Journal of Pollution Effects & Control
Open Access
ISSN: 2375-4397
ISSN: 2375-4397
Research Article - (2023)Volume 11, Issue 1
The various treatment processes are being used for the wastewater treatment, including the air induced method for the decomposition of wastewater pollutants mainly the organic compounds and heavy metals. In this content, lab scale experiments were conducted for the comparisons of the results by using a natural oxidation process and the air induced method for the organic matter decomposition. The air was introduced into the wastewater for 3 hours in an open container, and the other container containing wastewater was left open for 1-3 days for the natural oxidation process. It is estimated that after treatment of induced air oxidation the concentration of heavy metals was decreased while the concentration of nitrate and phosphate was increased. The results of the wastewater samples revealed that the concentration of Iron (Fe), Zinc (Zn) and Manganese (Mn) were decreased, as for Iron (Fe) was found about 42-54%, zinc (Zn) was found about 33-50%, and the Manganese (Mn) was 63-75% in samples of air induced oxidation process after the of 24, 48 and 72 hours respectively.
Wastewater treatment; Natural oxidation; Air induced oxidation; Earth’s surface
Water is an important natural resource which is in limited amount. About 70% of the earth’s surface covered by water in which only 2.66% of the total global water resources are fresh water and for drinking purpose only 0.6% of fresh water is usable [1]. Water plays an important role not only in human’s life but it is also important for agriculture, industrial purposes, for power generation, for wildlife and also for recreation. Now a day’s water shortage becomes the main issue because of rapid population growth which cause increase in industries, agriculture and also domestic waste and resulted in elevated level of water pollution (about 85%) which is directly transported into fresh water bodies and affects the quality of fresh water [2]. This wastewater contributed to organic and inorganic materials, heavy metals, salts, pathogens and other contaminations.
Heavy metals are naturally found in the earth and defined on the basis of density, atomic number or their chemical properties [3]. They are concentrated as a result from industrial wastewater and other human activities which are directly or indirectly released into the environment. If the heavy metals are present in excessive concentration, they may cause several toxic effects to expose plants, animals and also human being. High levels of these metals also affect the aquatic living organisms [4].Heavy metals are the most dangerous pollutants in industrial and domestic wastewater. In fact, they are the main causes of degradation when they are directly transported in freshwater streams without any treatment. Increase in industrializations and urban population have direct impact on the environment. The resultant degradation and contamination of ecosystem become a major threat toward all living organisms worldwide; in particular, human beings [5].
Treatment process
Decomposition of wastewater pollutants (organic+inorganic) is quite necessary before being discharged into the fresh water bodies. Therefore it is a tough task for scientists and environmental engineers to treatment the wastewater with cost effective treatment techniques. There are various conventional methods for the treatment of wastewater containing heavy metals such as chemical precipitation, chemical oxidation, ion exchange, membrane separation, reverse osmosis, electro dialysis etc. in addition to this, for the removal of nutrients different groups of microorganisms like algae, fungi and bacteria are able to convert these nutrients into organic matter. Apart from this, induced air oxidation process also used for the removal of various contaminants present in domestic wastewater. As per work of aeration process was found to be an effective treatment method in reducing the characteristics of wastewater including pH, Biochemical Oxygen Demand (BOD), Chemical Oxygen Demand (COD), Oil, phosphorus and nitrogen [6,7]. They conclude that BOD, COD, Turbidity were found to be removed by 95.88%, 95.71%, and 37.72% respectively at the flow rate of 4 L/min for a detention time of 72 hours. Another work was done and stated that for the removal of nutrients and odor problems, aeration can be an effective method for the treatment of domestic wastewater without causing any environmental problem [8]. They conducted experimental work on pig manure to study the effect of continuous intermittent aeration at an airflow rate of 11 L/ min. Aeration process was effective for the removal of ammonia and nitrate [9]. Kazuyoshi Suzuki, et al. have also done work on the swine water to study the removal efficiency of PO4-P, Mg and Ca with the help of artificial crystallization by aeration [10]. They concluded that during 50 days of operation the removal percentage of phosphate, magnesium and calcium was 65%, 51% and 34% respectively. Another scientist has developed Induced Air Flotation (IAF) with the aid of a rotating-flow micro-bubble generator which produces micro fine bubbles having a diameter in micrometer for the treatment of wastewater [11-13]. This generator shows the best performance because air and water are induced at the same time and mixed with a pump.
Air induced oxidation process
For the decomposition of wastewater pollutants it is essential to increase the oxidation of organic and inorganic compounds. For this purpose the air is induced into wastewater under pressure which increases the oxidation of chemical compounds and due to this oxidation process various organic and inorganic compounds become reduce or decomposed. This process gives better results than normal oxidation of the wastewater and also provides the decomposition of the organic waste faster than the open oxidation system.
Sample collection
The domestic wastewater samples were collected from the junior staff colony Mehran University Jamshoro, Sindh, Pakistan (Figure 1). For the removal of large particles the waste water sample was filtered through a plastic screen before transferring into oxidation tanks. The wastewater sample was analyzed before treatment and it was found that the wastewater was contaminated with high level of organic and inorganic chemical compounds such as trace metals, nitrate and phosphate. Directly disposal of this wastewater into fresh water bodies leads to harmful effect on environment.

Figure 1: Collection of domestic wastewater.
Experimental setup
Induced air oxidation system was designed on lab scale to decompose the wastewater pollutants. In this system two oxidation tanks was used, one tank used for natural oxidation and other used for induced air oxidation. The 10 liters of wastewater sample were filled below the 1/4th part of the tanks. For the natural oxidation the wastewater was left for the given period of time (1-3 days), and for the induced air oxidation process the air was pumped in the tank containing same wastewater at the constant flow rate of 2 L/min with the help of controlled vale of air pump with different aeration and detention time. The air was introduced into wastewater oxidation tank for 1, 2 and 3 hours. The sampling and analyses were followed by taking natural oxidation and oxidized wastewater. The results of natural and induced air oxidation process were determined after detention time of 24, 48 and 72 hours. The process flow diagram is shown below Figure 2.
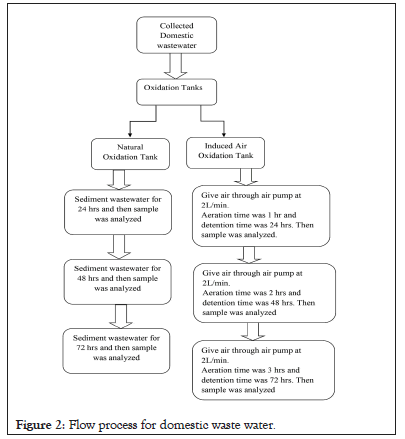
Figure 2: Flow process for domestic waste water.
In this study domestic wastewater was treated through air induced oxidation and natural oxidation process. Table 1 shows the initial readings of wastewater pollutants before treatment that shows the elevated level of pollutants that may affect the environment when directly disposed into fresh water streams (Table 1).
| Parameters | Initial value (mg/L) |
|---|---|
| Nitrate | 33.27 |
| Phosphate | 0.057 |
| Iron (Fe) | 2.303 |
| Manganese (Mn) | 1.521 |
| Zinc (Zn) | 31.02 |
Table 1: Characteristics of raw domestic wastewater.
Effect of natural oxidation on heavy metals
In this process the wastewater was sediment in an iron tank. The samples were analyzed after detention of 24, 48 and 72 hours. The natural oxidation process has shown minimum reduction in the concentration of trace metals. The results obtained from natural oxidation treatment are shown in Figure 3.
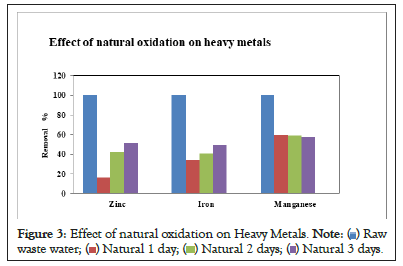
Figure 3: Effect of natural oxidation on Heavy Metals.  waste water;
waste water; 
Effect of induced air oxidation on heavy metals
In this treatment process the air was introduce in the wastewater tank through air pump at a constant air flow rate of 2 L/min. For the decomposition of wastewater pollutants the aeration time was set as 1, 2 and 3 hours and detention time was 24, 48 and 72 hours. After treatment it was concluded that as the aeration time was increased the concentration of iron, manganese and zinc was decreased. The induced air oxidation process shows the better removal of heavy metals as compared to natural oxidation. Because due to aeration process iron and manganese converts from solid to precipitate form and can be removed by filtration or sedimentation. The results was obtained from this method are shown in Figure 4. The comparison between natural oxidation and induced air oxidation was shown in Table 2.
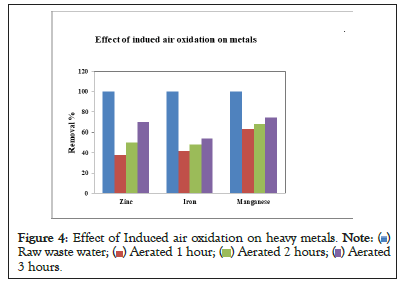
Figure 4: Effect of Induced air oxidation on heavy metals.  Raw waste water;
Raw waste water;  3 hours.
3 hours.
| Metals | Maximum removal percentage | |
|---|---|---|
| Natural oxidation process | Induced air oxidation process | |
| Zinc (Zn) | 30% | 49% |
| Iron (Fe) | 46% | 51% |
| Manganese (Mn) | 25% | 40% |
Table 2: Comparison between natural oxidation and induced air oxidation process.
Mechanism of removal (Heavy metals)
In aeration process the ferrous iron is converted in ferric iron which precipitates then in iron hydroxide Fe(OH)3 and then removed by filtration or sedimentation.
4Fe2 +3O2 → 2Fe2O3
2Fe2O3 + 3H2O → 2Fe(OH)3
Mn2+oxidationMn4+precipitationMnO2
Similar to iron, after aeration process the manganese removal by the oxidation of Mn2+ in Mn4+, which precipitates then in manganese dioxide (MnO2). The precipitation is then separated from water by filtration.
In this study domestic wastewater was treated through air induced oxidation and natural oxidation process it was concluded that induced air process gives faster decomposition of wastewater pollutants (organic+inorganic). As compared to natural oxidation process it also provides better results for the removal of heavy metals from domestic wastewater. After treatment with air induced process at the air flow rate of 2 L/min, it was concluded that as the aeration and detention time increases the percentage removal of metals also increases with the passage of time. The removal percentage of zinc was 38-70%, iron was 42-%54% and manganese was 63-75% respectively.
I would like to express my deepest thanks to Mr. Imdad Ali Kandhar who supported me throughout the course of this project. I am thankful for his aspiring guidance, invaluably constructive criticism and friendly advice during the project work. I also like to deepest thank to Dr. Sheeraz Ahmed Memon who had always been source of motivation for me.
[CrossRef] [Google scholar] [PubMed]
[CrossRef] [Google scholar] [PubMed]
Citation: Jamali GA, Kandhar IA, Memon SA, Saeed S (2023) Decomposition of Wastewater Pollutants through Natural Oxidation and Air Induced Oxidation Process. J Pollut Eff Cont. 11:358.
Received: 01-Mar-2023, Manuscript No. JPE-23-21993; Editor assigned: 03-Mar-2023, Pre QC No. JPE-23-21993 (PQ); Reviewed: 17-Mar-2023, QC No. JPE-23-21993; Revised: 24-Mar-2023, Manuscript No. JPE-23-21993 (R); Published: 31-Mar-2023 , DOI: 10.35248/2375-4397.23.11.358
Copyright: © 2023 Jamali GA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.