Journal of Women's Health Care
Open Access
ISSN: 2167-0420
ISSN: 2167-0420
Research Article - (2023)Volume 12, Issue 2
Background: Polycystic ovarian syndrome (PCOS) accounts for over 75% of anovulatory infertility. Letrozole is an effective alternative to clomiphene citrate as a first-line drug for ovulation induction in women with PCOS. This study aims to compare the effectiveness and safety profile of 2.5mg to 5mg daily oral doses of letrozole for ovulation induction in infertile women with PCOS.
Methods: The study was a nonrandomized control trial (NRCT). One hundred and ten eligible consenting infertile women with PCOS were consecutively assigned into two treatment groups. The intervention group received oral letrozole 2.5mg daily and the control group received oral letrozole 5mg daily, both groups starting from the 2nd day of menses for 5 days. Participants in both groups were followed up to determine the primary and secondary outcome measures. Data were analyzed using SPSS version 20.0 for windows (IBM Corporation). Continuous and categorical variables were analyzed using the student t-test, Chi-square test and Fisher's exact respectively. P<0.05 was considered as statistically significant.
Results: The Ovulation rate for the 2.5mg oral letrozole group and 5mg oral letrozole group were similar (96.4% Vs 92.7%). P=0.401. The number of matured follicles was significantly higher in the 5mg oral letrozole group (1.44±0.81) than 2.5mg oral letrozole group (1.07±0.37).
Conclusion: This study has shown that both 2.5mg and 5mg oral letrozole doses have equal effectiveness for ovulation induction. This study recommends the use of 2.5mg letrozole dose for initiation of ovulation induction.
Comparison; Effectiveness; Doses; Infertile women; PCOS, NRCT
Infertility is a worldwide problem, occurring in 8-15% of couples in their reproductive age. Anovulatory disorders are one of the commonest causes of infertility, accounting for about 30-40% of cases. PCOS is the leading cause of anovulation and is responsible for about 75% of cases of an ovulatory infertility [1-8].
Though the prevalence of PCOS varies among races and ethnicities, it has been quoted to affect about 5-10% of reproductive age group women [9,10]. A prevalence of up to 10-26% has however been reported in the western world and a high prevalence of 52% has been found among South Asian immigrants in Britain [6,9,11]. The prevalence of PCOS in Nigeria also varies across geographical zones with a prevalence range of 2.2%-18% reported [6,9,11].
Several methods have been effective for ovulation induction and fertility treatment in women with PCOS. These include weight reduction, exercise, lifestyle modifications, and drugs such as clomiphene citrate (CC), letrozole, metformin and gonadotrophins. Other measures include ovarian drilling and in-vitro fertilization (IVF) [13].
For over 40 years, CC which is a selective estrogen receptor modulator was considered the first-line drug in ovulation induction [5,13,14].
It is however associated with a lower cumulative pregnancy rate of 40-45% despite an ovulation rate of about 70-80% after 6 months of treatment with its side effects. There is, therefore, a need for a simple, safe and effective alternative drug for ovulation induction particularly in patients with PCOS [15-18].
Letrozole, a non-steroidal third-generation aromatase inhibitor, has been introduced as a new treatment option which can be used as an alternative for CC for ovulation induction in women with anovulatory infertility [5,15,19-21].
Several studies have shown the superior efficacy of letrozole over CC or at least equal efficacy to CC in ovulation induction among infertile women with PCOS [4,5,8,10,15,18]. Different doses of letrozole ranging from 2.5mg to 7.5mg daily have been used in these studies.
Only few studies have compared the effects of different doses of letrozole for ovarian stimulation in infertile women with PCOS [18]. However, there are conflicting opinions in the literature regarding the ideal dosage of letrozole for ovulation induction in terms of effectiveness and minimum side effects [8,20]. This leaves a knowledge gap which needs to be bridged. Therefore, the purpose of this study is to compare the effectiveness and safety profile of 2.5mg to 5mg daily oral doses of letrozole for ovulation induction in infertile women with PCOS.
Aim
To compare the effectiveness and safety profile of 2.5mg versus 5mg daily doses of oral letrozole administered from 2nd to 6th day of the menstrual cycle for ovulation induction in infertile women with PCOS.
Objectives
1. To compare the ovulation rates per cycle following the administration of oral letrozole 2.5mg (intervention) versus 5mg (control) daily doses for five days from day 2 of the menstrual cycle
2. To compare the numbers of days to achieve follicular maturity, the number of matured follicles, the endometrial thickness on the day of hCG administration, and pregnancy rate per cycle between the oral letrozole 2.5mg daily dose and oral letrozole 5mg daily dose
3. To determine and compare the common side effects and complications between the oral letrozole 2.5mg daily dose and oral letrozole 5mg daily dose
This was a non-randomized control trial conducted in the Department of Obstetrics and Gynaecology of the Federal Medical Centre Makurdi, Benue State, North Central Nigeria from 1st of July, 2019 to 29th of February, 2020. Ethical clearance was obtained from the Hospital Research Ethics Committee (HREC) of the Federal Medical Centre Makurdi. One hundred and ten consenting participants with PCOS who fulfilled the inclusion criteria and who are already aware of a particular letrozole dosage or already counseled on the use of a particular letrozole dose regimen by their gynaecologist, were assigned into two treatment groups (intervention and control groups) of 55 participants each.
Inclusion criteria
All consenting infertile women between the ages of 18-35 years that were diagnosed with PCOS at the study center during the study period. The diagnosis of PCOS was made in this study mainly by clinical and ultrasound findings in keeping with 2003 Rotterdam criteria [22].
Exclusion criteria
These include:
• Women with infertility due to submucous uterine fibroid, Asherman's syndrome and cervical stenosis
• Women with infertility due to bilateral tubal blockage
• Women with other causes of anovulatory cycle such as hyperprolactinaemia, thyroid dysfunction (hypothyroidism and hyperthyroidism)
• Women whose husbands have abnormal seminal fluid analysis (SFA) parameters according to WHO 2010 reference values for SFA [23]
• Women with allergies or contraindications to letrozole use
• Women who declined consent or refused to have TVS
Sample size determination
The sample size per group was determined using the formula for sample size calculation for comparison between two groups or proportions [24,25].
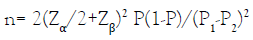
Where:
n = minimum sample size per group.
Zα/2=The value of type I error when α is 5% (0.05) at 95% confidence interval, which is 1.96.
Z β=The value of type II error when β is 0.20 at 80% power, which is 0.84.
P2=Ovulation induction rate in the control group (use of 5mg oral letrozole). Using a mean ovulation induction rate of 77% as P2 from a previous study with ovulation induction rate of between 70-84% [26].
P1=Ovulation induction rate for the intervention group (use of 2.5mg oral letrozole). Assuming that there will be a change in the control group by 25%, the value of P1 is 96.3%.
P=Pooled prevalence or proportion of events in two groups. P=P1+P2/2= 96.3+77/2= 86.7%
P1-P2= Difference in proportion of two events in two groups=96.3%-77%= 19.3%
Therefore, the minimum sample size per group,
= 2(1.96+0.84)20.867(1-0.867)/ (0.193)2
= 49
Anticipating up to 10% of the study participants will be a loss to follow up, an attrition rate of 10% was added to the above sample size, Therefore the minimum sample size (n) to be selected for each group in this study will be approximately 55. The total sample size (N) will be 110 study participants.
Sample recruitment
One hundred ten infertile women between the ages of 18-35 years that were diagnosed with PCOS who were eligible and consented to participate in the study were recruited. Eligibility was based on the inclusion and exclusion criteria for the study. All study participants were subjected to full history taking, clinical examination, and fertility work up (Transvaginal scan, seminal fluid analysis and hysterosalpingography). The diagnosis of PCOS was made in this study mainly by clinical and ultrasound findings in keeping with 2003 Rotterdam [22].
Sampling technique
Participants, who are already aware of a particular letrozole dosage or already counseled on the use of a particular letrozole dose regimen by their gynecologists, were assigned to that regimen. The two treatment groups were: Intervention (55 participants taking 2.5mg oral dose of letrozole) and control (55 participants taking 5mg oral dose of letrozole; control group).
Treatment protocol
Infertile women with PCOS who meet the inclusion criteria were recruited into the treatment protocol after obtaining informed written consent. Each participant in intervention and control groups received oral letrozole 2.5 mg and 5.0 mg respectively (TEVA, Teva UK Limited, East Bourne, BN22 9AG) starting from day 2 to day 6 (for 5 days) of their menstrual cycle [occurring spontaneously or induced with 10mg of oral Medroxyprogesterone acetate (MPA) daily for 10 days]. A baseline TVS (using LOGIQ V5 General Electric (GE) Medical System, Serial Number: 459631WX8, Made in China, Year of Manufacture: 2015/11) was done on the second day of their menstrual cycle to measure the number, size, and location of the follicles on each ovary as well as endometrial thickness (ET) and to ensure the absence of ovarian cysts before starting treatment [18,27].
A TVS was repeated on day 10 of their menstrual cycle to determine the presence, number, and size of dominant follicles (DF).
Follicles greater than 12mm on day 10 were regarded as dominant follicles [26]. If the dominant follicle was absent on day ten of the menstrual cycle, a repeat TVS was performed every 3-4 days later. The absence of a dominant follicle (DF < 12mm) by day 20 was regarded as failed induction and induction was repeated from day 2 of their next menstrual cycle [26]. If dominant follicles were present (DF>12mm), they were followed up until at least one follicle had a diameter of between 18 -24mm and then ovulation was triggered with 10,000IU of intramuscular β-hCG (Diclair-HP-HCG, BBT Biotech GmbH, Germany) [27,28]. The number of mature follicles (18-24mm) and endometrial thickness (normal endometrial thickness≥6mm) were measured on the day of administering β-hCG and number of days to achieve follicular maturity was noted [28]. The couple was counselled to have unprotected sexual intercourse 24-36 hours after administering intramuscular β-hCG [5]. TVS was done a week after triggering ovulation to determine if ovulation had occurred [presence of collapsed follicles, fluid in the Pouch of Douglas (POD), and corpus luteum cyst]. A serum Pregnancy test was done two weeks after given β-hCG administration. if positive, a TVS was done two weeks later to confirm clinical pregnancy. A single cycle was done for all the study participants. The size of each follicle was measured as a mean diameter by taking the average of three internal diameters measured in three orthogonal planes, while the endometrial thickness was measured at the greatest diameter perpendicular to the midsagittal plane in the fundal region, including both layers of the endometrial cavity [15,21,29]. The ovarian assessment (the sizes and number of follicles) was done manually with the use of a TVS. The diagnosis of OHSS in this study was made based on clinical criteria [30] (Figure 1)
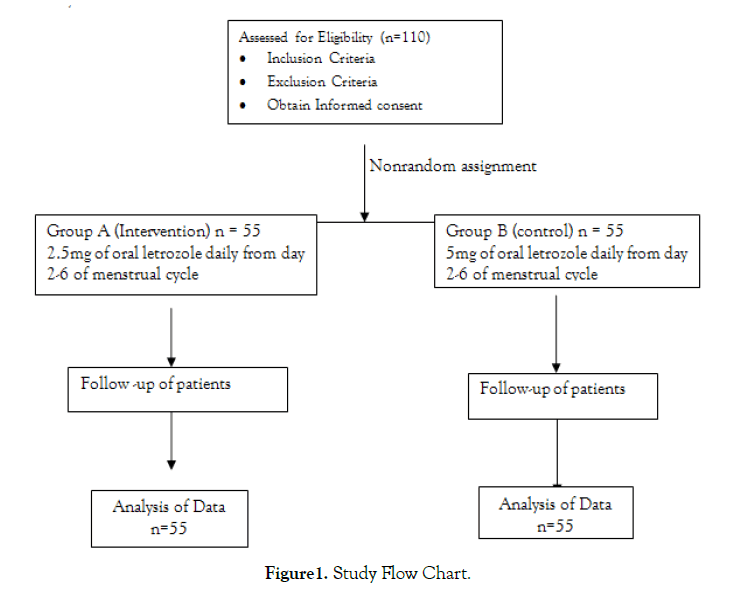
Figure 1: Study Flow Chart.
Data collection
Data was collected using a predesigned proforma to extract information from all consenting participants who meet the inclusion criteria from each group.
Outcomes measures
Primary Outcomes: Comparison of ovulation rates per cycle following administration of oral letrozole 2.5mg versus 5mg for five days from day 2 of the menstrual cycle of infertile women with polycystic ovarian syndrome.
Secondary Outcomes: Comparison of the numbers of days to achieve matured follicles, numbers of matured follicles, the endometrial thickness at hCG administration, pregnancy rates per cycle, sides effects and complications between the two groups.
Data were analyzed using statistical package for social sciences version 20.0 for windows (IBM Corporation). Continuous data were analyzed using the student t-test, while Chi-square(x2) test and Fischer's exact test was used for categorical data. P-value of less than 0.05 was considered as statistically significant. It was also considered statistically significant if the interval at 95% confidence interval (CI) does not include or cross the number one (1). Further statistical analysis was performed using multivariate regression analysis to account for the confounding factors identified in this study only when on bivariate analysis the P-value is less than 10%.
Socio-demographic characteristics of the study participants
The Table 1 below shows the socio-demographic characteristics of the study participants in both groups.
| Variables | 2.5mg Oral letrozole n=55 Frequency (%) |
5mg Oral letrozole n=55 Frequency (%) |
Both group N=110 Frequency (%) |
Test statistics | Df | p-value |
|---|---|---|---|---|---|---|
| Age(in year) | χ2=4.12 | 1 | 0.042* | |||
| <30 | 23(41.8) | 13(23.6) | 36(32.7) | |||
| ≥30-35 | 32(58.2) | 42(76.4) | 74(67.3) | |||
| Mean±SD | 29.95±4.64 | 31.84±3.54 | 30.89±4.22 | t=-2.40 | 108 | 0.018* |
| Educational status | Fisher’s exact=3.16 | 0.169 | ||||
| Primary education | 0(0.0) | 1(1.8) | 1(0.9) | |||
| Secondary education | 25(45.5) | 17(30.9) | 42(38.2) | |||
| Post-secondary education | 30(54.5) | 37(67.3) | 67(60.9) | |||
| Occupation | Fisher’s exact=6.95 | 0.220 | ||||
| Student | 7(12.7) | 8(14.5) | 15(13.6) | |||
| Civil servant | 10(18.2) | 19(34.5) | 29(26.4) | |||
| Trader | 21(38.2) | 21(38.2) | 42(38.2) | |||
| Farmer | 3(5.5) | 1(1.8) | 4(3.6) | |||
| House wife | 10(18.2) | 4(7.3) | 14(12.7) | |||
| Others | 4(7.3) | 2(3.6) | 6(5.5) | |||
| Ethnic group | Fisher’s exact=3.27 | 0.689 | ||||
| Tiv | 30(54.5) | 36(65.5) | 66(60.0) | |||
| Idoma | 10(18.2) | 9(16.4) | 19(17.3) | |||
| Igbo | 2(3.6) | 2(3.6) | 4(3.6) | |||
| Hausa | 3(5.5) | 2(3.6) | 5(4.5) | |||
| Yoruba | 1(1.8) | 2(3.6) | 3(2.7) | |||
| Others | 9(16.4) | 4(7.3) | 13(11.8) | |||
| Marital status | ||||||
| Married | 55(100.0) | 55(100.0) | 110(100.0) | - | - |
Table 1: Socio-demographic characteristics of PCOS patients for each group
Participants in the ≥30-35 age group were statistically significantly higher in both study groups than those in the age < 30 (58.2%, 76.4% versus 41.8%, 23.6%, χ2=4.12, p=0.042). The overall mean age of all the participants was 30.89±4.22 years. In the 2.5mg letrozole group, the mean age was 29.95±4.64 years, while in the 5mg letrozole group, the mean age was 31.84±3.54 years. There was a statistically significant difference between the ages of the study participants in both groups (t=2.40, p=0.018). All other socio-demographic characteristics were not statistically significant between the two study groups (P> 0.05).
Clinical characteristics of the study participants
The clinical characteristics of the study participants for each group. The overall mean BMI of all the participants was 28.12±5.30. In the 2.5mg letrozole group, the mean BMI was 26.74±5.44 kg/m2. In the 5mg letrozole group, the mean was 29.50±4.82 kg/m2. There was a statistically significant difference in the mean BMI between the study groups. (t=2.80, p=0.006). All other clinical characteristics shows no statistically significant difference (P<0.05) (Table 2).
| Variables | 2.5mg Oral letrozole n=55 Frequency (%) |
5mg Oral letrozole n=55 Frequency (%) |
Both group N=110 Frequency (%) |
Test statistics | Df | p-value |
|---|---|---|---|---|---|---|
| Menstrual cycle | χ2=0.06 | 1 | 0.801 | |||
| Spontaneous | 46(83.6) | 45(81.8) | 91(82.7) | |||
| Induced | 9(16.4) | 10(18.2) | 19(17.3) | |||
| Parity | χ2=2.33 | 1 | 0.127 | |||
| 0 | 33(60.0) | 25(45.5) | 58(52.7) | |||
| ≥1 | 22(40.0) | 30(54.5) | 52(47.3) | |||
| Mean±SD | 0.62±0.97 | 0.85±1.00 | 0.74±0.99 | t=-1.25 | 108 | 0.213 |
| Type of fertility | χ2=0.70 | 1 | 0.401 | |||
| Primary | 18(32.7) | 14(25.5) | 32(29.1) | |||
| Secondary | 37(67.3) | 41(74.5) | 78(70.9) | |||
| Duration of infertility | ||||||
| Mean±SD | 2.98±2.64 | 2.90±3.03 | 2.94±2.83 | t=0.14 | 108 | 0.889 |
| BMI (kg/m2) | Fisher’s exact=6.65 | 0.062 | ||||
| Underweight | 2(3.6) | 0(0.0) | 2(1.8) | |||
| Normal | 19(34.5) | 10(18.2) | 29(26.4) | |||
| Overweight | 20(36.4) | 22(40.0) | 42(38.2) | |||
| Obese | 14(25.5) | 23(41.8) | 37(33.6) | |||
| Mean±SD | 26.74±5.44 | 29.50±4.82 | 28.12±5.30 | t=-2.80 | 108 | 0.006* |
Table 2. Clinical characteristics of PCOS patients for each group
Comparison of ovulation rates per cycle among the study group
Table 3 below shows the ovulation rates per cycle among the study group.
| Variables | 2.5mg Oral letrozole N=55 Frequency(%) |
5mg Oral letrozole N=55 Frequency(%) |
Both group N=110 Frequency(%) |
χ2 | RR | 95% CI | p-value |
|---|---|---|---|---|---|---|---|
| Ovulation | 0.70 | 1.52 | 0.48– 4.8 | 0.401 | |||
| Occurred | 53(96.4) | 51(92.7) | 104(94.5) | ||||
| Not occurred | 2(3.6) | 4(7.3) | 6(5.5) |
Table 3. Comparison of ovulation rates per cycle among the study groups
The ovulation rate for 2.5mg letrozole group was 53(96.4%), while for 5mg letrozole group was 51(92.7%). There was no statistically significant difference between the ovulation rate of the study participants in both groups (χ2=0.70, p=0.401).
Comparison of ovulation rates per cycle between the study group, age and BMI
The comparison of ovulation rates per cycle between the study group, age and BMI in Table 4. The ovulation rate was more in the 2.5mg letrozole group 53(96.4%) compared to the 5mg letrozole group 51(92.7%). There was no statistically significant difference in the ovulation rate between the two study group (Y=0.70, P=0.401). The ovulation rate was more in the age group <30 years 35(97.2%) compared to the ≥30-35 years 69(93.2%). There was no statistically significant difference in the ovulation rate between the two age groups (Y=0.74, P=0.678). The ovulation rate was 2(100%) in the participants that were underweight. There was no statistically significant difference in the ovulation rate in the body mass index (Fisher’s exact=1.47, P=0.7880).
| Variable | Ovulation | χ2 | RR | 95%CI | P-value | |
|---|---|---|---|---|---|---|
| Yes n=104 Frequency (%) |
No n=6 Frequency (%) |
|||||
| Study group | Y=0.70 | 1.03 | 0.36– 11.84 | 0.401 | ||
| Letrozole (2.5mg) | 53(96.4) | 2(3.6) | ||||
| Letrozole (5mg) | 51(92.7) | 4(7.3) | ||||
| Age (in years) | Y=0.74 | 1.04 | 0.96– 1.13 | 0.678 | ||
| <30 | 35(97.2) | 1(2.8) | ||||
| ≥30-35 | 69(93.2) | 5(6.8) | ||||
| Body Mass Index | Fisher’s Exact=1.47 | - | - | 0.788 | ||
| Underweight | 2(100.0) | 0(0.0) | ||||
| Normal | 28(96.6) | 1(3.4) | ||||
| Overweight | 40(95.2) | 2(4.8) | ||||
| Obese | 34(91.9) | 3(5.5) | ||||
Table 4. Comparison of Ovulation rates per cycle between study group, age and BMI
Comparison of numbers of days to achieve follicular maturity, the numbers of matured follicles and the endometrial thickness at hCG administration between the study group
The comparison of the number of days to achieve follicular maturity, the number of matured follicles and the endometrial thickness at hCG administration among the study participants shown in Table 5.
| Variables | 2.5mg Oral Letrozole n=55 Mean (SD) |
5mg Oral letrozole n=55 Mean (SD) |
95%CI | T | Df | p-value |
|---|---|---|---|---|---|---|
| Number of days to achieve follicular maturity | 111.19(1.97) | 11.34(1.51) | -0.81 – 0.51 | -0.44 | 108 | 0.654 |
| Number of matured follicles | 1.07(0.37) | 1.44 (0.81) | -0.60 – -0.12 | -3.01 | 108 | 0.003* |
| Endometrial thickness at hCG administration | 9.39(1.80) | 9.36 (2.04) | -0.69 – 0.76 | 0.09 | 108 | 0.921 |
Table 5. Comparison of numbers of days to achieve follicular maturity, the numbers of matured follicles and the endometrial thickness at hCG administration between the groups
The overall mean number of days to achieve follicular maturity was 11.27±1.75 days. The mean number of days to achieve follicular maturity was 11.19±1.97 for 2.5mg letrozole group and 11.34±1.51 for the 5mg letrozole group. There was no statistically significant difference in the mean number of days to achieve follicular maturity (t=0.44, p=0.654).
The overall mean number of matured follicles was 1.25±0.65. The mean number of matured follicles in the 5mg letrozole group was 1.44±0.81. This was higher when compared with the mean number of matured follicles 1.07±0.37 in the 2.5mg letrozole group. The difference was statistically significant (t=3.01, p=0.003).
The overall mean endometrial thickness at hCG administration was 9.37±1.91. The mean endometrial thickness at hCG administration was 9.39±1.80 and 9.36±2.04 for 2.5mg letrozole group and 5mg letrozole group respectively. This was not statistically significant between both groups (t=0.09, p=0.921).
Comparison of pregnancy rates per cycle between the study groups
The pregnancy rate in the 2.5mg Letrozole group was 7 (13.2%), while in the 5mg letrozole group was 9(17.6%) in Table 6. There was no statistically significant difference between the pregnancy rate of the study participants in both groups (p=0.530).
| Variables | 2.5mg Oral letrozole n=53 Frequency (%) |
5mg Oral letrozole n=51 Frequency (%) |
Both group N=104 Frequency (%) |
χ2 | RR | 95% CI | p-value |
|---|---|---|---|---|---|---|---|
| Pregnancy | 0.39 | 0.83 | 0.46-1.51 | 0.530 | |||
| Occurred | 7(13.2) | 9(17.6) | 16(15.4) | ||||
| Not occurred | 46(86.8) | 42(82.4) | 88(84.6) |
Table 6. Comparison of pregnancy rate between the study groups (N=104)
Side effects and complication between the study groups
Participants in the 5mg letrozole group who had nausea were 5(9.1%), while 50 (90.9%) did not have nausea. In the 2.5mg letrozole group, only 1 (1.8%) had nausea, while 54 (98.2%) did not have nausea. There was no statistically significant difference between the two groups in terms of the side effect of nausea (χ2=2.82, p=0.093).
Only 2 (3.6%) of the participants had dizziness in the 5mg letrozole group, while 53 (96.4%) did not report dizziness. In the 2.5mg letrozole group, no participants reported having dizziness. There was no statistically significant difference between the two groups in terms of side effect of dizziness (χ2=2.03, p=0.154).
Only 1 (1.8%) had ovarian cyst formation in both groups. This was not statistically significant (χ2=0.00, p=1.000) (Table 7).
| Variables | 2.5mg Oral letrozole n=55 Frequency (%) |
5mg Oral letrozole n=55 Frequency (%) |
Both group N=110 Frequency (%) |
χ2 | RR | 95% CI | p-value |
|---|---|---|---|---|---|---|---|
| Nausea | 2.82 | 0.32 | 0.05 – 1.93 | 0.093 | |||
| Yes | 1(1.8) | 5(9.1) | 6(5.5) | ||||
| No | 54(98.2) | 50(90.9) | 104(94.5) | ||||
| Dizziness | 2.03 | - | - | 0.154 | |||
| Yes | 0(0.0) | 2(3.6) | 2(1.8) | ||||
| No | 55(100.0) | 53(96.4) | 108(98.2) | ||||
| Ovarian cyst formation | 0.00 | 1.00 | 0.24 – 4.05 | 1.000 | |||
| Yes | 1(1.8) | 1(1.8) | 2(1.8) | ||||
| gNo | 54(98.2) | 54(98.2) | 108(98.2) |
Table 7. Side effects and complication between the study groups
Our findings in this study shows that the ovulation rate, the number of days to achieve follicular maturity, the endometrial thickness on the day of hCG administration, pregnancy rates as well as side effects and complications were not statistically significant between the two study groups (P> 0.05). However, the number of matured follicles were found to be statistically significantly more in the 5.0mg letrozole group than in the 2.5mg letrozole group (1.07±0.37 versus 1.44±0.81 t=3.01, p=0.003)
Although, the ovulation rate in the 2.5mg letrozole group (96.4%) was higher than the 5mg letrozole group (92.7%) in this study. However, there was no statistically significant difference between the two groups in terms of ovulation rate (χ2=0.70, p=0.401). This report agrees with the studies by Badawy et al and Yang et al, [19,32].
The ovulation rate was more among the participants in the age group <30 years (97.2%) and underweight participants (100%). However, this difference was not statistically significant (Age in years Y=0.74, p=0.678; BMI Fischer’s exact =1.47, p=0.7880). On further analysis, the two confounding variables (age and BMI) did not significantly affect the ovulation rates between the two study groups (2.5mg oral letrozole versus 5mg oral letrozole), This therefore implies that the 2.5mg oral daily doses of letrozole and 5mg oral daily doses of letrozole have similar effectiveness for ovulation induction in infertile women with PCOS.
This present study demonstrated that the mean number of days to achieve follicular maturity was similar in the 2.5mg letrozole and in 5mg letrozole groups [(11.19±1.97 vs 11.34±1.51) (t=0.44, p=0.654)]. This supports the findings by Al-Fadhli [21]. However, our findings differ from the study by Badawy et al, who found a statistically significant difference in both groups [32]. This is probably due to differences in the study populations.
The mean number of matured follicles in the 5mg letrozole group (1.44±0.81) was higher than that of 2.5mg letrozole group (1.07±0.37), and this difference was statistically significant (t=-3.01, p=0.003). Our findings support the studies by Al-Fadhli, Yang and Badawy [21,31,32]. But contrary to this study, Badawy et al found the mean number of matured follicles higher in the 2.5mg letrozole group compared to the 5mg letrozole group [19]. This may be due to differences in the treatment protocols.
In this study, the mean endometrial thickness at hCG administration in both groups was similar, 9.39±1.80 and 9.36±2.04 for 2.5mg letrozole and 5mg letrozole groups respectively (t=0.09, p=0.921). This finding collaborates with the studies by Badawy et al, Al- Fadhli, Yang [19,21,31]. But this differ from the study by Badawy et al, who found a statistically significant difference in the mean endometrial thickness at hCG administration [32]. This may be attributed to the recruitment of different study population.
Although this study reported a higher pregnancy rate in the 5mg letrozole group (17.6%) compared to 2.5mg letrozole group (13.2%), there was however no statistically significant difference between the two study groups (p=0.530). This finding agrees with the study by Badawy et al [32]. But this differs from other studies by Badawy et al, Al-Fadhli, Yang, who all found statistically significant differences in pregnancy rate between the two study groups [19,21,32]. This may be related to the differences in study populations and the treatment protocols.
The common side effects and complications noted in this study were nausea, dizziness and ovarian cyst formation. Nausea occurred in 1.8% of the 2.5mg letrozole group and 9.1% of the 5mg letrozole group. Dizziness was experienced by 3.6% of the 5mg letrozole group and none in the 2.5mg letrozole group. Ovarian cyst formation was 1.8% in both the 2.5mg letrozole group and the 5mg letrozole group. These sides effects and complications were not statistically significant between the two groups (nausea χ2=2.82, p=0.093, dizziness χ2=2.03, p=0.154, ovarian cyst formation, χ2=0.00, p=1.000). This indicates that both treatment groups were associated with minimal side effects and complications.
The limitations of this study were: firstly, drug administration was unsupervised as the patients took the medications at home. Thus, it was difficult to know if patients were in compliant with the drugs and if they were taking dose correctly. Secondly, the timing and frequency of coitus was not under the control of the research team. Also, there was a likelihood of introducing bias (selection, information, and confounding) during the study due to not matching and non-random allocation of the participants in the control group with that in intervention group.
This present study has shown that there were no statistically significant differences in the ovulation rates, the mean number of days to achieve follicular maturity, the endometrial thickness at hCG administration and pregnancy rate between the 2.5mg letrozole group and the 5mg letrozole group. However, this study has shown a statistically significant difference between the two groups in the number of the matured follicle with the 5mg letrozole group having more matured follicles compared to the 2.5mg letrozole group. This implies that the use of 5mg letrozole dose is associated with the production of multiple follicles with increased risk of multiple pregnancies and OHSS, thus 2.5mg letrozole is the ideal dose of letrozole for ovulation induction because it is associated with monofollicular production, single pregnancy and reduces risk of OHSS which is the goal of ovulation induction. Also, the study has shown that there were minimal side effects and complications between the two study groups. This study therefore has demonstrated that 2.5mg dose of letrozole is safe and effective for ovulation induction and should be used as the ideal dose for initiation of ovulation induction in infertile women with PCOS.
This study recommends 2.5mg dose of letrozole as the ideal dose for initiation of ovulation induction in infertile women with PCOS. The doses can be increased based on the patient's response with an incremental dose of 2.5mg. Randomized control trials are needed to further establish the initial starting dose of 2.5mg letrozole for ovulation induction.
To all the study participants who willingly consented and participated in this study and to Mr Tersoo Joshua Apaa of the Federal Medical Centre who was involved in data organization and analysis during this study period.
Self
None to report
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Citation: Irowa O, Abu P, Mohammad H, Shaahu VN, Obulu MA, Ochejele S, et al. (2023) Comparison of the Effectiveness of 2.5mg versus 5mg Doses of Letrozole for Ovulation Induction in Infertile Women with Polycystic Ovarian Syndrome: A Non-Randomized Control Trial. J Women's Health Care. 12(2):626
Received: 03-Feb-2023, Manuscript No. JWH-23-21692; Editor assigned: 04-Feb-2023, Pre QC No. JWH-23-21692(PQ); Reviewed: 18-Feb-2023, QC No. JWH-23-21692; Revised: 21-Feb-2023, Manuscript No. JWH-23-21692 (R); Published: 28-Feb-2023 , DOI: 10.35248/2167-0420.23.12.626
Copyright: © 2023 Omoregie I et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited