Editor assigned: 17-Jul-2023, Pre QC No. IMT-23-25655 (PQ); Reviewed: 31-Jul-2023, QC No. IMT-23-25655; Revised: 07-Aug-2023, Manuscript No. IMT-23-25655 (Q); Published: 14-Aug-2023 , DOI: 10.35248/2471-9552.23.09.231
Copyright: © 2023 Zhang Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.