Journal of Medical & Surgical Pathology
Open Access
ISSN: 2472-4971
ISSN: 2472-4971
Case Report - (2022)Volume 7, Issue 4
Dermal duct tumors are uncommon and mostly benign. It manifests clinically as a firm papule, plaque, or nodule, most commonly on the lower limbs or the head and neck region. Dermal duct tumors are classified as poromas. These tumors can have divergent differentiation, but prominent ductal and sebaceous differentiation is a rare entity. Here we present a right thigh dermal duct tumor with prominent ductal differentiation. A 63-year-old man presented with a 2-year-old painless, slow-growing papilloma-like lesion over the upper anterior part of the right thigh. As no definitive opinion could be made on fine needle aspiration, an excision biopsy was done and diagnosis was confirmed through histopathology.
Ductal; Poroma; Soft cystic; Benign
Poroma is defined as benign glandular adnexal tumor that usually originates from the cells of the outer layer of the acrosyringium and terminal eccrine duct involving both eccrine and apocrine lineage. If poroid and cuticular cells involve the dermis, the neoplasm would be better classified as dermal duct tumor rather than as classic poroma. A poroid hidradenoma contains a mix of all the three cell types and is also limited to the dermis. Divergent differentiation of poroid tumors can occur, including sebaceous, follicular, and apocrine differentiation. We report here in rare thigh dermal duct tumor with prominent ductal differentiation.
A 63 year old otherwise healthy male presented with asymptomatic swelling over upper anterior 1/3rd of right thigh for 2 years .The lesion started as a tiny swelling and gradually increased to reach the current size. Prior to the onset of lesion,no trauma was reported and the lesion never bled. There were no notable antecedents in patient’s family or medical history. On cutaneous examination, it was-non tender, measuring 3.5 × 2 × 1.5 cm solitary soft, cystic and was attached to thigh with a peduncle and was almost like a papilloma, light colored tumor with clinically distinct margins. Systemic examination was not contributory. Initially sonography claimed it be a benign vascular lesion with internal echoes. Fine Needle Aspiration Cytology (FNAC) revealed only blood mixed material on repeated aspirations. No definite opinion could be made with the available material. Hence an excision biopsy was done under aseptic precautions. The excised tumor biopsy specimens were fixed in 10% buffered formalin and paraffin-embedded.
Grossly, the lab received a skin covered polypoid soft tissue mass measuring 4 × 3 × 1 cm. External surface was unremarkable except for hair .Cut surface shows a superficial cystic part filled with haemorrhage and the deeper solid grey/white areas (Figure 1).
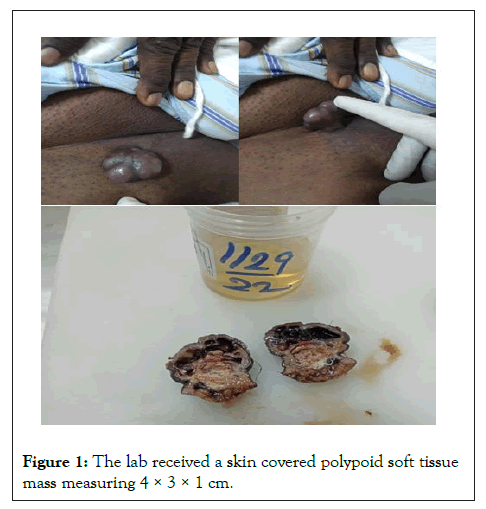
Figure 1: The lab received a skin covered polypoid soft tissue mass measuring 4 × 3 × 1 cm.
Microscopy
Sections studied show a well circumscribed, un-encapsulated solid-cystic lesion occupying the upper deep dermis with overlying epidermis being free of lesion. Predominantly solid with nests and sheets of monomorphic appearing cells having eosinophilic cytoplasm and round to oval nucleus with inconspicuous nucleoli. These sheets are intervened by hyalinised stroma and numerous ducts. Focal apocrine decapitation and secretions, mucinous change and squamous differentiation seen. Cystic spaces are lined by columnar to polyhedral cells (hob-nail type) with abundant eosinophilic cytoplasm and prominent nucleus with foci of discohesion in the lining. The region below the deeper dermis is free of tumor comprising only loose connective tissue ad few congested vessels. There is no evidence of atypia but occasional mitosis seen. Based on these findings, a diagnosis of dermal duct tumor with prominent ductal differentiation was made (Figures 1-6).
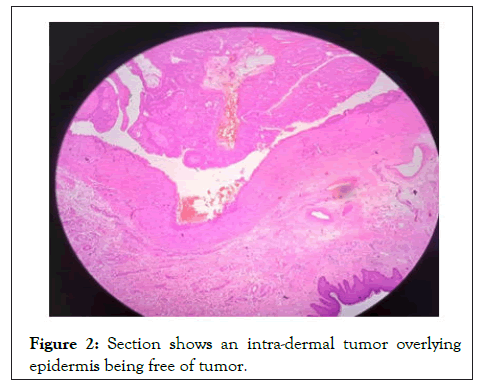
Figure 2: Section shows an intra-dermal tumor overlying epidermis being free of tumor.
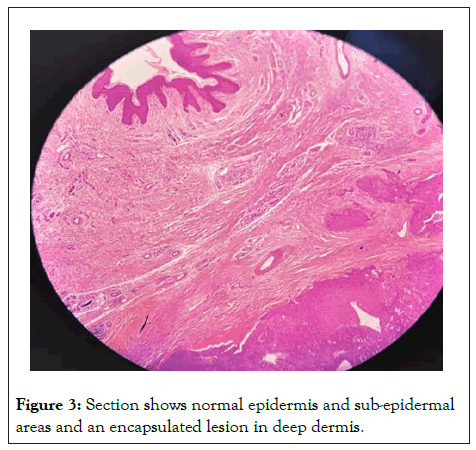
Figure 3: Section shows normal epidermis and sub-epidermal areas and an encapsulated lesion in deep dermis.
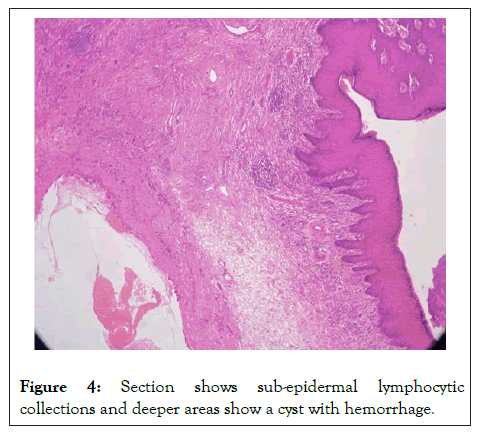
Figure 4: Section shows sub-epidermal lymphocytic collections and deeper areas show a cyst with hemorrhage.
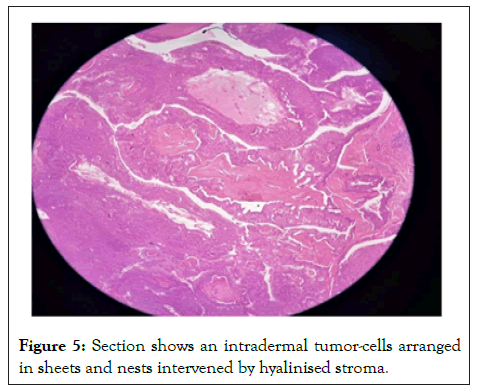
Figure 5: Section shows an intradermal tumor-cells arranged in sheets and nests intervened by hyalinised stroma.
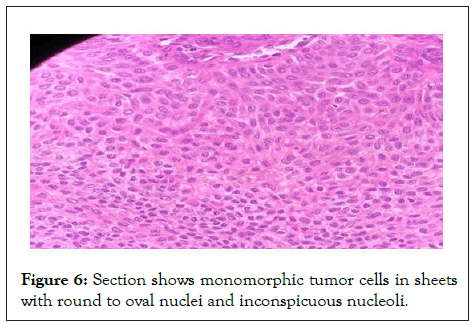
Figure 6: Section shows monomorphic tumor cells in sheets with round to oval nuclei and inconspicuous nucleoli.
Dermal duct tumor was first used in 1966. Eccrine acrospiroma is a term used to describe a morphologically diverse group of tumors that are thought to differentiate toward the distal part of the sweat duct. There are three types of acrospiroma: intraepidermal (hidroacanthoma simplex), juxta epidermal (poroma), and intradermal (dermal duct tumor) [1-7].
Benign cutaneous adnexal tumors with different differentiation are rare, with only a few well- documented cases described in the literature. Clinically, all the tumors appeared as solitary, slowly enlarging cutaneous or subcutaneous nodules located in the head and neck region and on the extremities. Histologically, they were characterized by well-marked, un-encapsulated nodules composed of lobular proliferation of epithelial cells expressing a spectrum of trichogenic, sebaceous, apocrine, and eccrine differentiation. The histological spectrum included lobules and trabeculae of basaloid cells with glandular and ductal elements, well- formed folliculos-vesicular units, primitive follicles and foci of trichome keratinization. Immuno-histochemical evaluation in the four cases showed similar staining profiles for cytokeratin, carcinoembryonic antigen and epithelial membrane antigen as in sweat gland adenomas; in addition, focal S-100 protein positivity and GCDFP-15 positivity can also be demonstrated, suggesting eccrine-apocrine differentiation.
According to a review of the literature, benign poroma-like adnexal neoplasms with divergent differentiation have been reported. For such type differentiation, Hanau proposed complex poroma-like adenoma and Zaim proposed sebocrine adenoma. The folliculocentric nature of the poroma with divergent differentiation, as well as the embryologic relationship between follicular and apocrine structure, indicate that those neoplasms were "apocrine" rather than "eccrine" poromas [2].
Poromas can develop from follicullosebaceous-apocrine germs and thus show apocrine rather than eccrine differentiation. In this case, predominant ductal differentiation suggested an apocrine lineage8, which could be explained by a phenomenon of multidirectional differentiation involving pluripotent cells with an adnexal structure9. These findings support the theory of a common embryological origin for the three adnexal components as a folliculosebaceous-apocrine unit and an eccrine gland from embryonal stratum germinatum.
These tumors typically occur in the elderly population with slight male predilection. Poromas may occur in patients with underlying skin conditions, including chronic radiation dermatitis, hypohidrotic ectodermal dysplasia, Bowen’s disease, and nevus sebaceous. Poroma eruptions have also occurred during pregnancy and after bone marrow transplantation.
Variants of Poroma are classified based on the predominant cell type and the degree of epidermal/dermal involvement. Also the identical lesion can have several variants. DDTs are mostly confined to the epidermis and contains small solid and cystic, nodular aggregates of poroid, cuticle, and clear cells. Eccrine pores also are composed of all the three cell types, but are located primarily within the epidermis and dermis. Hidroacanthoma simplex which is restricted only to epidermis consists mostly of poroid cells, less cuticular cells, and no clear cells. A poroid hidradenoma contains a mix of all the three cell types and is also limited to the dermis. Unlike DDT, poroid hidradenomas have the large aggregates of solid and cystic components and extend deeper into the reticular dermis and even subcutaneously.
Malignant transformation to porocarcinoma is the major complication with minimal risk. It might present with similar features like that of a benign poroma as a firm asymptomatic nodule but with the increased exophytic and ulcerative nature. Malignant transformation in poroma can be identified by bleeding, itching, ulceration, pain and rapid growth of that lesion [8-10].
Poromas are a type of skin adnexal tumors that develops from cells in the terminal eccrine or apocrine sweat gland duct. Dermal duct tumor is relatively an uncommon benign adnexal neoplasm. Excision surgery is curative with a prior histopathology confirmation, as poroma variants can mimic many other dermatologic conditions. These tumors were most frequently confused histologically with other adnexal neoplasms, including sebaceoma, sebaceous adenoma, basal cell carcinoma, chondroidsyringoma, and trichoepithelioma. As a result, a thorough understanding of poromas is required to ensure proper diagnosis and treatment.
This research received no external funding.
The authors declare no conflict of interest.
Informed consent obtained from patient
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
Citation: Dhanalakshmi MNK, Rajendran K, Fathima AT, Srirangaramasamy J (2022) Dermal Duct Tumor- An Atypical Presentation- A Case Report. J Med Surg Pathol. 7:252.
Received: 11-Nov-2022, Manuscript No. JMSP-22-20054; Editor assigned: 14-Nov-2022, Pre QC No. JMSP-22-20054 (PQ); Reviewed: 29-Nov-2022, QC No. JMSP-22-20054; Revised: 16-Dec-2022, Manuscript No. JMSP-22-20054 (R); Published: 23-Dec-2022 , DOI: 10.35248/2472-4971.22.07.252
Copyright: © 2022 Dhanalakshmi MNK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.