Enzyme Engineering
Open Access
ISSN: 2329-6674
ISSN: 2329-6674
Research Article - (2022)Volume 11, Issue 1
Biofuels are gaining popularity as a viable alternative to petroleum-based energy. Second and third-generation advanced biofuels, which can be made from renewable materials like agricultural waste, appear to be more competitive. Even though there was a minimum concentration of reducing sugars and high-value waste in the existing solutions. Therefore, the proposed research focused on the bioethanol feedstock potential of Potato Peel Waste (PPW), a zerovalue waste. Where, starch, cellulose, hemicellulose, and fermentable sugars are all present in sufficient amounts in PPW. On the other hand, bioethanol made from potato peel waste has a lot of market potential. Consequently, the policy goal is to ensure that a minimum amount of biofuel is readily available in the market at all times to meet demand. To yield large quantities of reducing sugars, a combination of enzymatic treatment with amylase production by Bacillus sp. UEB-S and Amyl glucosidase is used. For effective hydrolysis of PPW liquefaction, termamyl 120 L was utilised, and amyloglucosidase was used for saccharification. Furthermore, hydrolysis of starch from fresh potato tubers by HCl and H2SO4 is used to boost the activity of hydrogen ions participating in the reaction as catalyst. Hence, H2SO4 was more efficient to hydrolyse potato peel than HCl. Also, the supernatants were tested for reducing sugar using the 3,5-dinitrosalicylic acid (DNS) technique. As a result, the production of biofuel from potato peel waste as feedstock by different enzymes achieves commercial bioethanol production at the lowest possible cost, also reducing waste by-products.
Enzymatic hydrolysis; Fermentation; Bioethanol; Potato peel waste
In recent years, more research and development efforts have been focused on minimizing the usage of fossil fuels and lowering carbon dioxide emissions. With the world energy supply inevitably depleting, there has been a surge in interest in alternative energy sources around the world [1]. Where, bioethanol has been identified as the most promising biofuel derived from renewable resources, prompting increased research and development activities. Sugarcane, maize, wheat, and barley are the most common sources of bioethanol. However, the production of bioethanol from these crops competes with their usage as food sources [2-5]. This stage is usually carried out at high temperatures (80-110°C). The partially hydrolyzed starch glucoamylase is saccharified in the second stage, yielding a somewhat clean glucose stream that is subsequently fermented to ethanol by Saccharomyces yeasts [6]. Also, the traditional bioethanol manufacturing technique necessitates a hydrolysis phase to obtain fermentable sugars. As a result, an acid hydrolysis or enzymatic hydrolysis is required [7]. Acid hydrolysis procedures necessitate extreme conditions such as high temperature and low pH. Such circumstances necessitate the use of costly corrosiveresistant equipment. However, enzymatic processes carried out under less severe reaction circumstances can convert all biomass to glucose due to the great specificity of enzyme reactions [8-10]. Enzymatic hydrolysis of starchy feedstock occurs in three steps. Liquefaction is the initial phase, followed by saccharification. Fermentation is the final stage. Amylases hydrolyze the chemical bond (1-4) of starch to produce maltodextrins and maltose in the first step of liquefaction [11,12]. Whereas, the possibility of hydrolyzing starch at low temperatures was examined to cut costs and avoid large energy usage. The use of renewable resources, particularly agricultural and industrial by-products, is given considerable focus. Sugarcane bagasse and rice straw are just two examples of renewable resources. Potatoes are primarily consumed raw, but they can also be processed into several items, including mashed potatoes, chips, fries, and dehydrated foods. 20-50 percent of the raw product is discharged during processing, primarily as peels, posing a serious disposal concern for the potato sector. Figure 1 shows that peel contain enough starch, cellulose, hemicellulose, lignin, and fermentable sugars to be used as an ethanol feedstock. As a result, there is a need to develop a framework that should achieve commercial bioethanol production at the lowest possible cost by using potato peel waste as a feedstock and other enzymes. The contribution of this paper,
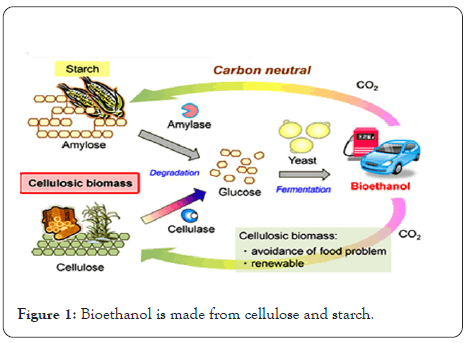
Figure 1: Bioethanol is made from cellulose and starch.
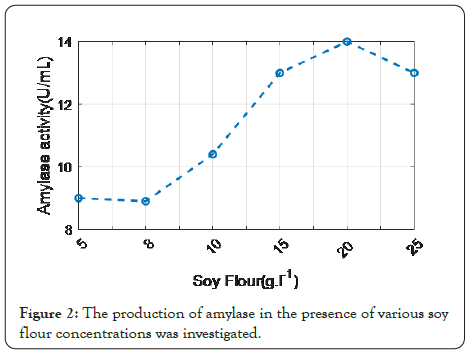
Figure 2: The production of amylase in the presence of various soy flour concentrations was investigated.
• To investigate the effect of different concentrations of sulphuric acid and hydrochloric acid on PPW hydrolysis was examined.
• To reduce sugar released after enzymatic hydrolysis by termamyl 120 L at different concentrations.
• To demonstrate that PPW, a potato industry by-product, could be used efficiently for ethanol production while also reducing waste by-products.
The remaining of the paper has been organized as follows: Section 2 presents the recent literatures; section 3 depicts the detailed description of the proposed methodology; section 4 discusses the implementation results; finally, section 5 concludes the paper.
Literature survey
Every year, an estimated 1.3 billion tonnes of food are produced worldwide, with about a million tonnes of food going to waste due to issues such as a faulty food supply chain, insufficient storage, and transportation delays.
The concept of Zero Waste is a practical strategy to combat food waste by repurposing or recycling whole foods or by-products. The notion of Zero Waste is currently gaining popularity around the world. When food is no longer edible, it should be disposed of using a waste hierarchy, such as composting, biofuel, or other methods.
Obtaining high-quality pellets, however, is challenging, according to many researchers, since it is controlled by various aspects like as density, humidity, calorific value, and hazardous emissions. All of these indicators must adhere to a set of criteria and guidelines.
In During the preparation of potatoes, a lot of trash is produced. About 35–46 percent of the recycled mass is wasted in the production of potato products. The volume of potato pulp, the principal by-product of potato starch production, has been estimated to exceed 1 million tonnes per year in Europe.
The high level of potato, the pulp can be used as a pre-pelleting binder ingredient for plant materials with a lesser sensitivity to densification. Potato pulp and combinations of potato pulp with other plant waste were researched by researchers, who discovered that the pellets have excellent energy characteristics. Potato pulp pellets have a heat of combustion of 16.33 MJ kg1 in dry matter and a calorific value of 15.41 MJ kg1.
Physical and chemical indications must be examined when producing novel solid biofuels. These parameters are critical for biofuel transportation, storage, and burning. Before the granulation process, the material should be prepared by cutting and grinding to obtain the finer fraction of the flour, as the density of biofuels is determined by the flour quality striving for a compromise between heat combustion and harmful emissions while producing novel biofuels (liquid or solid). This compromise pushes researchers to hunt for fuels with the needed calorific value while emitting fewer pollutants.
Traditionally, potato peel waste has been utilized to make lowvalue animal feed, fertiliser, or the raw material for biogas, resulting in the waste of copious nutritional components with antioxidant, antibacterial, apoptotic, chemo preventive and antiinflammatory characteristics. However, the core dogma of this research is proper waste management in conjunction with the economic feasibility of processing development.
Deal with industrial potato peel waste and promote its nutritional and industrial applications Potato peel waste can be converted into bio-fuels, dietary fiber, bio fertilizer, biogas, bio sorbent, antioxidants, and food additives by a variety of processes such as fermentation, extraction, and other treatments. Waste potatoes are used as a raw material in the manufacturing of potatobased bioethanol. Potato cultivation produces waste potatoes as a by-product. Waste potatoes are of sufficient quality for food production, but their size is inappropriate. A lot of solid potato mash is produced in the food potato sector, which can be used as a raw material in bio-ethanol production.
From the survey, estimated 1.3 billion tonnes of food are produced each year, with approximately a million tonnes of food going to waste. Where food is no longer edible, it should be disposed of according to a waste hierarchy, which includes composting, biofuel production, and recycling. During the preparation of potatoes, a large amount of waste is generated. In the manufacture of potato goods, between 35-46 percent of the recycled material is wasted. Because the density of biofuels is governed by the flour quality, cutting and grinding to acquire the finer fraction of the flour is necessary to achieve a balance between heat combustion and harmful emissions. Still, there is a lack of research for the production of biofuel from potato peel waste. To compromise this motivation, a novel security method needs to be developed.
The proposed methods will contribute to the production of biofuel from potato peel waste as feedstock by different enzymes. After that, it developed a methodology that chooses different datasets, which greatly reduces the possible cost, also reducing waste by-products. The next section explains the techniques and benefits of the algorithms in the proposed method.
In substrate preparation, PPW (Potato Peel Waste) was obtained from a local market. It was dried for 48 hours at 50 degrees Celsius, ground, and sieved with a particle size of 500 to 100 microns. It was kept at an ambient temperature (between 25 and 50 degrees Celsius). Also, the contents of total nitrogen, lipid, moisture, and ash in potatoes were determined using the American Association of Cereal Chemists standard procedures, respectively. To determine the starch content, the enzymatic calorimetric technique was used. Furthermore, the high starch content of this waste can encourage its use as a feedstock for ethanol production, but the low fermentable reducing sugar content 0.32 percent 013 percent) makes raw material fermentation impractical, necessitating initial hydrolysis (acidic or enzymatic) of carbohydrates.
For liquefaction, termamyl 120 L was utilized, and amyloglucosidase was used for saccharification, which was supplied by Sigma-Aldrich. The activity of UEB-S amylase was measured and its activity peaked at pH 5 and 700°C. Where 100 mL distilled water was mixed with dry potato peel waste (1.25- 17.5%). At pH 7, the mixture was treated with Ternamyl 120 L (15 U, 30 U, or 45 U), followed by amyloglucosidase (3 U, 6 U, or 9 U) at pH 4.5. At various periods, samples were taken out of the process. These samples were heated for 5 minutes at 100°C, cooled, then centrifuged for 10 minutes at 8000 rpm. These reactions were carried out with 15% PPW and at three distinct enzyme concentrations: 45, 90, and 135 U. At 70°C and pH 5, the reaction was incubated. Also, the saccharification stage was carried out for 24 hours at 600°C, pH 4.5, with 9 units of amyloglucosidase. By using the 3, 5-dinitrosalicylic acid (DNS) technique, the supernatants were tested for reducing sugar. Whereby, replacing Termamyl 120 L with amylase UEB-S, PPW hydrolysis was also achieved. Therefore, the reaction was carried out with 15% PPW and varied enzyme concentrations of 45, 90, and 135 units. At 70°C and pH 5, the reaction was incubated and 9 units of amyloglucosidase were used in the saccharification stage, which took 24 hours at 600°C and pH 4.5. 20-50 percent of the raw product is discharged during the processing process, primarily as peels, posing a serious disposal problem for the potato industry. As demonstrated in Figure 1, peels contain adequate amounts of starch, cellulose, hemicellulose, lignin, and fermentable sugars to be used as an ethanol feedstock (Figure 1).
In the proposed framework, potato peel, a zero-value waste, has been used to make ethanol in research. As a result, a comparison study was conducted to evaluate the effects of acid hydrolysis and various enzymes on the effective transformation of potato peels as a feedstock for bioethanol production. Finally, a comparative study for the production of biofuel from Potato Peel Waste as feedstock by different enzymes is described in the subsequent subsections.
Fermentation of PPW hydrolysates with ethanol
Ethanol is made in two ways,the first is the synthesis of ethanol petroleum. The other is microbial carbohydrate fermentation, which requires the presence of fermentable sugars (especially five-carbon and six-carbon sugars). Consequently, there are three major processes in the synthesis of ethanol utilizing microbial fermentation. The first step is to create a fermentable sugar solution. The second stage involves fermenting the carbohydrates in the solution to produce ethanol under particular microbiological conditions. The next stage is to separate and purify the ethanol from the solution, which is commonly accomplished using the distillation procedure. The following equation depicts the general conversion of carbohydrates to ethanol by anaerobic microbial fermentation:1 glucose →2 CO2+2 ATP+2 Ethanol
Bioethanol is a great biofuel alternative to fossil fuels, whether it's used as a pure fuel with high efficiency or combined with gasoline, or as a gasoline additive.
In 250 mL Erlenmeyer flasks, ethanol fermentation was carried out. 1% yeast extract and 2% peptone were added to the hydrolysates. They were autoclaved for 20 minutes at 120°C. After cooling to room temperature, they were fermented with ethanol by a 20-hour-old Saccharomyces cerevisiae culture in a 250 ml Erlenmeyer flask under agitated conditions (pH 5.0, 300°C, 100 rpm). Then the supernatants were then centrifuged and analyzed for reducing sugar using the 3,5-dinitrosalicylic acid (DNS) method and ethanol concentration using the Diagnostic System International ethanol FS kit. The following formula is used to compute the percent yield:
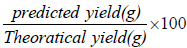
When the substrate is pure glucose, the maximal theoretical yield corresponds to the predicted yield of ethanol. Ethanol has a theoretical yield of 0.51 ethanol/kg glucose. The next section explains the production of amylase by Bacillus sp. UEB-S.
Development of a cost-effective medium for amylase production by Bacillus sp. UEB-S: The ability of Bacillus sp. UEB-S to degrade potato peel and create amylase activity was investigated. As a result, various concentrations of potato peel were tested. Potato peel at a concentration of 10 g L-1 produced the highest enzyme activity (9.75 U/ml). There was a decrease in amylase activity above this concentration. Different nitrogen sources were evaluated at a concentration of 10 g L-1 to boost amylase activity, and the results showed that soy flour improved amylase activity to 11 U/ml. Increasing the percentage of soy flour, while employing a concentration of 20 g L-1 soy flour, amylase production improved and reached a maximum level (15.64 U/ml). Bacillus sp. UEB-S was able to produce amylase in a medium containing 10 g L-1 PPW and 20 g L-1 soy flour are shown in Figure 3.
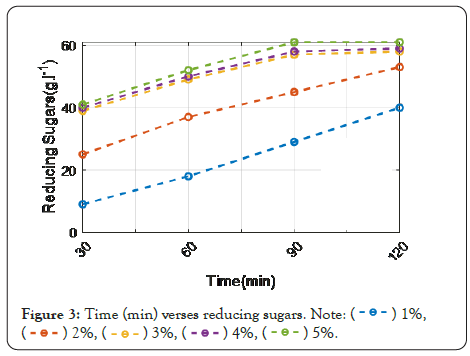
Figure 3: Time (min) verses reducing sugars.
The tested concentration is 1%-5%. In the instance of H2SO4, glucose yield was raised at 5% concentration until 90 minutes, when the maximum yield of sugars was reached (65 g L-1). There was no substantial rise in hydrolysis after this period. This shows that after 90 minutes at 90°C, full hydrolysis of starch was achieved using 5% H2SO4. Using a 5% acid concentration for 120 minutes, a high yield of glucose (62 g L-1) was achieved via HCl hydrolysis. This yield is slightly lower than when H2SO4 was used. As a result, H2SO4 at a concentration of 5% was chosen to hydrolyze PPW at 90°C for 120 minutes. During PPW hydrolysate fermentation, the time course profile of ethanol production (g L-1) is shown in Figure 4. The next section explains the results obtain from the potato peel Waste as feedstock by different enzymes is discusses it in detail.
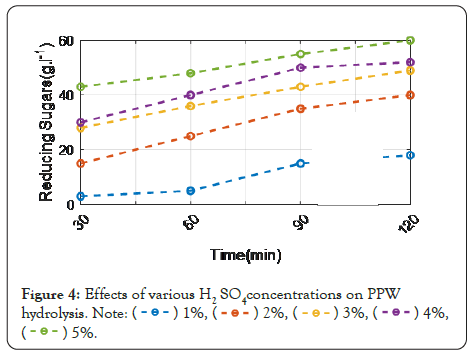
Figure 4: Effects of various H2 SO4concentrations on PPW hydrolysis.
This segment provides a detailed description of the implementation results as well as the performance of the proposed system and a comparison section to ensure that the proposed system performs valuable.
Results obtained from each methodology
The data used as an input and output of various processes carried out for the production of biofuel from potato peel waste as feedstock by different enzymes are provided and explain the methodology in a detailed manner.
Analysis of potato peel waste: Potato peel waste contained proteins(16 ± 0.85 percent, w/w), fat (0.54 ± 0.10 percent, w/w), moisture (6.79 ± 0.20 percent, w/w),starch (48.32 ± 1.90 percent), and ashes (7.1 ± 0.31 percent, w/w) are shown in Table 1. Compared to the existing techniques, the proposed method's proteins, ashes, and lipids were all higher.
| Parameters | Dry weight (%) |
|---|---|
| Moisture | 6.79 ± 0.20 |
| Reducing Sugar | 0.31 ± 013 |
| Starch | 48.32 ± 1.90 |
| Nitrogen | 0.32 ± 014 |
| Protein | 16 ± 0.85 |
| Fat | 0.54 ± 0.10 |
| Ash | 7.1 ± 0.31 |
| Fibre | 26.52 ± 0.23 |
Table 1: Potato Peel Waste (PPW) chemical composition.
Thus, PPW might be thought of as a rich medium containing all of the essential ingredients for microbe growth and amylase synthesis. Furthermore, the waste's high starch content may encourage its use as a feedstock for ethanol production, but the low fermentable reducing sugar content (0.32%, 0.14%) makes fermentation of the raw material impractical, necessitating an initial step of carbohydrate hydrolysis (acidic or enzymatic) (Figure 2).
Figure 2 shows that, by increasing the percentage of soy flour while employing a concentration of 20 g L-1 soy flour, amylase production improved and reached a maximum level (15.64 U/ ml). Bacillus sp. UEB-S was able to produce amylase in a medium containing 10 g L-1 PPW and 20 g L-1 soy flour.
Production of ethanol from PPW: In Acid hydrolysis, the effect of varying amounts of sulphuric acid and hydrochloric acid on PPW hydrolysis was investigated in the proposed method (Figures 3-12).
Figure 3 shows the effect of various hydrochloric acid (HCI) concentrations on PPW hydrolysis. The tested concentration is 1%, 2%, 3%, 4%, 5%. Whereby by increasing the time, the reduced sugar is at its maximum. Compared to the 1% level of concentration, 5% of HCI concentration gets increased.
Figures 3 and 4 indicates that H2SO4 was more efficient than HCl at hydrolyzing potato peel at a low concentration (1%). An increase in sugar production was obtained by increasing the concentration of the acids at a fixed period of heating, and the greatest glucose yields were obtained at a concentration of 5%. These findings are reinforced by the hydrolysis of starch from fresh potato tubers using HCl and H2SO4 and found that the rate of hydrolysis increased with the acid concentration. This is most likely owing to an increase in the activity of hydrogen ions acting as catalysts in the reaction.
Figure 5 shows that the reducing sugar produced after enzymatic hydrolysis by termamyl 120 L at various doses. The results showed that the higher the enzyme concentration, the bigger the amount of fermentable reducing sugar. After 60 minutes of incubation with termamyl 120 L (45U), 14.16 g L-1 reducing sugar was released.
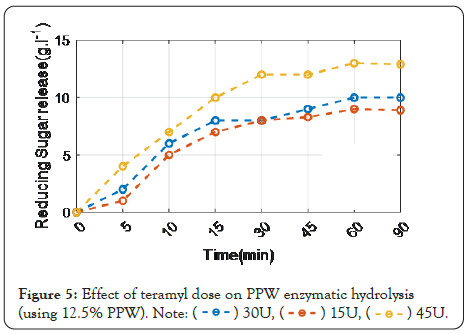
Figure 5: Effect of teramyl dose on PPW enzymatic hydrolysis (using 12.5% PPW).
Figure 6 shows that the liquefaction stage alone was insufficient, necessitating the addition of a saccharification stage. As a result, a two-enzyme combination (termamyl and amyloglucosidase) was required for effective PPW hydrolysis. The release of reducing sugars was measured using 45 U of termamyl 120 L in combination with different doses of amyloglucosidase.
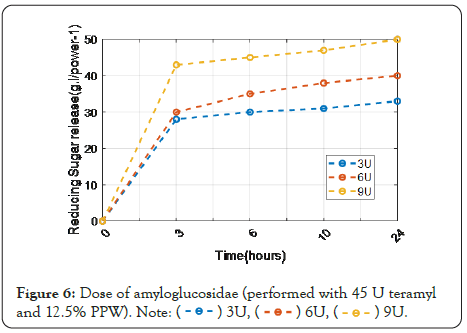
Figure 6: Dose of amyloglucosidae (performed with 45 U teramyl and 12.5% PPW).
Figure 7 shows that the enzyme combination (termamyl 120 L 45 U and amyloglucosidase 9 U) produced the maximum release sugar (51 g L-1). There is no substantial increase in reducing sugars with higher enzyme dosages. As a result, this combination was kept to get the greatest PPW concentration for optimal lowering sugar release. Various concentrations (ranging from 1.25 percent to 17.5 percent) were investigated.
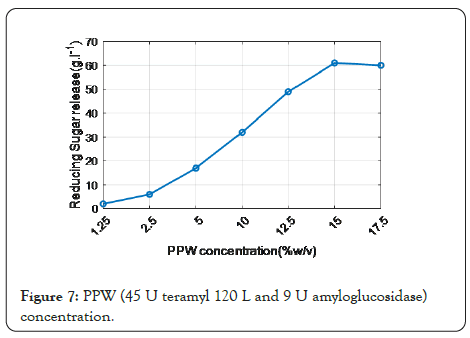
Figure 7: PPW (45 U teramyl 120 L and 9 U amyloglucosidase) concentration.
Figure 8 shows that the findings revealed that a little amount of reducing sugars was released at low PPW concentrations, but that raising this concentration resulted in a higher fermentable reducing sugar content. The most effective concentration was 15%, which yielded 63 g L-1 of reducing sugars. The possibility of hydrolyzing starch at a lower temperature was studied by swapping UEB-S amylase for termamyl 120 L amylase in the hopes of lowering the cost of production. In comparison to acidic hydrolysis, enzymatic hydrolysis of PPW liberates a greater amount of fermentable reducing sugar.
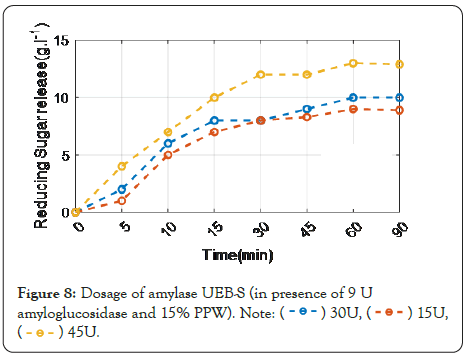
Figure 8: Dosage of amylase UEB-S (in presence of 9 U amyloglucosidase and 15% PPW).
The best ethanol productions were attained with a 6% hydrolysate concentration, as shown in Figure 9, however, it appears evident that the combinations UEB-S/amyloglucosidase produced 21 g L-1 of ethanol vs. 19.6 g L-1 for the teramyl 120 L/ amyloglucosidase combination.
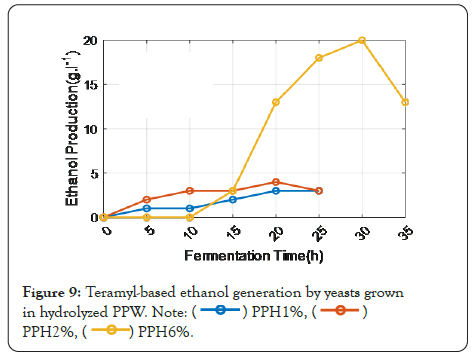
Figure 9: Teramyl-based ethanol generation by yeasts grown in hydrolyzed PPW.
Figure 10 shows that the graph is plotted between fermentation time and ethanol production. Compared to PPH 1% and PPH 2%, PPH 6% ethanol production is at its maximum. Consequently, hydrothermal pre-treatment of PPW resulted in a larger release of reducing sugars than acid or alkali pre-treatment. Also, the nitrogen content of hydrothermally pre-treated PPW hydrolysates supplemented with ammonium sulphate as a nitrogen source was increased by reducing sugars and ethanol.
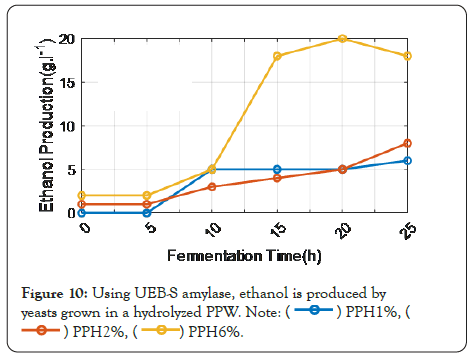
Figure 10: Using UEB-S amylase, ethanol is produced by yeasts grown in a hydrolyzed PPW.
The impact of shaft speed on the throughput capacity of potatoes is highly increased compared to the existing technique (yam, cocoyam, cassava) (Figure 11).
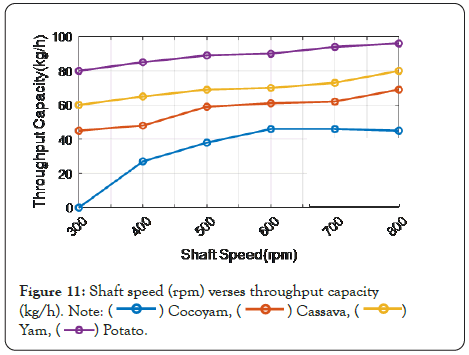
Figure 11: Shaft speed (rpm) verses throughput capacity (kg/h).
Figure 12 depicts the comparative analysis of the product for Ethanol production with the existing technique. The ethanol production of the proposed technique achieves 2 g . 1−1 (-1), which is 1 g . 1−1 (-1), higher than the cocoyam, 1.3 g . 1−1 (-1), higher than the yam, and 0.7 g . 1−1 (-1), higher than cassava.
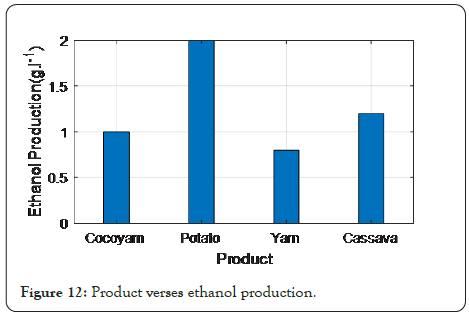
Figure 12: Product verses ethanol production.
The proposed method reveals that, following adequate hydrolysis, potato peel waste can be used as a cost-effective source of amylase and a feedstock for bioethanol production. The reducing sugar concentrations produced during PPW hydrolysis using termamyl 120 L, amylase UEB-S, and H2SO4 (varying between 63 anfd 69 g L1) were similar. Furthermore, after fermentation of the sugars produced during hydrolysis with termamyl 120 L and amylase UEB-S, substantially equivalent ethanol concentrations were achieved. The result of the proposed method shows that PPW, a by-product of the potato industry that has been processed with a locally made enzyme, has a high potential for ethanol production.
[Cross Ref], [Google Scholar].
[Cross Ref], [Google Scholar].
[Cross Ref], [Google Scholar].
[Cross Ref], [Google Scholar].
[Cross Ref], [Google Scholar].
[Cross Ref], [Google Scholar].
[Cross Ref], [Google Scholar].
[Cross Ref], [Google Scholar].
[Cross Ref], [Google Scholar].
[Cross Ref], [Google Scholar].
Citation: Deshmukh M, Pande A (2022) Comparative Study for Production of Biofuel from Potato Peel Waste as Feedstock by Different Enzymes. Enz Eng. 11: 175
Received: 07-Jan-2022, Manuscript No. EEG-22-46419; Editor assigned: 10-Jan-2022, Pre QC No. EEG-22-46419 (PQ); Reviewed: 24-Jan-2022, QC No. EEG-22-46419; Revised: 28-Jan-2022, Manuscript No. EEG-22-46419 (R); Published: 04-Feb-2022 , DOI: 10.35841/2329-6674.22.11.1000175
Copyright: © 2022 Deshmukh M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.