
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Research Article - (2020)Volume 11, Issue 1
Introduction: Contused lungs threaten life of the polytrauma patients. Accumulation of fluid in the lungs markedly reduces oxygenation and facilitates development of Ventilator associated pneumonia (VAP). VAP is a subtype of health care acquired pneumonia with high mortality rate (45%). Antibiotics are considered as the corner stone of management in treatment of VAP.
Aim: To evaluate and compare the effect of use of combination of meropenem/gentamicin versus the use of ceftazidime/avibactam in the treatment of ARDS induced by both VAP and lung contusion and early weaning from the ventilator and compare their effect on the mortality rate.
Patients and methods: Prospective double blind study conducted on 200 patients of King Abdul-Aziz Specialized Hospital. Patients were allocated in two groups: Group A included 100 patients received meropenem/ gentamicin while Group B included another 100 patients received ceftazidime/avibactam. The duration of the study was 14 days. In this study, diagnosis of lung contusion confirmed by computerized axial tomography (CT) of the chest, while VAP Diagnosed by modified clinical pulmonary infection scores (CPIS).
Results: 15, 38 and 46 patients in group A showed controlled tracheal secretion respectively in 3 studied period compared to 28, 75 and 83 in group B, less parenchymatous lung infiltration in the chest X-ray 12, 40 and 48 patients in group A compared to 24, 88 and 91 patients in group B, improvement of the hypoxic index 48, 76 and 85 patients in group A compared to 66, 90 and 98 patients in B, normalization of temperature 16, 36 and 54 patients in group A, while 40, 76 and 90 patients in B and reduction of total Leucocytic count 18, 35 and 57 patients in group A, while 38, 70 and 87 patients in group B, there was 15 out of 98 patients in group A not weaned while only 5out of 100 patients in group B cases failed to be weaned from mechanical ventilation within the studied period (2 weeks).
Conclusion: The use of ceftazidime/avibactam more rapidly control all parameters of CPIS and provide faster weaning from the ventilator with non-significant lower mortality rate than meropenem/gentamicin.
Ceftazidime; Clinical pulmonary infection score; Pneumonia
Severe contused lungs considered a major challenge in the critical areas. The acute and severe reduction in the tissue oxygenation due to high fluid accumulation in lungs jeopardies both local lung immunity and systemic immunity of the patients and facilitate superadded infection (VAP) [1-3]. The pertinacious material from the exudative phase of traumatic inflammation and minor hematomas create an excellent media for bacterial growth and development of VAP. Ventilator associated pneumonia (VAP) which occurs in such patients characterized by being rapidly developed (in only 48 hours from ICU admission) and resistant to conventional antibiotics with difficult weaning from the ventilator and higher mortality [4-6]. Hospital mortality of ventilated patients who developed VAP is 46% [6]. The art of choosing the best antibiotic combination that can cover the most common pathogen together with rapid emerging of resistant bacterial strains that cause this fatal type of pneumonia is still a point of research.
Bacteriologically, the most common pathogen causing VAP is the gram negative micro-organisms especially Klebsiella pneumoniae , Enterobacter cloacae , Escherichia coli, Serratia marcescens , Proteus mirabilis, Pseudomonas aeruginosa and Haemophilus influenza [7-9]. All those pathogen are responsible for more than 65% of VAP cases. Unfortunately 37% from the positive Klebsiella pneumonia cultures were carbapenem-resistant, 30% from the positive Pseudomonas aeruginosa were carbapenem-resistant, 28% from other Enterobacteriaceae culture were also carbapenem-resistant and all those pathogen were ceftazidime non-susceptible [9]. The increasing percent of those resistant bacteria to the conventional antibiotics emerge the need of new antibiotic combination to control these fatal types of pneumonias [10]. Meropenem and gentamicin enjoyed long history of being safe trustable drug in treatment of ARDS (adult respiratory distress syndrome) due to VAP and has become one of most common conventional antibiotics combination in this field.
Yet the emerge of new gram negative strain resistant to Meropenem with an increasing percent in intensive care units all over the world put the Intensivist in challenge and mandate searching for new antibiotics combination [11]. Ceftazidime inhibits bacterial peptidoglycan cell wall synthesis following binding to penicillin binding proteins (PBPs), which leads to bacterial cell lysis and death. Avibactam is a non β- lactam, β-lactamase inhibitor that acts by forming a covalent adduct with the enzyme that is stable to hydrolysis. It inhibits both Ambler class A and class C β-lactamases and some class D enzymes, including extended-spectrum β-lactamases (ESBLs), KPC and OXA-48 carbapenemases and AmpC enzymes. Avibactam does not inhibit class B enzymes (metallo-β-lactamases) and is not able to inhibit many class D enzymes [12,13]. This unique microbiological profile covers most carbapenem-non-susceptible Enterobacteriaceae and multidrugresistant P aeruginosa (excluding metallo-β-lactamase producers), and thus ceftazidime-avibactam is a potential alternative to carbapenems for the treatment of serious Gram-negative infections, including those caused by some carbapenemase-producing bacteria [14].
Clinically, the diagnosis of VAP can be done by application of modified clinical-pulmonary infection score (CPIS). VAP is diagnosed by CPIS score 6 or more [15]. The rapid control of both the systemic and local signs of VAP is considered the corner stone in the management of VAP even before complete bacteriological cure. The delay of starting the proper antibiotics combination in such cases carry high percent of morbidity in the form of difficult or failure of weaning from the ventilator and high mortality rate due to septic shock and multi-organ failure [16]. All strategies of chemotherapy combinations try to achieve good control of VAP as soon as possible to shorten the duration of mechanical ventilation and stop septic inflammation which delays the healing of the contused lung [17].
Aim
To evaluate and compare the effect of use of combination of meropenem/gentamicin versus the use of ceftazidime/avibactam in the treatment of ARDS induced by both VAP and lung contusion and early weaning from the ventilator and compare their effect on the mortality rate.
This was a prospective double blind study conducted on 200 polytrauma patients admitted to King Abdul-Aziz Specialized Hospital, Taif, KSA between July 2018 and December 2019 in surgical ICU. All patients were having severe chest trauma, contused lungs either with or without severe head trauma. King Abdul-Aziz Specialized Hospital research and ethical committee approved the project and a written consent for all the patients was taken from the first degree relatives of the patients.
Inclusion criteria
• Age group between 18-50 years.
• Computerized tomography (CT) chest is considered the only diagnostic tool for lung contusion.
• Intubated and ventilated patients due to respiratory failure from severe lung contusion were enrolled into the study once admitted from the ER and all of them evaluated by the parameter of CPIS every 5 days for 14 days.
• Respiratory failure was diagnosed by arterial blood gases (ABG) with partial pressure of oxygen (PaO) ≤ 60 mmHg and/or partial pressure of carbon dioxide (PaCO) ≤ 60 mmHg.
• Any patient with Glasgow Coma Scale (GCS) more than 8 from head trauma was intubated and included in our study only if he had severe lung contusion diagnosed by CT chest and had the previous blood gases.
All patients enrolled in this study in both groups were diagnosed first as having lung contusion by CT chest and evaluated after 2 days from intubation and mechanical ventilation for development of VAP by modified clinical pulmonary infection score (CPIS).Those that get a score of 6 or more were eligible to enter into our study.
Exclusion criteria
• Age less than 18 years or more than 50 years.
• Any patient with renal impairment.
• Any patient with acquired or congenital immunodeficiency syndrome.
• Any patient with ischemic or congenital heart disease.
• Any patient receiving corticosteroid treatment.
• Pregnant or lactating females.
Routine survey was done to all patients according to our hospital policy in the form of Chest X-ray, Abdominal ultrasound, and CT brain as a routine in our hospital policy. Full laboratory work (CBC, complete chemistry and coagulation profile). All patients were intubated in ER or in our surgical ICU, then put on full ventilatory support and were sedated with intravenous analgesia using fentanyl 50-100 μg/hour and intravenous sedation using midazolam 5 mg/hour till we achieve a Ramsay score of 2-3. All the patients were randomly allocated in two groups (Group A and Group B).Randomization sequence was created using Excel 2007 (Microsoft, Redmond, WA,USA) with a 1:1 allocation using random block sizes of 2 and 4 by an independent doctor. In this way, sequence generation and type of randomization can be expressed at the same time. Each group included 100 patients.
Patients of Group A received meropenem one gram slowly IV infusion every 8 hours for 14 days and gentamicin 7 milligram (mg)/ Kilogram body weight per day slowly IV infusion once daily only for one week, while patients of Group B received ceftazidime 2 grams and avibactam 500 mg every 8 hours for 14 days in solution for injection(sodium chloride 9 mg/mL (0.9%), sodium chloride 4.5 mg/mL, dextrose 25 mg/mL, 0.45% sodium chloride, 2.5% dextrose and/or Lactated Ringer’s solution). The solution for injection should be administered over 120 minutes.
• Temperature was measured every 3 hours for 2 weeks.
• ABG was done every 8 hours for 2 weeks.
• CBC including white cell count was done daily and for 2 weeks.
• Complete renal functions daily (urea and creatinine serum level) for 2 weeks.
Any patient showing rise in the creatinine level was recorded and excluded from the study.
• Chest X-ray for all the patients was ordered after intubation and with onset of ventilation and every 24 hours for 2 weeks.
• All patients received anti-stress (Omeprazole 20 mg IV every 12 hours).
• Oro-gastric tubes were inserted to all patients and feeding was started within 24 hours.
• Daily evaluation for conscious level and need for sedation and ventilation were done for all patients.
• Tracheobronchial lavage was obtained by bronchoscopy 2 times a week and sent for qualitative culture for 2 weeks.
• Blood culture was also taken 2 times per week for 2 weeks.
• The planned duration of the study is two weeks from diagnosis of VAP and starting the antibiotics so any patients who failed to be weaned within this period were considered morbidity and recorded.
The 5 points of bundle for VAP prevention were strictly applied to all patients in both groups A and B:
• Elevation of the head of the bed 30º to 45º.
• Daily evaluation for possible extubation.
• The use of endotracheal tube with subglottic secretion drainage.
• Oral care with oral antiseptics.
• Initiation of safe enteral nutrition, if possible, within 24-48 hours from ICU admission, intubation and mechanical ventilation.
Failure of weaning from the ventilator within 2 weeks considered morbidity in our study. Two patients died from group A in the first 5 days from starting our study from septic shock and multi-organ failure.
Statistical analysis
Sample size calculation was conducted using Epi-save software to conduct a comparative study. The estimated sample size is made at assumption of 95% confidence level and 80% power of study. The primary outcome measure percent of improvement in modified clinical-pulmonary infection score (CPIS) (Table 1).
| CPIS | 0 | 1 | 2 |
|---|---|---|---|
| Tracheal secretion | Rare | Abundant | Abundant and purulent |
| Chest X-ray infiltrate | No infiltrate | Diffuse | Localized |
| Temperature ºC | >36.5 and <38.4 | >38.5 and <38.9 | >39 or <36 |
| Leucocytic count per mm3 | >4000 and <11000 | <4000 or >11000 | <4000 or >11000 +band form >500 |
| Hypoxic index PaO2/FIO2 mmHg | >240 or ARDS | -- | <240 and no evidence of ARDS |
| Microbiology | Negative | -- | Positive |
Table 1: Modified Clinical-Pulmonary Infection Score (CPIS).
The Data were collected and entered into the personal computer. Statistical analysis was done using Statistical Package for Social Sciences (SPSS/version 20) soft-ware. The statistical tests used were as follows: Number, percent, arithmetic mean and standard deviation for categorized parameters Chi square test was used, while for two groups t-test was used for parametric data. The level of significance was considered 0.05.
Tables below represent the demographic data of patients in both groups and showed no significant difference between the two groups as regard the age while there was significant difference as regard the sex as male are more common than female in road traffic accidents in both groups (Tables 2-4).
| Group A | Group B | p | |||
|---|---|---|---|---|---|
| Age group | (n=98) | % | (n=100) | % | |
| 18-22 years | 35 | 35.7 | 32 | 32 | 0.817 |
| 23-35 | 26 | 26.5 | 28 | 28 | |
| 36-45 | 28 | 28.6 | 27 | 27 | |
| 46-50 | 9 | 9.2 | 13 | 13 | |
| Sex | (n=98) | (n=100) | |||
| Male | 60 | 61.2 | 84 | 84 | 0.0032* |
| Female | 38 | 38.8 | 16 | 16 | |
| Surgical causes of ventilation | (n=98) | (n=100) | |||
| GCS>8 ≤12 with severe lung contusion | 28 | 28.6 | 35 | 35 | 0.102 |
| GCS>12 trauma with severe lung contusion | 10 | 10.2 | 4 | 4 | 0.089 |
| Severe lung contusion without flail chest | 43 | 43.9 | 45 | 45 | 0.465 |
| Severe lung contusion with flail chest | 17 | 17.3 | 16 | 16 | 0.551 |
p<0.05 is considered statistically significant*, GCS is Glasgow coma scale
Table 2: Demographic data of the studied patients’ groups.
| Group A (n=98 patients) | p value | ||||||
|---|---|---|---|---|---|---|---|
| APACH II Score | >25 | 16-25 | <15 | ||||
| No. | % | No. | % | No. | % | ||
| End of 1st 5 Days | 46 | 46.9 | 37 | 38 | 15 | 15.3 | 0.013* |
| End of 2nd 5 Days | 15 | 15.3 | 65 | 66 | 18 | 18.4 | 0.0159* |
| End of 3rd 4 Days | 2 | 2 | 50 | 51 | 46 | 46.9 | 0.001* |
| Group B (n=100 patients) | |||||||
| APACH II Score | >25 | 16-25 | <15 | ||||
| No. | % | No. | % | No. | % | ||
| End of 1st 5 Days | 15 | 15 | 57 | 57 | 28 | 28 | 0.013* |
| End of 2nd 5 Days | 11 | 11 | 51 | 51 | 38 | 38 | 0.0159* |
| End of 3rd 4 Days | 0 | 0 | 17 | 17 | 83 | 83 | 0.001* |
Table 3: Represent number and percentage of APACH II score in all patients in both groups in the studied period.
| Group A (n=98 patients) | Group B (n=100 patients) | p value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CPIS Score | 0 | 1 | 2 | 0 | 1 | 2 | |||||||
| End of 1st 5 Days | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Tracheal secretion | 15 | 15.3 | 37 | 37.8 | 46 | 46.9 | 28 | 28 | 57 | 57 | 15 | 15 | 0.013* |
| Chest x-ray infiltrate | 12 | 12.2 | 54 | 55.1 | 32 | 32.7 | 24 | 24 | 28 | 28 | 48 | 48 | 0.027* |
| Temperature | 16 | 16.3 | 67 | 68.4 | 15 | 15.3 | 40 | 40 | 50 | 50 | 10 | 10 | 0.001* |
| Leucocytic count/mm3 | 18 | 18.4 | 65 | 66.3 | 15 | 15.3 | 38 | 38 | 51 | 51 | 11 | 11 | 0.0159* |
| PAO2/FIO2 mmHg | 48 | 49 | ---- | 0 | 50 | 51 | 66 | 66 | ---- | 0 | 34 | 34 | 0.102 |
| Microbiology | 33 | 33.7 | ---- | 0 | 65 | 66.3 | 49 | 49 | ---- | 0 | 51 | 51 | 0.215 |
| End of 2nd 5 Days | |||||||||||||
| Tracheal secretion | 38 | 38.8 | 49 | 50 | 11 | 11.2 | 75 | 75 | 24 | 24 | 1 | 1 | 0.0224* |
| Chest x-ray infiltrate | 40 | 40.8 | 50 | 51 | 8 | 8.2 | 88 | 88 | 12 | 12 | 0 | 0 | 0.003* |
| Temperature | 36 | 36.7 | 54 | 55.1 | 8 | 8.2 | 76 | 76 | 24 | 24 | 0 | 0 | 0.015* |
| Leucocytic count/mm3 | 35 | 35.7 | 61 | 62.2 | 2 | 2 | 70 | 70 | 30 | 30 | 0 | 0 | 0.025* |
| PAO2/FIO2 mmHg | 76 | 77.6 | ---- | 0 | 22 | 22.4 | 90 | 90 | ---- | 0 | 10 | 10 | 0.096 |
| Microbiology | 45 | 45.9 | ---- | 0 | 53 | 54.1 | 69 | 69 | ---- | 0 | 31 | 31 | 0.016* |
| End of 3rd 4 Days | |||||||||||||
| Tracheal secretion | 46 | 46.9 | 50 | 51 | 2 | 2 | 83 | 83 | 17 | 17 | 0 | 0 | 0.001* |
| Chest x-ray infiltrate | 48 | 49 | 48 | 49 | 2 | 2 | 91 | 91 | 9 | 9 | 0 | 0 | 0.001* |
| Temperature | 54 | 55.1 | 42 | 42.9 | 2 | 2 | 90 | 90 | 10 | 10 | 0 | 0 | 0.001* |
| Leucocytic counts/mm3 | 57 | 58.2 | 39 | 39.8 | 2 | 2 | 87 | 87 | 13 | 13 | 0 | 0 | 0.0025* |
| PAO2/FIO2 mmHg | 85 | 86.7 | ---- | 0 | 13 | 13.3 | 98 | 98 | ---- | 0 | 2 | 2 | 0.625 |
| Microbiology | 78 | 79.6 | ---- | 0 | 20 | 20.4 | 100 | 100 | ---- | 0 | 0 | 0 | 0.002* |
Data are presented as number and percentage of patients; P<0.05 is considered statistically significant*
Table 4: Represent number and percentage of patients who had either a score of 0, 1 or 2 for all CPIS parameters in the studied period.
As regards the control of local signs of VAP in contused lung:
Tracheal secretion: Scanty tracheal secretion significant in group B compared to group A during the period of the study. At the end of the first 5 days, end of the second 5 days and end of the last four days; The control of purulent tracheal secretion was significantly better in group B compared to group A allover the duration of the study; Negative qualitative bacteriological culture from tracheal secretion was significantly better in group B compared to group A within the duration of the study. At the end of the first 5 days, end of the second 5 days and end of the last four days.
Chest X ray: Disappearance of parenchymatous lung infiltrate in the chest X ray was significantly better in group B compared to group A within the duration of the study; Hypoxic index PaO2/FIO2; Number of patients had hypoxic index more than 240 (or no ARDS) is significantly higher in group B compared to group A all over the duration of the study.
As regards the control of systemic signs of VAP in contused lung:
APACH II Score: Number of patients improved and had APACH II Score<15 in group B were significantly higher compared to group A in all the duration of the study.
Leucocytic count: Number of patients had normal Leucocytic count in group B was significantly higher compared to group A in all the duration of the study.
Temperature: Number of patients had core temperature between 36.5 to 38.4°C in group B was significantly higher compared to group A in all the duration of the study (Figures 1-6).
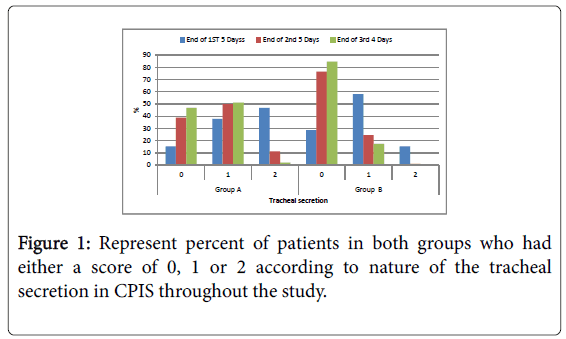
Figure 1: Represent percent of patients in both groups who had either a score of 0, 1 or 2 according to nature of the tracheal secretion in CPIS throughout the study.
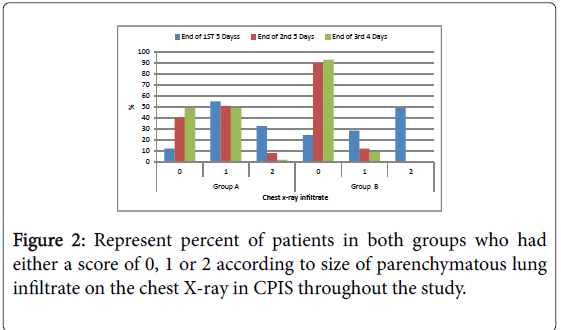
Figure 2: Represent percent of patients in both groups who had either a score of 0, 1 or 2 according to size of parenchymatous lung infiltrate on the chest X-ray in CPIS throughout the study.
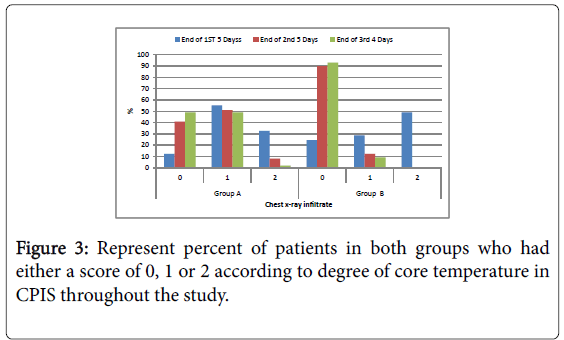
Figure 3: Represent percent of patients in both groups who had either a score of 0, 1 or 2 according to degree of core temperature in CPIS throughout the study.
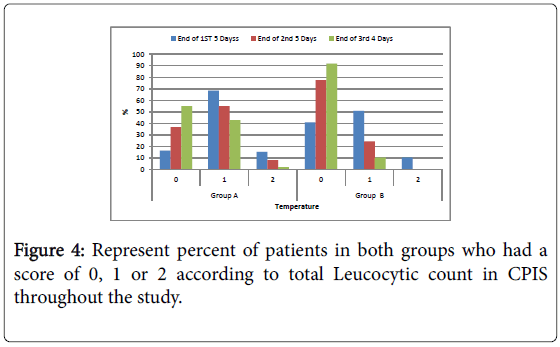
Figure 4: Represent percent of patients in both groups who had a score of 0, 1 or 2 according to total Leucocytic count in CPIS throughout the study.
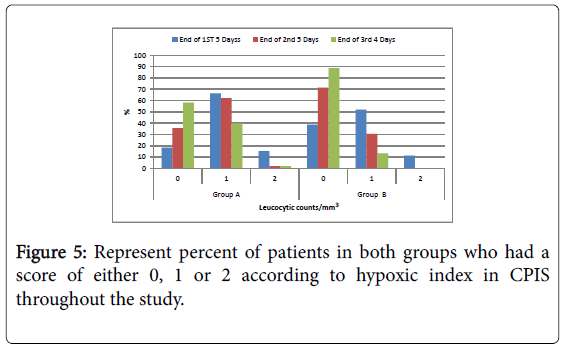
Figure 5: Represent percent of patients in both groups who had a score of either 0, 1 or 2 according to hypoxic index in CPIS throughout the study.
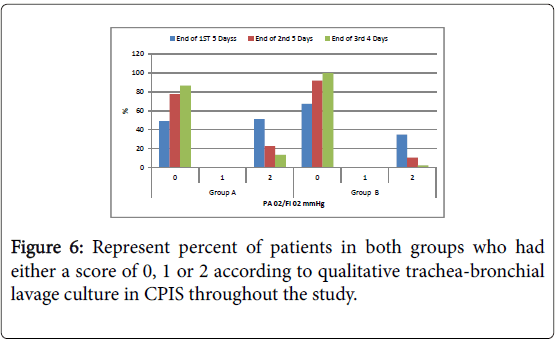
Figure 6: Represent percent of patients in both groups who had either a score of 0, 1 or 2 according to qualitative trachea-bronchial lavage culture in CPIS throughout the study.
Number of patients weaned from the ventilator within the studied period was significant higher in group B compared to group A in first 5 days, second 5 days and third 4 days while the total number of weaned patients at the end of the studied period was non-significant higher in group B compared to group A (Tables 5 and 6).
| Patients weaned from ventilator | Group A | Group B | p | ||
|---|---|---|---|---|---|
| (n=98) | % | (n=100) | % | ||
| First 5 days | 10 | 10.2 | 28 | 28 | 0.042* |
| Second 5 days | 33 | 33.7 | 59 | 59 | 0.002* |
| Third 4 days | 40 | 40.8 | 8 | 8 | 0.001* |
| Total | 83 | 84.7 | 95 | 95 | 0.101 |
Table 5: Represent Number and percentage of patients weaned from mechanical ventilation in the studied period.
| Group A (n=98 patients) | Group B (n=100 patients) | p value | |
|---|---|---|---|
| Morbidity (failure of weaning within 2 weeks) | 15 patients out of 98 failed to be weaned within the period of the study (15.3%) | 5 patients out of 100 failed to be weaned within the period of the study (5%) | 0.098 |
| Mortality rate | 2 patients (2%) | 0 patients (0%) | 0.465 |
Table 6: Represent the recorded morbidity and mortality in all patients in both groups in the studied period.
Number of patients had both morbidity (could not be weaned in the studied period) and mortality and both was non-significant higher in group A compared to group B (Figure 7).
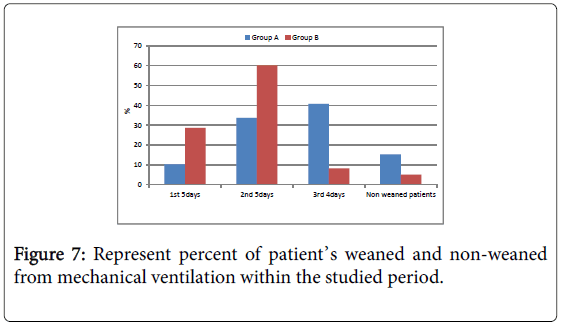
Figure 7: Represent percent of patient’s weaned and non-weaned from mechanical ventilation within the studied period.
The decision for both diagnose and empirical treatment of severe life threatening hospital acquired pneumonia especially on the top of severely contused lungs is challenging to Intensivists. The rapid administration of proper effective combination antibiotics considered the corner stone of management. The weaning decision and thus lowering both morbidity and mortality rates depend mainly on weaning from the ventilator. In our study tracheal secretion return back to its normal color and amount and there was significant disappear of its purulent content in group B compared to group A during all duration of the study (at the end of the first 5 days, end of the second 5 days and end of the last 4 days). Negative qualitative bacteriological culture from tracheal secretion was significantly better in group B compared to group A within the duration of the study. Disappearance of parenchymatous lung infiltrate in the chest X ray and getting clear lung field in the chest X ray (radiological cure) was significantly better in group B compared to group A within the duration of the study. Number of patients had hypoxic index more than 240 (or no ARDS) is significantly higher in group B compared to group A all over the duration of the study.
These improvement in the former parameters considered control of local signs of VAP which was significant better in group B compared to group A. This could be explained by the ability of ceftazidimeavibactam to control infection done by carbapenem-resistant gram negative bacteria especially Klebsiella pneumoniae and Pseudomonas aeruginosa . Those two species responsible for most of resistant VAP cases to the ordinary antibiotics combination especially carbapenem containing combinations. Another explanation is the avibactam component of the drug which is a non-betalactam beta-lactamase inhibitor that inactivates certain beta-lactamases that degrade ceftazidime. Avibactam does not decrease the activity of ceftazidime against ceftazidime-susceptible organisms.
The bacteriological cure followed by rapid control of the inflammatory reaction against the trauma and thus complete lung healing from contusion with prevention of superadded bacterial infection in the near future for the contused lung. This mechanism accelerates lung healing from both contusion and VAP and improve hypoxic index due to lung tissue cure from both trauma and infection.
Number of patients had APACH II score <15, normal Leucocytic count and normal core temperature in group B was significantly higher compared to group A in studied period, These improvement in the former parameters considered control of systemic signs of VAP which was significant better in group B compared to group A. This could be explained by control of local inflammatory response of both lung contusion and VAP after bacteriological cure and thus rapid and efficient control of their systemic manifestation in the form of total Leucocytic count and systemic core temperature. To our knowledge it is one of the first prospective randomized controlled trial that showed clinical improvement, compared with a carbapenem linezolid which was the most famous standard antibiotics in treatment of life threatening health care acquired pneumonia. Our results show noninferiority for the treatment of nosocomial pneumonia by ceftazidime avibactam caused by suspected carbapenem resistant Gram-negative aerobic pathogens [18]. In spite that we design our study to exclude any patient with marked deterioration in renal function in both groups yet we found that the safety profile of ceftazidime-avibactam in this study was similar to that of carbapenem/linezolid and no detectable major renal dysfunction side effect reported in our cases in both groups this could be due to small sample size (only 100 patients was chosen in each group) or/and the young age group randomly selected in our patients in both groups as more than 90% of our patients considered young adults (below 45 years of age) without any comorbidity. The high percent of young age group in the sample explained by our locality in Saudi Arabia (KSA) as the people start to drive motor vehicle in very young age and road traffic accident (RTA) are very common in this country according to KSA Ministry of Health hospitals, 81% of annual deaths are due to RTA and 20% of KSA hospitals beds are occupied by traffic accidents victims [19]. Other side effects difficult to be assessed in our study as all patients intubated, ventilated and sedated e.g. nausea and vomiting, change bowel habits and insomnia which is very difficult to be assessed in our cases.
A key limitation of this trial is that we could not establish optimum duration of treatment with either ceftazidime-avibactam or meropenem, and thus it does not provide any additional information that affects the standard of care with respect to these aspects of patient management. Furthermore, various aspects of the design, particularly the duration of study treatment of 14 days, although consistent with guidelines available at the start of the study, might not be representative of clinical practice and guidelines, which typically involve antibiotic de-escalation based on culture results [20].
The results of our study support a study done by Antoni et al. in 2017 on adults patients involve 808 patients with nosocomial pneumonia (including ventilator-associated pneumonia), enrolled at 136 centers in 23 countries, were randomly assigned (1:1) to 2000 mg ceftazidime and 500 mg avibactam (by 2 h intravenous infusion every 8 h) or 1000 mg meropenem (by 30-min intravenous infusion every 8 h) for 7-14 days; and stated that the use of ceftazidime/avibactam combination were non inferior in its safety and its efficacy compared to meropenem alone and ceftazidime alone [21]. Another study done by Zilberberg et al. in 2017 and its labeled under Carbapenem resistance, inappropriate empiric treatment and outcomes among patients hospitalized with Enterobacteriaceae urinary tract infection, pneumonia and sepsis and proven that mortality in the critical areas increasing especially between patients with life threatening infection like pneumonia, urinary tracts and sepsis due to Carbapenem resistance Enterobacteriaceae and open the gate for research for new antibiotics to control this problems that cause high mortality [22]. Other studies related to life threatening infection but not pneumonia done by Mazuski et al. in 2016 on Efficacy and safety of ceftazidimeavibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection and proved the superiority of ceftazidime-avibactam with metronidazole over meropenem in this life threatening condition [23].
The use of ceftazidime/avibactam more rapidly control all parameters of CPIS and provide faster weaning from the ventilator with non-significant lower mortality rate than meropenem/ gentamicin.
Citation: Alam MGIM (2020) Comparative Study between Usage of Meropenem/Gentamicin versus ceftazidime/Avibactam in the Treatment of ARDS Induced by both Lung Trauma and VAP. J Anesth Clin Res 11: 933. DOI: 10.35248/2155-6148.20.11.933
Received: 08-Jan-2020 Accepted: 22-Jan-2020 Published: 29-Jan-2020 , DOI: 10.35248/2155-6148.20.11.933
Copyright: © 2020 Allam MGIM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.