Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
ISSN: 1948-5964
Review Article - (2021)Volume 13, Issue 1
Coronavirus disease 2019 (COVID-19) is caused by a novel coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). It has been declared as a pandemic by the World Health Organization (WHO) on March 11, 2020. Due to the lack of complete understanding of SARS-CoV-2, the disease continues to accelerate at a fast pace affecting millions around the globe. But inspiringly, unanimous efforts by researchers globally, have led to repurposing of several antiviral drugs that have proven successful in saving millions of lives. This review sheds light upon SARS-CoV-2 viral pathogenesis and describes clinically favorable drugs currently being used in the treatment of infected patients. In addition, it gives a comprehensive insight of the current status of different types of vaccines under trial worldwide that might help us to tide over the pandemic.
SARS-CoV-2; Co-morbidities; Therapeutics; Vaccines; Vitamins
Currently accelerating COVID 19 pandemic caused by Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2) is an unprecedented challenge known in the history of pandemics faced by humans. In spite of united efforts by researchers globally, the pandemic continues to be a threat to millions of lives across the world. At present, according to WHO estimates, there has been a drastic impact of COVID 19 on 216 countries with more than 28 million confirmed cases, nearly 1 million related deaths and it has hampered the mental and social well-being of humans worldwide.
SARS-CoV-2 is a novel Beta coronavirus from subgenus Sarbecovirus. It was first reported in Wuhan, China in late December 2019, following previous pandemic of SARS in Southern China (2002) and MERS in Saudi Arabia (2012). Phylogenetic analysis of initial isolates revealed that the novel virus was more genetically identical to the two bat derived–coronavirus strains-bat- SL-CoVZC45 (87.99%) and bat-SL-CoVZXC21 (87.23%) than SARS (79%) and MERS (50%). However, there may be a possible involvement of an intermediate host between bats and humans [1]. This review highlights our current understanding of SARS-CoV-2 virus and focuses on various therapeutic drugs being used for treatment. In addition, the review summarizes the global COVID 19 vaccine development strategies.
SARS-CoV-2 is an enveloped virus with positive sense single stranded, non-segmented RNA genome of 26-32 kb in length and 60-120 nm in diameter [2]. The virus is characterized by the presence of projections of spike proteins on its surface giving it a crown like appearance under electron microscope, hence named ‘Coronavirus’ [3]. The genome of SARS-CoV-2 contains 14 Open Reading Frames (ORFs), encoding various structural and non-structural proteins necessary for viral virulence and survival depicted in Figure 1.
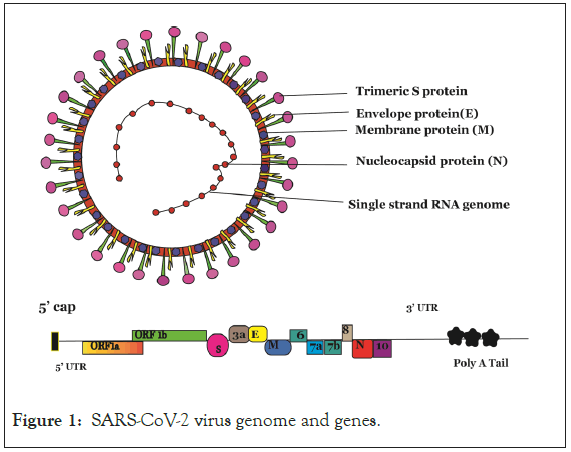
Figure 1: SARS-CoV-2 virus genome and genes.
The genome of SARS-CoV-2 contains 14 Open Reading Frames (ORFs), encoding various structural and non-structural proteins. Viral genome encodes two large genes ORF 1a (orange), ORF 1b (green), which are translated through ribosomal frame shifting into 2 large overlapping polyproteins pp1a and pp1b that eventually encode 16 non-structural proteins (NSPs1-NSPs16). The viral genome encodes 4 structural proteins that include spike (S) glycoprotein (Pink), envelope (E) protein (Yellow), membrane (M) (Blue) and nucleocapsid (N) (Red) proteins and several accessory proteins.
Following entry of virus into target host cells, viral RNA is uncoated and released in the cytoplasm. The first ORFs- ORF1a and ORF1b are translated through ribosomal frame shifting into 2 large overlapping polyproteins pp1a and pp1b.These proteins are further cleaved into Non-structural proteins (Nsps) 1-11 and 1-16 respectively which codes for RNA dependent RNA polymerase (RdRp), Helicase, exonucleases, and proteases [4]. These proteins form the essential RNA replicase-transcriptase complex. This complex localises to Double Membrane Vesicles (DMVs) in the perinuclear region derived from Rough Endoplasmic Reticulum (RER) and initiates synthesis of negative sense RNA. The full length negative sense genome serves as a template for synthesis of the positive sense RNA genome. Additionally, the RNA genome undergoes discontinuous transcription to produce sub genomic negative sense RNAs that encode structural proteins. These proteins facilitate virus assembly at the ER-Golgi Intermediate Compartment (ERGIC), followed by release of nascent virion particles [2]. The viral RNA polymerase and helicase are the major determinants of viral replication and thus serve as the first line of antiviral targets against Coronaviruses. The life cycle of SARSCoV- 2 is diagrammatically depicted in Figure 2.
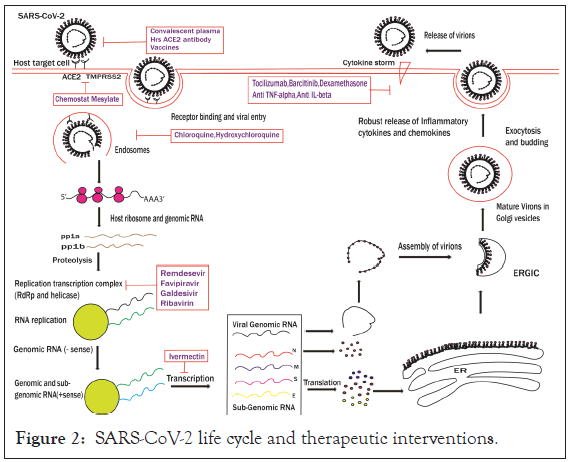
Figure 2: SARS-CoV-2 life cycle and therapeutic interventions.
SARS-CoV-2 gains entry primarily into the target cells through attachment and binding of its envelope Spike (S) glycoprotein with the cellular ACE2 receptors (endosomal pathway). Following entry, viral RNA is uncoated and released in the cytoplasm. ORF1a and ORF1ab are translated to produce pp1a and pp1ab polyproteins, which are cleaved by the proteases of the RNA replicasetranscriptase complex. RNA replicase-transcriptase initiates synthesis of negative sense (−) RNA. The full length negative sense genome serves as a template for synthesis of the positive sense RNA genome. Additionally, the RNA genome undergoes discontinuous transcription to produce sub genomic negative sense RNAs that encode structural proteins (S, E, M and N). These proteins facilitate virus assembly at the ER-Golgi Intermediate Compartment (ERGIC), followed by release of nascent virion particles through exocytosis.
The viral genome encodes 4 structural proteins that include Spike (S) glycoprotein, Envelope (E) protein, Membrane (M) and Nucleocapsid (N) proteins and several accessory proteins [4,5]. The spike protein is a transmembrane protein that mediates binding of the virus to ACE2 receptors on host cells followed by membrane fusion similar to SARS-CoV. The S protein hence, determines the host tropism as well as transmission efficiency [1]. The Nucleocapsid (N)protein is bound to genomic RNA and plays a role in the viral replication cycle whereas the Membrane (M) proteins confers shape to the virus envelope and promotes viral assembly by stabilizing the N protein and RNA complex. Lastly, the Envelope or (E) proteins are the smallest proteins responsible for production and maturation of viral particles [2].
The Spike protein (S) of SARS-CoV-2 has been identified as the major determinant of viral infection in humans and is responsible for virus entry and disease manifestation [6]. ACE2 receptors are type 1 transmembrane glycoproteins that are actively expressed in wide range of cells such as nasal epithelial cells, especially goblet and secretory cells, lung alveolar epithelial cells, intestinal enterocytes, endothelial cells, arterial smooth muscle cells, renal as well as cardiovascular tissues which explains the plethora of symptoms experienced by infected patients from respiratory failure to multiple organ failure [7]. In addition, progression of the disease primarily depends upon several risk factors such as age, pregnancy as well as underlying moderate to severe co-morbidities. The clinical manifestations include asymptomatic to mild infection which are primarily characterized by fever, cough, headache, fatigue, loss of taste or smell. Other symptoms include but are not limited to myalgia, lymphopenia, dyspnoea, diarrhoea, haemoptysis to more severe pneumonia that can further progress to ARDS (Acute Respiratory Distress syndrome) or multiple organ failure and ultimately loss of life [8,9].
Upon viral exposure, SARS-CoV-2 gains entry primarily into the nasal epithelium cells through attachment and binding of its envelope spike (S) glycoprotein with the cellular ACE2 receptors. This is followed by fusion of virus membrane with the host cell. After fusion, the type II transmembrane serine protease 2 (TMPRSS2) primes and activates the spike S proteins inducing conformational changes that enables virus entry. The binding affinity between ACE2 and viral S glycoprotein hence determines the extent of infection and disease severity [10]. This is the initial phase of infection which might be asymptomatic or pre-symptomatic but infectious viruses can be detected in nasal swabs and can be transmitted also [11]. The virus spreads down the respiratory tract and infects alveolar type 2 cells (major target) in lungs, leading to cellular apoptosis that can further progress to ARDS. But only 20% of infected patients with underlying risk factors and complications reach this stage [12].
The different phases of infection are dependent upon a plethora of host immune responses that are triggered by replication of virus inside the host cells resulting in pathogenesis. Antigen presenting cells such as macrophages and dendritic cells recognize viral proteins through PRR (pattern recognition receptors) and induce production of pro-inflammatory cytokines such as interferon- α and TNF β as the initial defence against the invading viruses. These type 1 interferons activate JAK-STAT pathways to initiate transcription of Interferon Stimulated Genes (ISGs) that function to suppress viral replication [2]. The exaggerated release of cytokines such as IFN-α, IFN-γ, IL-6, IL-12, IL-1β, IL-33, TNF-α, TGF-β, IL-17 and various chemokines- CCL2,CCL3,CCL5,CXCL8,CXCL9 and CXCL10 lead to a cytokine storm triggering hyper-inflammation and excessive tissue damage and results in multiple organ failure or respiratory failure in severe cases (Acute Respiratory Distress syndrome) [13]. Furthermore, activation of Th1 cells via MHC class1 stimulates cytotoxic CD8+ T cells that kill virally infected cells. The CD4+ T cells activate humoral immune response eliciting antigen specific IgM and IgG antibodies. The presence of these SARS-CoV-2 specific antibodies in patients is a biomarker for disease diagnosis. Remarkably, administering convalescent serum from recovered patients, which contains neutralising antibodies, has been shown as a promising approach in saving lives of critically ill patients. But few patients who had undergone convalescent serum therapy developed more severe disease due to ADE (Antibody Dependent Enhancement) of SARS-CoV-2 infection [14]. Hence, additional studies are required to completely understand the role of convalescent sera in treatment of COVID 19 patients.
Why some people are more prone than others to COVID 19? Is the disease prognosis dependent on age, gender or any other medical condition? Several reports suggest that certain medical conditions such as heart disease, hypertension, malignancy, COPD, Diabetes, Chronic lung disease, Kidney disease and Obesity exacerbates the disease progression [15-17]. A list of various such co-morbidities is illustrated in Figure 3. Among most prevalent co-morbidities, hypertension, diabetes, Cardiovascular disease and respiratory disorders accounts for 21.1%, 9.7%, 8.4%, 1.5% respectively [18]. Nearly 1.7 billon people (22% of total world population) have at least one medical condition that makes them more vulnerable to COVID 19. Among them 349 million people would require hospital admission [19].
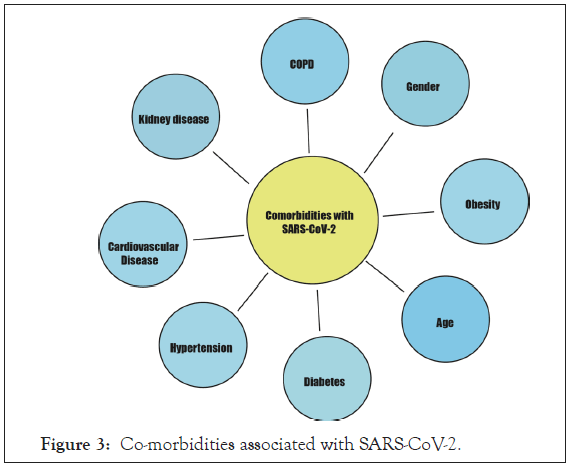
Figure 3: Co-morbidities associated with SARS-CoV-2.
However, interestingly, it was observed that there is a bidirectional relationship between COVID 19 and diabetes [20]. COVID 19 not only causes onset of diabetes but also causes severe metabolic complications including diabetic ketoacidosis and hyperosmolarity in pre-existing diabetic patients [21]. Binding of SARS coronavirus to its receptors on islet cells can damage them and cause onset of diabetes in COVID 19 patients [22]. Further studies on SARS- CoV-2 associated diabetes could reveal novel mechanism of action of the virus.
Besides these medical conditions, age also plays a vital role in clinical manifestations and severity of the disease. People aged ≥ 60 years have higher severity than younger people [23]. It was observed that susceptibility of infection is reduced to half in individuals aged lower than 20 years as compared to those aged above 20 years [24].
There is also an association between gender and COVID 19 in terms of severity and mortality. Irrespective of age, males with COVID 19 display two time’s higher severity and mortality as compared to females [25]. Furthermore, mortality increases with every 10 years age interval [16]. The gender specific effects of SARS-CoV-2 disease were studied using a mice model and it was observed that oestrogen receptor signalling played a protective role in infected female mice [26]. In addition, the presence of extra X chromosome in females which encodes majority of genes related to immunity such as TLR7 and 8, FOXP3, CD40L and also expresses various micro RNAs (miR-233,miR-106A,miR-424,miR542, and miR-503) may regulate immunity [27]. Thus, gender specific severity and mortality in SARS-CoV-2 warrants further research.
The rapid globalized spread of SARS-CoV-2 worldwide necessitates the search for effective therapeutics to curb the spread of the virus. So far, no specific and effective treatment strategy has been approved. Current treatment protocols provide patients with supportive care by administering antibiotics and/or providing oxygen therapy to improve ventilation and treat any co-morbid conditions. The patients may be given repurposed drugs and plasma therapy, depending upon the clinical stage of the disease. There are primarily three promising strategies being focused to treat SARS- CoV-2 infection and combat its spread- Rapid repurposing of approved drugs, Neutralizing Antibody therapy, and development of vaccines. These approaches are described in details further.
Therapeutics
Drug repurposing is one of the quickest and promising approaches that can facilitate drug discovery process by reinventing the off-label potential of existing pharmaceuticals in addition to their approved role to treat specific diseases. Various computational modeling, in silico and other approaches have led to repurposing of various drugs targeting viral and host factors essential for viral entry and replication resulting in reduction of viral load and suppressing inflammatory responses. Some of the repurposed drugs and their mechanism of action are listed in Table 1 and described below.
| Drugs | Targets | Reference |
|---|---|---|
| Virus Based Therapeutics | ||
| Remdesevir | RNA dependent RNA polymerase ( RdRp) | [28] |
| Favipiravir | RdRp | [29] |
| Galdesivir | RdRp | [30] |
| Ribavirin | RdRp | [31] |
| Host Based Therapeutics | ||
| Tocilizumab | IL-6 receptor | [32] |
| Sarilumab | IL-6 receptor | [32] |
| Camostat mesylate | TMPRSS2 | [10] |
| Barcitinib | JNK inhibitor | [33] |
| Chloroquine | Endosomal acidification | [28] |
| Anti-TNFα drugs | TNF α | [34] |
| Convalescent plasma | Antibody therapy | [35] |
| Human recombinant soluble ACE2 antibody ( hrs ACE2) | Antibody therapy | [36] |
| Dexamethasone | Corticosteroid | [37] |
| Ivermectin | IMPα/β1 | [38] |
| Aviptadil (RLF-100) | Anti-inflammatory | [39] |
Table 1: List of clinically effective drugs against SARS-CoV-2.
Drugs targeting viral factors
Remdesevir: Remdesevir is an adenosine analogue that can target SARS-CoV-2 RNA dependent RNA polymerase (RdRp) enzyme and inhibit its activity. It gets incorporated in place of adenosine triphosphates, evades the proofreading activity of viral ribonucleases and blocks viral RNA synthesis. Initially developed against Ebola virus, it showed potent activity against SARS-CoV and MERS viruses [40]. It has been administered to several hundred patients with confirmed, severe SARS-CoV-2 infections in the United States, Europe, and Japan through “Expanded Access” or “Compassionate Use” programs. Various preclinical trials did not find any evidence of resistance mutations and preliminary data proved that Remdesevir helps in reducing clinical recovery of patients from 15 days to 11 days. Similar results were obtained using a combination of Remdesevir and chloroquine [28]. Recently, 2 generic versions of Remdesevir namely Covifor and Cipremi have been launched in India by Cipla and Hetero which can be given intravenously to inhibit viral replication in mild cases of infection under ‘restricted emergency use drug’.
Favipiravir: Favipiravir is a widely known anti-influenza drug which was first approved for use in Japan in 2014 [41]. It is a purine nucleoside analogue which can competitively inhibit the viral RNA dependent RNA polymerase enzyme [42]. Favipiravir is a broad spectrum drug which has been reported to block SARSCoV- 2 viral replication [29]. In an open label-non randomized trial, rapid decline in SARS-CoV-2 virus was observed as compared to patients treated with lopinavir and ritonavir combinations [43]. Just like Remdesevir, Favipiravir has been approved as a drug with ‘restricted emergency use’ as oral medication to treat SARSCoV- 2 patients. It is commercially available as ‘Fabiflu’. Although, Favipiravir has demonstrated good safety profile during short term use more studies are required to assess its long term potential [44].
Drugs targeting host factors
Tocilizumab: Tocilizumab is an US FDA approved human monoclonal antibody that blocks IL-6 receptors [45]. This drug has been repurposed against SARS-CoV-2 to treat critically-ill patients with elevated serum IL-6 and lung injury [46]. In a retrospective study, the drug was found to improve the recovery period of infected patients over 5 days with reduction in inflammation and pulmonary complications. Due to positive clinical response, Tocilizumab holds great promise for use in SARS-CoV-2 patients [32].
Ivermectin: Ivermectin is a FDA approved broad spectrum, antiparasitic drug that has shown to be active against broad range of viruses such as Human Immunodeficiency Virus (HIV), Influenza, Dengue viruses, West Nile viruses and DNA virus pseudo rabies (PRV). It acts by inhibiting host importin protein IMPα/β1 which impedes the nuclear import of host and viral proteins [47]. Ivermectin has been reported as a potential antiviral drug against SARS-CoV-2 viruses. A recent study observed that incubation of Vero-h SLAM cells with this drug led to 500 fold reduction in viral RNA expression after 48 h [38]. Ivermectin is currently under clinical trials against the novel SARS-CoV-2 virus.
Barcitinib: Baircitinib is a JNK (Janus Kinase) inhibitor which has been approved for treatment of rheumatoid arthritis. This drug has been repurposed for treatment of COVID 19 as it can inhibit viral entry and decline cytokine surge associated with the disease [40,41]. Barcitinib-mediated inhibition of Numb kinases- AAK1 and GAK1 led to decline in clathrin mediated endocytosis of SARS-CoV-2 in vitro and in primary liver spheroids [48]. Furthermore, in a small case study of patients with bilateral pneumonia, Barcitinib therapy was associated with improvement in clinical and radiological markers along with decline in CRP and IL-6 levels [49]. However, this drug may be associated with lymphocytopenia and neutropenia or enhanced incidence of co-infection in COVID 19 patients [50]. Therefore, Barcitinib therapy holds promise if subjected to appropriate clinical testing and trials.
Camostat mesylate and nafamostat mesylate: The compound Camostat mesylate is a TMPRSS2 inhibitor which has been approved for treatment of pancreatitis in Japan. It has been reported to suppress MERS-CoV and SARS-CoV infection in vitro and is currently being tested against SARS-CoV-2 [10]. Another drug, Nafamostat mesylate which was approved by the FDA as an inhibitor of MERS-CoV, reduced SARS-CoV-2 entry in human lung cells with higher efficiency than Camostat mesylate [51].These drugs are currently under investigation.
Dexamethasone: Dexamethasone is a corticosteroid which has been used as an anti-inflammatory and immunosuppressive drug to treat asthma, rheumatoid arthritis, cancer and certain skin and eye infections [52-54]. In a study termed ‘‘RECOVERY’’ conducted by Oxford University on critically ill COVID 19 patients, it reduced death rate of patients on ventilator support by one-third and patients on oxygen therapy by one-fifth [37,55]. Dexamethasone has been approved for treatment of critically ill COVID 19 patients in the UK and recently in India.
Aviptadil (RLF-100): Aviptadil is a patented formulation of VIP (Vasoactive Intestinal Peptide), developed by Dr. Sami Said in 1970. COVID 19 patients with severe co-morbidities and placed on ventilators and ECMO (Extracorporeal Membrane Oxygenation) support recovered significantly after 3 days of treatment with this drug [56]. This neuropeptide has been reported to block the replication of SARS-CoV-2 virus in human lung epithelial cells and monocytes and decreased production of inflammatory cytokines in severe SARS-CoV-2 infected patients [39]. Aviptadil has been granted FDA fast track approval as first COVID 19 therapeutic under FDA emergency use IND authorization and expanded access protocol.
Antibody therapy against SARS-CoV-2 virus
Convalescent plasma therapy: Due to lack of an effective treatment to cure SARS-CoV-2 infection, administering convalescent plasma therapy has shown promising results in clinical management of critically ill patients and saved their lives [35]. Viral antigen specific IgM and IgG neutralizing antibodies are generated in response to viral infection. These antibodies have been detected in the serum of patients in the late stage of SARS-CoV-2 infection, along with higher level of serum IL-2, IL-7, IL-10, GCSF, MCP-1, MIP1A and TNF-α. Patients with severe infection have higher levels as compared to patients with mild disease. The antibodies from recovered patients can neutralize SARS-CoV-2 isolated from another patient. Therefore, convalescent plasma with an antibody titer ≥ 1:160 or ≥ 1:320 can be infused in the early course of infection, before the body produces its own IgG, to achieve robust infused IgG response [57]. This therapy although effective is not readily available and is bound by some pitfalls like variability in antibody titre, risk of contamination and low specificity.
Neutralizing monoclonal antibodies: Monoclonal antibodies are isolated from a single plasma B cell from a vaccinated or naturally infected animal or human donor and possess higher affinity towards specific epitopes as compared to polyclonal antibodies. These neutralizing antibodies can reduce virus infection by targeting essential epitopes required for virus binding to receptors on the host cells. The virus-antibody immune complexes may be phagocytized or degraded by complement proteins and trigger antiviral immune response. Due to high specificity, these antibodies hold vast potential in combating pathogenic viruses like Ebola virus. REGN-EB3, a novel, triple antibody cocktail of 3 monoclonal antibodies (REGN 3470, 3471 and 3479) developed by Regeneron pharmaceuticals against Ebola virus, is under regulatory review by FDA. Recently, REGN-CoV2, another investigational antibody cocktail showed reduced viral load and associated symptoms in non-hospitalized COVID19 patients after Phase1/2/3 descriptive analysis. In addition, this approach can help in the development of vaccines by identifying broadly cross reactive epitopes which can serve as basis for designing vaccines for future Coronaviruses outbreaks [7].
To combat SARS-CoV-2 virus pandemic, research is underway to isolate and develop clinically effective neutralizing monoclonal antibodies that can be used as prophylactic as well as therapeutics. Some mAbs under clinical trials are listed below.
LYCOV-555: It is a potent, neutralizing IgG1 monoclonal antibody directed against the spike protein of SARS-CoV-2 coronavirus. This antibody was identified in blood sample from a SARS-CoV-2 recovered patient and is currently being developed by Eli Lilly in collaboration with Abcellera. Recently, NIH has launched a phase 3 randomized controlled trials termed as ACTIV-3 to test LYCOV-555 in hospitalized COVID 19 patients.
REGN-COV2: Regeneron pharmaceutical has developed a dual antibody cocktail called REGN-COV2 to block infectivity of SARS-CoV- 2, which is a combination of REGN10933 and REGN10987 mAbs. It is a human neutralizing monoclonal antibody directed against spike protein of SARS-CoV-2 virus. Currently, this antibody is undergoing clinical trials to test safety and efficacy.
Mesenchymal Stem Cell (MSC) therapy
The severity of SARS-CoV-2 infection is manifested largely by aberrant pro-inflammatory cytokine response. This can be prevented by immunomodulatory functions of MSCs. These cells not only help in suppressing over activation of inflammatory immune response but also help in repair of damaged lung tissues thereby slowing down the rapid worsening of disease towards respiratory failure [57]. These positive outcomes of MSCs therapy have been observed in the treatment of SARS-CoV-2 infected patients [58].
Recombinant ACE2 protein
Several studies have reported a strong binding affinity between host ACE2 and RBD of SARS-CoV-2 leading to high infectivity of SARS-CoV-2. This implies that exogenously added ACE2 could competitively block the viral interaction with host ACE2, limiting the infection and potentially providing protection against ARDS development [59]. Recent in vitro studies have shown that soluble human recombinant ACE2 protein (hrsACE2) significantly reduced SARS-CoV-2 recovery from Vero-E6 cells and inhibit SARS-CoV-2 infection in engineered human capillary and kidney tissues [36]. The recombinant ACE2 APN01 blocked viral entry and also reduced lung injury and is currently under phase II trials [60].
Niclosamide
Niclosamide is an FDA approved anti-helminthic drug. It has been identified as a broad spectrum antiviral against multiple viruses such as SARS-CoV, MERS, EBV, HCV, and Ebola by drug repurposing screening strategies [61]. In pre-clinical research, this drug was reported to be more potent in inhibiting replication of SARS-CoV-2 than Remdesevir and Hydroxychloroquine but demonstrated poor bio-availability following oral administration [62,63]. Recently, Daewoong Pharmaceuticals (South Korea) and Mankind Pharma (India) have collaborated for conducting phase 1 clinical trial for long acting intramuscular formulation of Niclosamide (DWRX2003).This was based on finding by Daewoong that the injectable formulation potently removed SARS-CoV- 2 virus from lungs of animals tested and prevented cytokine storm. The US FDA has also granted approval to ANA therapeutics to initiate human clinical trials to test Niclosamide.
Vitamins and SARS-CoV-2 infections
Vitamins supplementation has long been approved for treating several viral and respiratory infections as it is safe, efficacious and easily accessible. Several ongoing studies have revealed clinically effective role of vitamins in treating SARS-CoV-2 infections as described below.
Vitamin D: Vitamin D plays a crucial role in regulation of antiviral and immunopathological inflammatory responses against several acute respiratory diseases [64]. Recent reports have also indicated that respiratory diseases are associated with dysregulated vitamin D metabolism, raising the possibility that vitamin D deficiency might arise as a consequence of pulmonary inflammation [64]. A systematic review and meta-analysis of randomized controlled studies revealed that vitamin D supplementation provided protection against acute respiratory tract infection [65]. The disparities in mortality rate due to SARS-CoV-2 among different age groups in populations worldwide could be attributed to deficiency of Vitamin D [66]. Lower circulating 25 (OH)D concentration has also been reported to be associated with susceptibility to SARS-CoV-2 infection and clinical severity [67,68]. Hence, it is justified to consider Vitamin D supplementation as a means of enhancing defence against viral infections including SARS-CoV-2 [69].
Vitamin C: High dose of vitamin C has been shown to improve recovery in patients with sepsis and suffering from viral infections. Administration of vitamin C in respiratory tract infections can shorten the duration of the infection. Vitamin C is an effective, safe and inexpensive therapeutic alternative which has been widely used against SARS-CoV-2 infections [70]. The potent antioxidant and immunomodulatory functions of vitamin C helped in reducing the robust cytokine storm and oxidative stress in SARS-CoV-2 patients [71].
The major concern with SARS-CoV-2 infections is care of patients requiring Intensive Care Unit (ICU). Several meta-analysis data have reported that vitamin C was found to reduce ICU stay by 8% and also shorten the duration of mechanical ventilation support [72,73]. High-dose intravenous injection of vitamin C improved oxygen index of moderate to severe COVID 19 patients in China and helped in their cure [71]. Several clinical trials are underway to evaluate the role of vitamin C in treatment of COVID 19. An ongoing phase 2 trial is evaluating the dose of vitamin C required for treatment of severe COVID 19 associated pneumonia [74,75]. These results will provide us with new insights into the role of vitamin C as treatment regimen against SARS-CoV-2.
Vaccines
Despite implementation of various safety measures such as surveillance, quarantine, proper sanitization and social distancing, SARS-CoV-2 is spreading at an alarming rate. More than 200 million people have been infected worldwide.
Due to lack of proper antiviral therapeutic drugs, there is an urgent need for development of vaccines. Generally, vaccine development is a rigorous and time consuming process. On average it takes 10- 15 years to develop a vaccine [78]. But, due to the severity of the current COVID 19 pandemic a rapid path has been implemented. There are several concerns regarding safety and long term efficacy of the current vaccine approach which need to be addressed.
Strategies for vaccine production
There is a huge race for making a safe, highly efficacious and cost effective vaccine against COVID 19. Currently four strategies have been being adopted for to achieve this as illustrated in Figure 4.
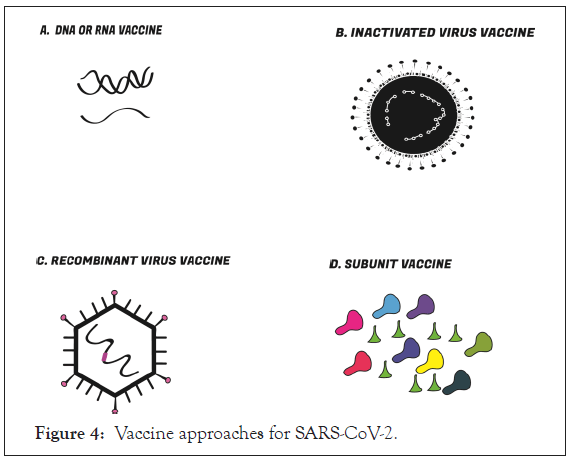
Figure 4: Vaccine approaches for SARS-CoV-2.
The S protein is the major target for vaccine development.SARSCoV- 2 vaccines are developed using different vaccine platforms such as DNA/RNA vaccine, inactivated virus vaccine, Recombinant vector vaccine and Subunit vaccine.
Gene based vaccines: Gene based vaccines include both DNA and RNA vaccines. They are simple, stable, economical and safe but have lower immunogenicity and efficacy. So, further technological interventions are needed to solve these problems. These vaccines can elicit both cell mediated and humoral immune response. They are extremely adaptable due to the ease of antigen manipulation. This approach can be used to develop vaccines against wide range of pathogens like virus, bacteria and parasites because they support antigen delivery from any pathogens [79]. But it is not the best approach for generation of immune responses against multiple proteins since these vaccines usually encode a single viral protein.
DNA vaccines: DNA vaccines are based on a plasmid encoding the viral antigen. They are simple, safe and highly stable. Currently, DNA vaccines for SARS-CoV-2 comprise of vectors expressing S, M and N protein. Currently, there are six DNA vaccines which are detailed in Table 2.
| Vaccine Name | Gene encoding | Manufacturer | Clinical trial |
|---|---|---|---|
| INO-4800 | S antigen | Centre for Pharmaceutical Research, Kansas City, University of Pennsylvania | Phase I/II (NCT04336410,NCT04447781) |
| AG0301-COVID 19 | S antigen | AnGes Inc., Japan Agency for Medical Research and Development | Phase I/II (NCT04463472). |
| GX-19 | S antigen | Genexine Inc | Phase I/IIa (NCT04445389) |
| ZyCoV-D | S antigen | Zydus Cadila,Cadila Healthcare Limited | Phase I/II (CTRI/2020/07/026352) |
| BacTRL-Spike | S antigen | Symvivo Corporation | Phase I (NCT04334980) |
Table 2: DNA Vaccines.
RNA vaccine: RNA vaccines encode a viral mRNA expressing an antigen. RNA vaccines have several advantages over DNA vaccines. They are relatively safer than DNA vaccines since they cannot get incorporated in the host genome. They elicit an immune response similar to biological infection. But, being mRNA they are less stable than DNA vaccine. Manufacturing of RNA Vaccines does not require cells or animal substrates. mRNA vaccine does not need to enter the nucleus and their immunostimulatory effects are higher as compared to DNA vaccines. But these vaccines have few disadvantages as compared to DNA Vaccines. These include decreased stability, decreased translation into proteins, and the requirement to escape endosomal uptake. Furthermore concomitant uses of other drugs may impact mRNA metabolism and the number of protein antigen molecules produced per molecule of mRNA delivered is less as compared to plasmid DNA vector [80-82]. There are currently six RNA vaccines in clinical trials, which are detailed in Table 3.
| Vaccine Name | Gene encoding | Manufacturer | Clinical trial |
|---|---|---|---|
| mRNA1273 | S antigen | Moderna TX, Inc | Phase I/II/ III (NCT04283461) |
| BTN162 | S antigen | Pfizer and BioNTec | Phase I/ II/III (NCT04368728) |
| LNP-nCoV saRNA | S antigen | Imperial College London, Morningside Ventures | Phase I/II (IRAS-Number: 279315CPMS-ID: 46068) |
| LUNARCOV19 (ARCT-021) | S antigen | Arcturus Therapeutics, Inc., Duke-NUS | Phase I/II(NCT04480957). |
| CVnCoV | S antigen | Cure Vac | Phase I(NCT04449276) |
| Unnamed mRNA vaccine | S antigen | Yunnan Walvax Biotechnology Co., Ltd | Phase I(ChiCTR2000034112) |
Table 3: RNA Vaccines.
Inactivated virus vaccine: Inactivated virus vaccines are generated by inactivating the virus by using chemical or physical agents to render them replication-incompetent. These are highly stable and economical although efficacy of vaccine may be low since the viral antigens may have been modified during the inactivation process. In general, these vaccines do not elicit strong immune response, and may require booster dose. Currently, there are six inactivated virus vaccines being developed which are detailed in Table 4.
| Vaccine Name | Agent of inactivation | Manufacturer | Clinical trial |
|---|---|---|---|
| BBIBP-CorV | Β-propionolactone | Beijing Institute of Biological Products, Sinopharm | Phase I/II/III(ChiCTR2000032459, ChiCTR2000034780) |
| CoronaVac | - | Sinovac Biotech Co.,Bhutanan institute | Phase I/II/III(NC04352608, NCT04383574,NCT0445659) |
| V-SARS | heat-inactivated | Immunitor LLC | Phase I/II (NCT04380532). |
| unnamed vaccine by Yunnan | β propionolactone |
Chinese Academy of Medical Sciences | Phase I/II(NCT04412538,NCT04470609) |
| Unnamed Inactive Vaccine-Wuhan | - | Wuhan Institute of Biological Products, Sinopharm | Phase I/II/III (ChiCTR2000031809,ChiCTR2000034780) |
| BBV152 A,B,C |
- | Bharat Biotech International Limited, ICMR | Phase I/II ( NCT04471519). |
Table 4: Inactivated Vaccines.
Recombinant virus vaccine: Recombinant virus vaccines are constructed by introducing a viral protein in the backbone of an actively replicating virus. They produce antigens inside cells which evoke a strong immune response. They are highly effective in generating long term immunity since the virus can infect cells and get incorporated in the genome thereby avoiding the need for a booster dose. Recombinant virus vaccines that are currently in clinical trials for SARS-CoV-2 use adenovirus and measles virus as a vector. AZD1222, Ad26.COV2.S, Gam-COVID-Vac, Ad5nCoV are examples of non-replicating adenovirus vectors whereas V591 and TMV-083 is replication competent measles vector used. There are currently six recombinant virus vaccines detailed in Table 5.
| Vaccine Name | Gene encoding | Manufacturer | Clinical trial |
|---|---|---|---|
| AZD1222 | S antigen | Consortium of the Jenner Institute, Oxford Biomedica, University of Oxford | Phase I/II/III (NCT04324606) |
| Ad26.COV2.S | S antigen | Janssen Vaccines and Prevention, Beth Israel Deaconess Medical Center, Johnson & Johnson | Phase I/II (NCT04436276). |
| Ad5-nCoV | S antigen | CanSino Biologics Inc., Institute of Biotechnology ,PLA of China | Phase I/II (NCT04313127, ChiCTR2000030906) |
| Gam-COVID-Vac | S antigen | Gamaleya Research Institute of Epidemiology and Microbiology | Phase I/II (NCT04437875, NCT04436471) |
| V591 | S antigen | Merck Sharp & Dohme Corp | Phase I (NCT04498247). |
| TMV-083 | S antigen | Institute Pasteur, Themis Bioscience GmbH. | Phase I (NCT04497298). |
Table 5: Recombinant virus Vaccines.
Protein subunit vaccines: Subunit vaccines encode the viral antigenic determinants. They are high specific and elicit elevated immune response. However, they may elicit low and short-lived immunogenicity. Thus, they may require use of adjuvants. There are currently eight protein subunit vaccines against SARS-CoV-2 in clinical trials which are detailed in Table 6.
| Vaccine Name | Gene encoding | Manufacturer | Clinical trial |
|---|---|---|---|
| COVAX19 | S protein | Gene Cure Biotechnologies, Vaxine | Phase I/II (NCT04428073, NCT04453852). |
| KBPCOVID 19 | Various viral genes | Kentucky BioProcessing, Inc. | Phase I/II (NCT04473690). |
| Unnamed Recombinant vaccine-Anhui | S protein | Anhui ZhifeiLongcom Biopharmaceutical, Institute of Microbiology of the CAS | Phase I/II (NCT04445194,NCT04466085) |
| SBC2019 | S protein | Clover Biopharmaceuticals | Phase I (NCT04405908) |
| NVX‑CoV2373 | S protein | Novavax, Takeda | Phase I (NCT04368988) |
| MVCCOV1901 | S protein | Medigen Vaccine Biologics Corp | Phase I (NCT04487210). |
| Unnamed Spike Protein Candidate | S protein | University of Queensland, Syneos Health, etc | Phase I (ACTRN12620000674932,NCT04495933) |
| AdimrSC-2f | S protein | Ad immune Corporation | Phase I (NCT04522089) |
Table 6: Subunit Vaccines.
Besides these there are other vaccines in clinical trials. These are either repurposed vaccines or virus like particle vaccines which are detailed in Table 7.
| Vaccine Name | Vaccines type | Manufacturer | Clinical trial |
|---|---|---|---|
| Oral Polio Vaccine | Repurposed | Bandim Health Project | Phase IV |
| Bacillus-Calmette-Guerin(BCG) | Repurposed | University of Melbourne and Murdoch Children’s Research Institute | Phase III/IV |
| Measles-Mumps-Rubella Vaccine | Repurposed | Kasr El Aini Hospital | Phase III/IV |
| IMM-101 | Repurposed | Canadian Cancer Trials Group, Immodulon Therapeutics Ltd | Phase III |
| BACMUNE (MV130) | Repurposed | Inmunotek S.L., BioClever 2005 S.L | Phase III |
| AlloStim | Bioengineered living cell | Immunovative Therapies, Ltd., Mirror Biologics, Inc | Phase I /II |
| COVID 19/aAPC Vaccine | Modified APC | Shenzhen Geno-Immune Medical Institute | Phase I |
| Unnamed VLP Vaccine | Virus Like Particle | Medicago Inc | Phase I |
| RUTI | Repurposed | Fundació Institut Germans Trias i Pujol | N/A |
Table 7: Other Vaccines.
Challenges of vaccine development
SARS-CoV-2 being an RNA virus is continuously changing and evolving. Major challenges for vaccine development include safety, generation of immunity and efficacy in all age groups and need to be addressed.
COVID-19 has emerged as one of the most devastating pandemic known till date. Hence, there is an urgent need to develop therapeutics and vaccines against it. Inspiringly, global efforts have been made to use repurposed drugs for clinical management of SARS-CoV-2 infection which have been successful in saving millions of lives worldwide. Co-morbidities such as heart diseases, hypertension, malignancy, COPD, Diabetes, Chronic lung disease, Kidney disease and Obesity pose increased vulnerability towards COVID-19. Hence it is important to develop strong immunity by adopting a healthy life style in order to decrease the susceptibility, severity and mortality associated with COVID-19. More stringent steps are needed to develop safe and effective therapeutics and vaccines to prevent successive emergence of other novel pandemics in future.
Citation: Madhu Rai, Yuvraj KC, Ritu Gaur (2021) Current Therapeutics and Prophylactics against COVID-19 J Antivir Antiretrovir. 13:207.
Received: 15-Dec-2020 Accepted: 30-Dec-2020 Published: 06-Jan-2021 , DOI: 10.35248/1948-5964.21.13.207
Copyright: © 2021 Rai M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.