
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Research Article - (2022)Volume 13, Issue 11
Background: Spinal anesthesia type of neuraxial regional anesthesia is widely used for inguinal hernia repair surgeries. Need of study raised due to evolution of non-opioid adjuvants to avoid risks of intrathecal opioids. Our aims were to study change in hemodynamic parameters intra and postoperatively, block characteristics, duration of analgesia and adverse effects intraoperatively and postoperatively.
Objectives: A type of prospective, double blinded randomized controlled trial study carried out at Tertiary Care Hospital, during 2018-2019 with 70 adult male patients in the age group of 18-65 years having unilateral inguinal hernia after taking Institutional Ethical Committee clearance (registration number: ECR/6/INST/GUJ/2013) and written informed consent were taken in their own language according to institutional protocols and explaining the cause, pathology and consequences of the disease process.
Methods: In this study, 70 patients, after matched inclusion criteria, posted for unilateral inguinal hernia were assessed. They were divided into two, Group M and Group N, 35 each who received Magnesium sulphate (100 mg) and Neostigmine methyl sulphate (75 mcg) respectively; along with 0.5% Bupivacaine (15 mg). Primary outcome was to study hemodynamic stability and secondary outcome was to study blockage characteristics and adverse effects. Statistical analysis done by using the SPSS Statistical Software version 24.0. Mean and Standard deviation were calculated for analysis. Unpaired 'T' test were applied between Group M and Group N.
Results: Significantly delayed onset of sensory block with neostigmine (2.19 ± 0.40 min, p<0.05), significantly delayed onset (2.85 ± 1.29 min, p<0.05) and longer duration in motor block (188.82 ± 14.5 min, p<0.05) observed with neostigmine. Significant bradycardia and hypotension with neostigmine and maximum at 1 min (P<0.01).There was significant hypotension with neostigmine at 10 min and 15 min (P<0.05). Duration of analgesia was longer with neostigmine as compared to magnesium Sulphate (Group M=98.4 ± 30.86 min, Group N=215.45 ± 17.4 min). Adverse effects were more with neostigmine.
Conclusion: Longer duration of blockage and analgesia seen by Neostigmine methylsulphate with significant hypotension, bradycardia and vomiting.
Spinal anaesthesia; Bupivacaine hydrichloride; Magnesium sulphate; Neostigmine methylsulphate
Regional anaesthesia has overcome risk of general anaesthesia and gave excellent benefits. Spinal anaesthesia is commonly used regional anesthetic technique for lower abdominal surgeries. Regional anaesthesia gives excellent pain relief and facilitates early postoperative mobilization of patients [1]. 1898 August Bier introduced spinal analgesia in clinical practice. Then, it is widely used [2,3]. Bupivacaine Hydrochloride, long acting Amide type of local anaesthetic is most commonly used in the lower abdominal surgeries like inguinal hernia repairs [4]. The duration of action can be prolonged by addition of substances called adjuvants. But no drug yet is ideal having advantage without side effects. We are comparing Non-opioid adjuvants-magnesium sulphate and neostigmine. Magnesium sulphate is non-competitive antagonist to NMDA (N-methyl-D-aspartate) receptors [5]. Neostigmine acts as analgesic by spinal mechanism-reversible inhibitor of the enzyme cholinesterase [6].
This study was done with 70 adult patients of ASA grade 1,2,3 after considering inclusion and exclusion criteria in 18-65 years age group having unilateral inguinal hernia after taking Institutional Ethical Committee clearance (registration number: ECR/6/INST/ GUJ/2013) and written informed consent in their own language according to institutional protocols and explaining the cause, pathology and consequences of the disease process. Patients were equally divided into 2 groups given same volume of drug in both groups. Magnesium sulphate 100 mg and Neostigmine 75 microgram added to Inj.Bupivacaine heavy (0.5 %) 15 mg intrathecally by Group M and Group N respectively. Patients were kept nil by mouth 8 hours prior to procedure for solids. Venous access was done. Multipara monitor attached and NIBP, pulse oximetry, ECG and vitals were recorded. Patients are pre-loaded with 15 ml/kg of appropriate IV fluid. Premedication given with Inj.Ondansetron 60 mcg/kg, Inj.Glycopyrrolate 4 mcg and Inj.Midazolam 20 mcg/ kg. After proper preparation subarachnoid block was given Inj. 0.5% Bupivacaine heavy (15 mg)+Inj.Magnesium sulphate 100 mg in Group M and Inj. 0.5% Bupivacaine heavy (15 mg)+Inj. Neostigmine methyl sulphate 75 μg in Group N intrathecally, with a 25 G Quince spinal needle in L3-L4 intervertebral space in lateral decubitus position under all aseptic and antiseptic precautions after clear and free flow of cerebrospinal fluid. Then patients were immediately placed in supine position. After giving subarachnoid block, we observed. Time of onset of sensory block, Time of onset and duration of motor block, Hemodynamic changes, total Duration of analgesia, adverse effects. Hypotension (MAP<20% of baseline systolic blood pressure) were treated with appropriate fluid (ringer lactate solution/colloid) and then Inj.Me phentermine 6 mg intravenous in incremental doses if needed. Bradycardia (HR<50/min) were treated with Inj.Glycopyrrolate 0.5 mg intravenous (up to maximum of 3 doses). Inj.Atropine 0.6 to 1.2 mg. Onset of sensory block was defined as the time from intrathecal injection to lack of pain with pin prick test at L1 level. Motor block was evaluated by Modified bromage scale. Onset of motor block was defined as the time from intrathecal injection to impossibility of knee flexion. When the score was zero in Modified bromage scale, it was considered as recovery from complete motor block. After motor and sensory recovery, patient was shifted from recovery room to ward.
Postoperative Pain assessment done by VAS score, when it was more than 3, first dose of analgesic given in form of Inj.Diclofenac sodium 75 mg intramuscularly and duration of analgesia by intrathecal drug had been completed. After study completed, data were collected and Statistical analysis done by using the SPSS Statistical Software version 24.0. Mean and Standard deviation were calculated for analysis. Unpaired 'T' test were applied between Group M and Group N. Associations with p value less than 0.05 was considered significant and less than 0.001 highly significant.
In our study, the mean age of patient in Group M is 48.26 ± 13.39 years and in Group N is 42.2 ± 13.22. This difference in age is not statistically significant as shown in Table 1. Onset of sensory block was significantly delayed (P<0.05) in Group N (2.19 ± 0.40) as compared to Group M (1.32 ± 0.41) as shown in Table 2. Onset of motor block was faster and duration of motor block was longer in neostigmine as compared to magnesium sulphate Table 2, Figures 1 and 2. Variation in duration of analgesia among both groups. Mean duration of analgesia was highly significant (p<0.0001) in Group N (215.25 ± 17.49) as compared to Group M (98.4 ± 30.46) as shown in Table 3 and Figure 3. We studied perioperative pulse rate changes from pre induction stage to 90 mins in both the groups. There was significant bradycardia in Group N (p<0.05). No significant pulse rate changes in Group M as shown in Table 4 and Figure 4. Significant hypotension was noted at 10 min (80.10 ± 4.78, p=0.0066) and 15 min (87.41 ± 4.13, p=0.0010) in Group N as shown in Table 5 and Figure 5. Cases of nausea, vomiting, hypotension were more with neostigmine and shivering observed more with magnesium sulphate as shown in Table 6 and Figure 6.
| Demographic data | Group M (MgSO4) |
Group N (Neostigmine) |
P value |
|---|---|---|---|
| Age (Mean ± SD) | 48.26 ± 13.39 | 42.2 ± 13.22 | 0.0621 |
| Weight (Mean ± SD) | 59 ± 4.9 | 55 ± 6 | 0.0657 |
Table 1: Basic information of CRA and burnout under investigation.
| Group M (Mean ± SD) |
Group N (Mean ± SD) |
P value | ||
|---|---|---|---|---|
| Sensory block | Onset in minutes | 1.32 ± 0.41 | 2.19 ± 0.40 | 0.0001 |
| Motor block | Onset in minutes | 1.52 ± 0.69 | 2.85 ± 1.29 | 0.0007 |
| Duration of motor block | 103.42 ± 11.44 | 188.82 ± 14.05 | 0.0001 |
Table 2: Sensory and motor block.
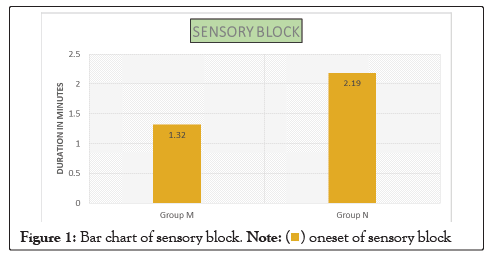
Figure 1: Bar chart of sensory block.
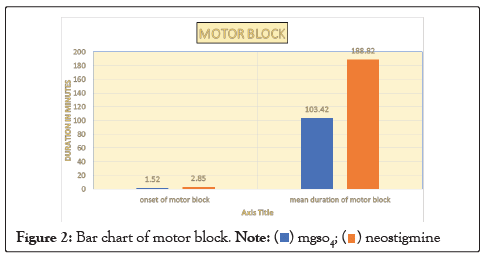
Figure 2: Bar chart of motor block.
| Group M | Group N | P value | |
|---|---|---|---|
| Total duration of analgesia in minutes (mean ± SD) | 98.4 ± 30.46 | 215.25 ± 17.49 | 0.0001 |
Table 3: Duration of analgesia.
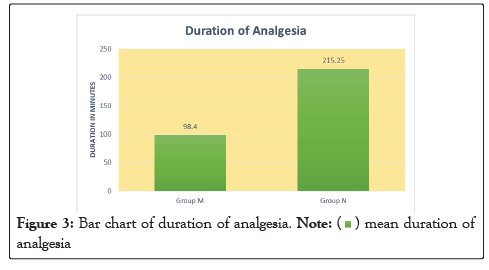
Figure 3: Bar chart of duration of analgesia.

| Group M | Group N | P value | |
|---|---|---|---|
| Pre induction | 80.45 ± 11.9 | 86.28 ± 7.21 | 0.1598 |
| 1 min | 79.65 ± 11.24 | 90.11 ± 11.40 | 0.1213 |
| 3 min | 78.40 ± 11 | 90 ± 11.61 | 0.0003 |
| 5 min | 77.42 ± 10.29 | 60.37 ± 10.09 | 0.0005 |
| 10 min | 77.88 ± 10.43 | 85.08 ± 9.03 | 0.0017 |
| 15 min | 77.14 ± 9.8 | 82.62 ± 8.18 | 0.0076 |
| 30 min | 82.11 ± 9.52 | 72.11 ± 7.1 | 0.0003 |
| 45 min | 79.14 ± 8.94 | 75.14 ± 6.46 | 0.0196 |
| 60 min | 80 ± 10.13 | 72.8 ± 6.16 | 0.0003 |
| 90 min | 76.29 ± 9.71 | 73.02 ± 5.4 | 0.0313 |
Table 4: Pulse rate variation.
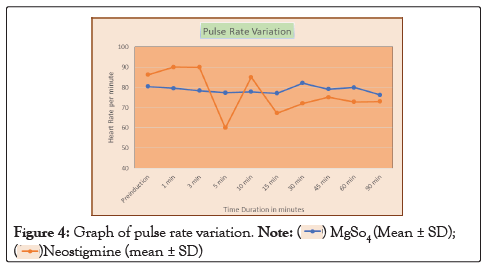
Figure 4: Graph of pulse rate variation.
| Group M (MgSO4) |
Group N (Neostigmine) |
P value | |
|---|---|---|---|
| Premedication | 95.94 ± 7.31 | 92.05 ± 5.21 | 0.4899 |
| 1 min | 94.43 ± 5.89 | 90.05 ± 6.04 | 0.1095 |
| 3 min | 92.22 ± 5.49 | 90.57 ± 5.31 | 0.2251 |
| 5 min | 90.45 ± 6.48 | 88.87 ± 4.98 | 0.1329 |
| 10 min | 90.13 ± 5.40 | 80.10 ± 4.78 | 0.0066 |
| 15 min | 90.13 ± 4.16 | 87.41 ± 4.13 | 0.0010 |
| 30 min | 90.72 ± 4.35 | 90.34 ± 3.78 | 0.3502 |
| 45 min | 89.58 ± 4.21 | 88.87 ± 2.79 | 0.2105 |
| 60 min | 89.33 ± 4.89 | 86.70 ± 4.59 | 0.294556101 |
| 90 min | 85.88 ± 15.26 | 82.72 ± 4.54 | 0.06291 |
Table 5: Mean arterial pressure variation.
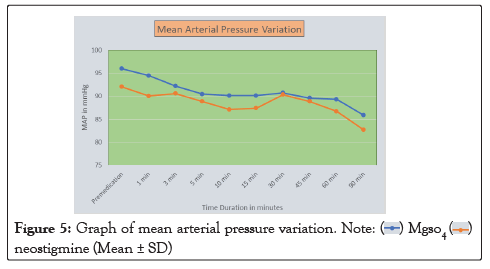
Figure 5: Graph of mean arterial pressure variation.
| Group M (MgSO4) (n =35) |
Group N (Neostigmine) (n =35) |
|
|---|---|---|
| Hypotension | 1 | 5 |
| Headache | 2 | 0 |
| Bradycardia | 0 | 7 |
| Nausea | 0 | 5 |
| Vomiting | 0 | 1 |
| Shivering | 6 | 0 |
| Dysphoria | 0 | 0 |
Table 6: Side effects.
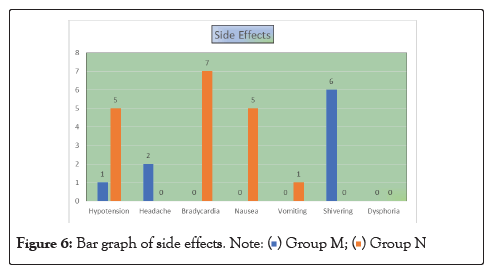
Figure 6: Bar graph of side effects.
Spinal anesthesia (SA) is commonly used anesthetic technique for lower abdominal surgery [7,8]. Larger dose of analgesic is required to provide effective analgesia when local anesthetic used without adjuvants [9,10]. Need of study of non-opioid adjuvants evolved due to significant adverse effects of Neuraxial opioids. Recent research has focused on non-opioid spinal receptors. Our study is to evaluate effectiveness, hemodynamic stability, postoperative analgesia of two non-opioid adjuvants added to intrathecal local anaesthetic. Use of NMDA receptor antagonists is emerged as significant advances in pain management in the last two decades. Magnesium sulphate is a non-competitive NMDA receptor antagonist and prevents central sensitization from peripheral nociceptive stimulation. Intrathecal magnesium sulphate (1000–2000 mg) had good motor and sensory block without neurological damage in study done by HAUBOLD and MELTZER5. Intrathecal magnesium sulphate has good safety profile at a dose less than 3 mg/kg Saxena, et al. compared intrathecal magnesium sulphate and neostigmine [11]. Both produced substantial antinociception without neurotoxicity in their study, potentiated analgesia of bupivacaine and opioids [12].
Our study results showed that onset of the sensory block is faster in group M as compared to group McPeak duration of sensory block more in Group N as compared to Group M. There was significant delay in onset of motor blockade in group duration of motor and sensory blockade was longer with neostigmine as compared to MgSo4. Chaudhry, et al., compared 50 mg and 100 mg MgSo4 as adjuvant in orthopaedic operation [13]. They found prolonged sensory and motor blockade with 100 mg without neurological side effects. So MgSo4 has better therapeutic profile. In contrast to our study, Seyed Hamid Reza Faiza, et al., studies between magnesium sulphate and neostigmine found no significant difference in onset and duration of sensory block [14].
Efficacy of spinal additives neostigmine and magnesium sulphate on characteristics of subarachnoid block compared by Sucheta [15].Prolongation of sensory block was not significantly different in both additives. Onset and duration of motor block were similar in all the groups.
Two different concentrations of neostigmine 50 and 150 microgram taken by Savita and postoperative analgesia evaluated [16]. Duration of motor block increased with higher concentration. Other characteristics were similar. Neostigmine as an intrathecal adjuvant 25 microgram prolonged only sensory block but 50-150 mics prolonged duration of motor block in study by Liu [17].
There was significant bradycardia and hypotension with additive neostigmine in our study but reversed with Inj.Glycopyrrolate 0.2 mg intravenously and fast crystalloid injection intravenously and Inj.Mephentermine 6 mg intravenously respectively. Less fluctuations in Mean Arterial Pressure with magnesium sulphate so, hemodynamic stability is better found with intrathecal Magnesium Sulphate than intrathecal neostigmine. Same results were found by Chaudhry done with magnesium Sulphate as our study, no significant hemodynamic instability [13]. Ahmad, et al., studied two different concentrations of intrathecal neostigmine 50 microgram and 150 micrograms in two groups respectively [18]. They observed less hemodynamic instability in both groups. Higher dose produced prolonged sensory and motor block. Nausea was more with high dose of neostigmine. Similar results found by Faiz, et al., study between magnesium sulphate and neostigmine an adjuvant with Inj.Bupivacaine Heavy (0.5%) in lower extremity surgeries [14].
Contrast results observed in rats studied by Pan, et al., of Intrathecal Neostigmine, Bupivacaine, and Their Combination on Sympathetic Nerve Activity [19]. They found that intrathecal injection of neostigmine increased blood pressure in rats because of increase in splanchnic nerve activity.
Three different concentrations 25,50 and 75 microgram studied by Lauretti et al., patients of vaginal hysterectomy [20]. Adverse effects (nausea) observed only with 75 micrograms but good analgesia in less concentration than 50 micrograms.
Liu did study on 6.25 to 50 micrograms of neostigmine [17]. Among these, they found 50 micrograms significantly increased the duration of sensory and motor block. In contrast to our study, good hemodynamic stability achieved in their results with dose dependent side effects.
Our study proved effectiveness of analgesia was good with neostigmine. More duration of analgesia with neostigmine than magnesium achieved. Similar results found with study by S. Gupta et al. done in 2009 with neostigmine of 50 and 75 microgram and Khadke, et al., neostigmine used as adjuvant intrathecally [21]. But, Saini, et al., observed greatly enhanced analgesia by intrathecal neostigmine in the 150 μg dose and less consumption of rescue analgesic but ineffective with 50 microgram concentration [16]. Similar results found with study on 50,75 and 150 micrograms. Good analgesia and fewer side effects with 75 micrograms by Vandana Pandey [22].
Our study showed more incidence of nausea and vomiting with neostigmine and incidences of shivering seen with Magnesium Sulphate [23]. In contrast to that, study done with caesarean patients for ant shivering effect of Magnesium Sulphate intrathecal by Faiz, et al., [24] .They observed less incidence of shivering with Magnesium sulphate 25 mg than normal saline as adjuvant.
In contrast to my study, Suchita Khadke et al., Low incidence of hypotension with neostigmine [16]. Other parameters were similar to our study. Similar findings seen by Vasantha et al., with 50 microgram neostigmine [25]. They understood hypotension secondary to α2-agonist can be prevented by stimulation of M2 spinal muscarinic cholinergic receptors and nitric oxide synthesis. Similar hemodynamic outcomes seen when Gupta et al., compared 50 and 75 micrograms neostigmine in 2009 [22]. In contrast to our study, no significant hemodynamic changes seen when Lauretta, et al., studied subarachnoid neostigmine in randomly allocated abdominal hysterectomy patients in 1996 [26].
Opposite results found by Angelo, et al., in United States. There was severe nausea but no prolongation of analgesia found [26].
Two different doses of magnesium sulphate were studied by Chaudhry, et al., [14]. Opposite results about effectiveness of analgesia and adverse effects observed to our study.
As per our study results, delayed onset and longer duration of motor and sensory block were observed with intrathecal neostigmine as compared to intrathecal magnesium sulphate. In context of hemodynamic profile, lesser pulse rate variation and lesser hypotension were observed with magnesium sulphate as compared to neostigmine methyl sulphate. Significantly longer duration of analgesia was provided with neostigmine as compared to magnesium sulphate but with greater adverse effects as compared to magnesium sulphate.
Cost effectiveness not analysed in this study. We cannot cover larger samples and all types of surgeries. Study does not cover every age group people, high risk patients.
[Crossref][Google Scholar][Pubmed].
[Crossref][Google Scholar][Pubmed].
[Crossref][Google Scholar][Pubmed].
[Crossref][Google Scholar][Pubmed].
[Crossref][Google Scholar][Pubmed].
[Crossref][Google Scholar][Pubmed].
[Google Scholar][Pubmed].
[Crossref][Google Scholar][Pubmed].
[Crossref][Google Scholar][Pubmed].
[Crossref][Google Scholar][Pubmed].
[Crossref][Google Scholar][Pubmed].
[Crossref][Google Scholar][Pubmed].
[Crossref][Google Scholar][Pubmed].
Citation: Upadhyay DY, Trivedi V (2022) Comparison of Effectiveness of Intrathecal Magnesium Sulphate 100 mg plus Inj.Bupivacaine Heavy 0.5% 15 mg Versus Intrathecal Neostigmine 75 Microgram Plus Inj.Bupivacaine Heavy 0.5% 15 mg in Unilateral Inguinal Hernia. J AnesthClin. Res. 13:1049.
Received: 28-Nov-2022, Manuscript No. JACR-22-16287; Editor assigned: 01-Dec-2022, Pre QC No. JACR-22-16287 (PQ); Reviewed: 16-Dec-2022, QC No. JACR-22-16287; Revised: 23-Dec-2022, Manuscript No. JACR-22-16287 (R); Accepted: 30-Dec-2023 Published: 31-Dec-2022 , DOI: 10.35248/2155-6148.22.13.1049
Copyright: © 2022 Upadhyay DY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.