International Journal of Physical Medicine & Rehabilitation
Open Access
ISSN: 2329-9096
ISSN: 2329-9096
Research - (2022)Volume 10, Issue 2
This article was not intended to be a complete report of a standard clinical trial. It is a report of the outcomes of preliminary data for validation of the CMECD® procedure (Coletti Method of EMG ChemoDenervation) protocol for the treatment of chronic pain resulting from chronic muscle spasm. Methods are here detailed on how to approach the patient with chronic pain, identify the presence of chronic muscle spasm and undertake the treatment protocol and how to perform the follow up process to confirm that chronic pain secondary to chronic muscle spasm was the accurate diagnosis. Furthermore, the results from a survey of a cohort of more than 90 patients treated by the CMECD® procedure are presented. This information regards the location and duration of prior pain, prior treatment strategies, degree of success in resolving pain and duration of relief. Outcome data consisting of patient and staff reporting of specific situations in which the chronic pain treatment was successful has been included to help establish the “believability” of outcome successes and to elucidate the potential life altering effects of successful treatment of chronic pain secondary to chronic muscle spasm. This article will hopefully enhance the interest in this treatment protocol and increase the chance that a classical international clinical trial will be undertaken.
EMG; Muscle; Spontaneous Electrical Activity (SEA); Spasm; Pain; CMECD® protocol; Phenoxybenzamine-lidocaine mixture
This article is not a complete report of a standard clinical trial. It is a report of the outcomes of preliminary data for validation of the CMECD® procedure (Coletti Method of EMG ChemoDenervation) for the treatment of chronic pain resulting from chronic muscle spasm that was compiled over a 15-year period [1-4]. Methods are here detailed on how to approach the patient with chronic pain, identify the presence of chronic muscle spasm, undertake the treatment protocol and how to perform the follow up process to confirm that chronic pain secondary to chronic muscle spasm was the accurate diagnosis. Furthermore, the survey results of a cohort of more than 90 patients treated by the CMECD® procedure regarding duration of prior pain, location of pain, prior treatment strategies and degree of success in resolving pain and duration of relief are presented. Outcome data consisting of patient and staff reporting of specific situations in which the chronic pain treatment was successful has been included to help establish the “believability” of outcome successes and to elucidate the potential life altering effects of successful treatment of chronic pain secondary to chronic muscle spasm. This article is intended to demonstrate that chronic pain when resulting from chronic muscle spasm can be treated successfully with long-term results with the use of the CMECD® procedure. It will hopefully increase the interest in this treatment protocol and increase the chance that a classical international clinical trial will be undertaken.
It is appreciated that there is reluctance if not marked skepticism when reviewing procedures and results that are unexpectedly positive especially when they are the product of a single individual without institutional support. As will be demonstrated from the patient survey and patient accounts, this procedure could relieve chronic pain without the use of opioid medications for many. This invited article is the first comprehensive presentation of this procedure. The conceptual support for this procedure is reported elsewhere [5] including a description of the 12 published abstracts in the peer-reviewed journal Muscle & Nerve that supported its procedural development. It is therefore presented to a potentially unreceptive audience with the hope that academic skepticism and distain can be overcome.
Diagnostic and treatment step by step procedural approach
Patient assessment: Chronic pain presents in a variety of forms. Static constant pain, pain on standing, on movement and pain following a given activity may all be secondary to chronic muscle spasm. Not uncommonly there is no appreciation by the individual that their chronic pain is secondary to a chronic muscle spasm. For example, tennis elbow is almost always secondary to a muscle proximal or distal to the elbow but virtually never recognized by the individual. Piriformis syndrome causes sciatica, but the piriformis spasm is not generally recognized as the source of the pain. Pain in the foot is commonly caused by spasm of muscles in the lower leg especially the anterior muscles. Several good works provide a key to the possible muscle spasm that may be responsible for a given pain. My preferred source is a work by Clair and Amber Davies is and is referenced [6]. Their work follows up on the classic work by Travel and Simons “Myofascial Pain and Dysfunction”.
Patient examination: Nearly all muscles in chronic spasm exhibit tenderness on compression and seem incompressible. Deeper muscles, such as lumbar muscles, may require significant pressure to elicit discomfort. Comparison of one side to the other can help distinguish true tenderness versus an excessive compression effort. The individual must be placed in position such that the muscle is not in use during the examination. Chronic spasms are frequently found in the calf muscles and in the fore-arm muscles without the individual being aware of such and represent an excellent learning tool.
Diagnostic procedure: The simplest of EMG devices can suffice for identifying Spontaneous Electrical Activity (SEA) in a muscle in chronic spasm. The presence of SEA in a muscle placed at rest, in a patient without an underlying neurologic condition, is the identifying signature of chronic muscle spasm. Devices with a screen are more satisfying for feedback but devices with sound alone can be used successfully. The EMG device that was originally used and shown in the images below was a Cadwell 5200 A. The videos that were placed on YouTube were with use of an Intronix Myoguide EMG. Sensitivity and loudness settings are generally placed in mid-range. On the Myoguide device, settings of 7 and 7 respectively were used. Needle insertion can be performed with or without a skin-freezing agent for pain prevention. Needle insertion always causes some degree of electrical activity. Occasionally, increased insertional activity is encountered which typically is 1-2 second rapid-fire spike that can almost never be recreated by a second insertion or movement of the needle tip. This increased insertional activity, when present, has been found to correlate with muscle membrane instability in muscle not yet in full-blown chronic spasm. Until recently, treatment of those sites was avoided, but good outcomes have resulted in treating selective individuals with that presentation. The classic finding of chronic muscle spasm is a highly chaotic, high potential electrical activity in a muscle placed at absolute rest by posture.
Treatment procedure: A cocktail of previously prepared phenoxybenzamine 5 mg/ml and Dexamethasone 1.5 mg/ml is then diluted with equal amounts of Lidocaine 2%. An EMG injecting needle 1.5 to 3 inches is typically used and very small aliquots of the solution is distributed throughout the muscle until all SEA is abolished. A classic finding is that sites as small as a quarter inch/one half centimeter away from an injection site will remain strongly active despite the nearby injection. This is a markedly different approach than trigger point injection or use of botulinum toxin. The needle should be advanced and injections made until all but a small baseline activity remains. Then the needle should be pulled back and directed several degrees away from the initial orientation searching for SEA. Ultimately, a full 360 degree of exploration should be made to assure the muscle is fully treated. Not un-commonly SEA can be found continuing off in an unexpected direction and should be followed often requiring a separate skin penetration. Normally, a maximum of 20 ml of the combined solution should be utilized in a single procedure. Phenoxybenzamine is an alpha-blocker and can cause hypotension as a systemic effect for up to 36 hours. Images seen below are typical pre and post treatment EMG findings. A few amateurish treatment videos have been posted online and may be a useful teaching aid (Figure 1) [7-9].
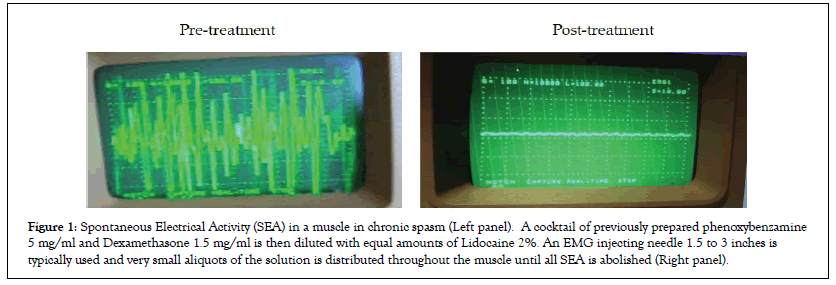
Figure 1: Spontaneous Electrical Activity (SEA) in a muscle in chronic spasm (Left panel). A cocktail of previously prepared phenoxybenzamine 5 mg/ml and Dexamethasone 1.5 mg/ml is then diluted with equal amounts of Lidocaine 2%. An EMG injecting needle 1.5 to 3 inches is typically used and very small aliquots of the solution is distributed throughout the muscle until all SEA is abolished (Right panel).
Acute treatment assessment: If chosen properly, the presenting complaint of pain will be resolved when the individual is asked to recreate it. A good method is to interrupt the procedure after half of the allotted injectate has been used and have the patient stretch or do any movement that would normally elicit the presenting pain. One must be mindful of the phenomena “Hierarchy of Pain” recently reported [9] wherein a subject will immediately sense a second less severe pain as soon as the most significant pain is resolved. Therefore, careful attention to exact sites of pain must be questioned. If the initial site of pain has been resolved and another is then reported, it is perfectly acceptable to perform the EMG examination of that site for treatment. Limitation of the total safe dose of medication used in one sitting may require a subsequent procedure. In the case of tendonopathies, it generally requires 2-3 days for resolution. The pathophysiology of this occurrence is discussed elsewhere [10].
Follow up treatment assessment: It is a key to know that while the combined effects of Lidocaine in pain relief and blockade of electrical activity are nearly immediate, the effects of phenoxybenzamine take up to an hour to be effective. Unless the individual is a fast metabolizer of Lidocaine, (often seen in individuals with red hair) there will be a fortuitous overlap of the pain and muscle spasm relief. A follow up visit is therefore strongly recommended to get a full assessment of procedural success. This will be especially important if attempts are made to use an even less concentrated solution of phenoxybenzamine to minimize its irritant effects. This is a consideration for future research.
Timing of subsequent treatments: Individual sites that fully resolve SEA almost never require a second injection. Discomfort at the site of injection can last up to one week but usually not more than 2-3 days. Treatment of a second site therefore should be done no sooner than one week from the initial injection.
Post treatment patient direction: The longer a muscle has been in chronic spasm, the more injured it has become. Loss of mitochondria with chronic muscle spasm has been reported [11]. Rehabilitation must therefore take into account the degree of muscle atrophy and not require excessive use that would recreate the overuse injury that was responsible for the chronic muscle spasm in the first place.
Risk profile: Hypotension up to 36 hours and site discomfort represents the primary risk for the use of phenoxybenzamine. Injection procedure deep into lumbar muscles without ultrasound or x-ray guidance has been found to be of minimal risk with no adverse events in at least a hundred lumbar injections. Deep muscle injections with EMG guidance in the lumbar region can be performed with the knowledge that a needle tip penetration into the peritoneum is unlikely to have a significant adverse effect. Injections in the thoracic and cervical regions require significantly more caution. Respiratory variation of the EMG signal indicates that further penetration of that muscle has a significant risk of lung penetration.
Data collection and analysis
In the development of the CMECD® procedure a population of roughly 100 the most recently treated individuals were surveyed by a questionnaire collecting information on the duration of pain prior CMECD®, on the results following other treatments (if performed) and on the outcome in terms of relief of pain following CMECD®. An initial reporting of the responses was published in abstract form [4] and a selection of these data was presented in a recent article [9].
All the obtained data are categorical variables. They were organized in contingency tables and the association between pairs of variables was statistically tested by Fisher’s exact test or Chi-square test. The SPSS 13.0 software (SPSS Inc., Chicago, USA) was used for the analysis and p<0.05 was always considered as the limit for statistical significance.
During the development of a new treatment strategy, it is not expected that every individual treated will have dramatic outcome results. In the development of the CMECD® procedure over 30 different muscle groups were treated [1,12-14].
This procedure involves the identification of chronic muscle spasm by the presence of Spontaneous Electrical Activity (SEA) by EMG and the complete resolution of SEA with EMG guided chemodenervation by use of a phenoxybenzamine/lidocaine/ dexamethasone mixture. The likelihood of universal positive outcomes in a newly developed procedure is highly statistically unlikely.
As has been previously reported [1], a population of roughly 100 of the most recent individuals that were treated with the CMECD® procedure was surveyed. An initial response to the survey was only 21 individuals. A second survey request, specifically asking for responses of individuals with no pain relief, yielded another 23 responses, 5 of which had no pain relief. The second request was to encourage patients with no benefit to respond to correct the inherent bias of only good outcomes being reported. An initial reporting of the responses was published in abstract form [4]. A selection this data was presented in a recent article “The Ischemic Model of Chronic Muscle Spasm and Pain” [9]. The full data set is below in Table 1.
| Sex | Initials | Age | Pain: Site | Pain: Duration |
Pain: Severity |
Pain Relief |
Relief: Partial |
Relief: Moderate |
Relief: Complete |
Relief: Duration |
Relief: Impact |
Medicine change |
Medicine type |
Referral | Have referred |
Prior surgery |
Prior massage |
Epidural | Acupuncture | Phys. therapy |
Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | SD | 68 | low back | years | severe | Y | Y | 1 to 3 months |
major | mild | opioid | strong | N | N | Y | N | Y | Y | N | ||
| M | JW | 49 | low back and leg | 20+ years | severe | Y | Y | > 3 months |
major | moderate | opioid | strong | Y | N | Y | N | N | Y | self help | ||
| M | LT | 63 | low back and leg |
2-3 months |
severe | Y | Y | > 3 months |
major | off meds | opioid | Y | N | N | N | N | Y | injections | |||
| M | FB | 74 | neck | years | severe | Y | Y | > 3 | major | off meds | opiod | strong | N | Y | Y | Y | N | Y | N | ||
| M | PM | 57 | low back | months | severe | Y | Y | > 3 | major | off meds | Aleve | strong | N | N | Y | Y | Y | N | N | ||
| F | DS | 42 | R ankle and foot |
3+ years | severe | Y | Y | >3 months |
major | off +/- all meds |
opioid | Y | N | Y | Y | N | Y | boot, chiropractor |
|||
| M | JB | 73 | low back | 4 years | mld | Y | Y | 1 wk to 1 month |
none | moderate | Aleve | strong | N | Y | Y | Y | Y | N | |||
| F | CS | 71 | low back and leg |
2+ years | severe | Y | Y | > 3 months |
major | off meds | opioid | Y | N | Y | N | N | Y | N | |||
| F | BF | 70 | low back and hip |
years | moderate | Y | Y | > 3 months |
major | off meds | Aleve | Y | N | Y | Y | N | Y | yoga, chiropractor |
|||
| F | JM | 74 | leg | 1 year | moderate | Y | Y | > 3 | major | none | Tylenol | strong | Y | N | Y | N | N | Y | N | ||
| M | JL | 65 | low back | 10 years | moderate | Y | Y | > 3 | major | none used | Aleve | strong | N | N | Y | N | Y | Y | N | ||
| M | DD | 61 | low back and leg |
8 years | severe | Y | Y | > 3 months |
major | off meds | Tylenol | strong | Y | N | Y | Y | Y | Y | N | ||
| M | JS | 60 | low back | 10 + years | moderate | Y | Y | > 3 | major | off meds | opioid | strong | N | N | Y | N | N | Y | N | ||
| M | JV | 67 | leg | 3 years | severe | Y | Y | 1 to 3 months |
major | off meds | Aleve | stong | N | N | Y | N | N | Y | N | ||
| M | GO | 76 | legs | 2 years | severe | Y | Y | > 3 | major | off meds | opioid | Y | N | N | Y | N | N | N | |||
| M | BM | 65 | neck, shoulder, arm, leg |
years | severe | Y | Y | > 3 months |
major | off meds | Aleve | Y | Y | Y | N | Y | Y | N | |||
| M | RF | 71 | low back and leg |
years | severe | Y | Y | 1 to 3 months |
major | off meds | opioid | strong | Y | N | Y | N | Y | Y | chiropractor | ||
| M | LW | 61 | upper and low back, leg |
years | severe | Y | Y | > 3 months |
major | off meds | Aleve | strong | N | N | Y | N | N | Y | N | ||
| F | VC | 62 | low back and leg | years | severe | Y | Y | > 3 months |
major | off meds | Aleve | Y | N | Y | N | Y | Y | N | |||
| M | AQ | 87 | upper back | years | severe | Y | Y | > 3 | major | none used | strong | N | N | Y | N | N | Y | heat | |||
| M | DB | 58 | low back and leg |
5+ years | severe | Y | Y | > 3 months |
major | off meds | Aleve | Y | N | Y | Y | N | Y | N | |||
| F | AL | 78 | low back and leg |
years | severe | Y | Y | 1 to 3 months |
major | moderate | Aleve | strong | N | N | Y | Y | Y | Y | N | ||
| M | PT | 82 | upper back | months | moderate | Y | Y | 1 to 3 | none | off meds | Tylenol | strong | N | N | N | N | N | Y | N | ||
| F | JH | 34 | low back | years | moderate | Y | Y | > 3 | minor | mild | opioid | strong | N | N | N | Y | N | Y | N | ||
| M | FS | 94 | neck | months | moderate | Y | Y | > 3 | moderate | Tylenol | possibly | N | N | Y | Y | Y | Y | heat and cold | |||
| F | RP | 43 | low back | 9 years | moderate | Y | Y | 1 wk to 1 month |
minor | mild | opioid | strong | N | N | Y | Y | Y | Y | chiropractor | ||
| F | CM | 42 | low back | years | moderate | Y | Y | > 3 | major | off meds | Aleve | Y | N | Y | Y | N | Y | N | |||
| F | DF | 87 | low back | years | severe | Y | Y | < 1 week | none | Aleve | possibly | Y | Y | Y | Y | Y | Y | N | |||
| M | CS | 59 | neck and low back |
years | moderate | Y | Y | > 3 months |
minor | moderate | Aleve | strong | N | N | N | Y | N | Y | N | ||
| F | EH | 56 | neck | years | moderate | Y | Y | none | minor | none | none | no | N | N | Y | Y | N | Y | N | ||
| M | JC | 67 | low back | years | moderate, severe |
Y | Y | none | none | none | Aleve | possibly | N | N | N | N | N | N | N | ||
| M | WS | 71 | neck and upper back |
months | severe | Y | Y | > 3 months |
major | off meds | Tylenol | strong | Y | Y | N | N | N | N | N | ||
| F | CS | 52 | arm | months | severe | Y | Y | > 3 | none | none | strong | N | N | Y | N | N | N | N | |||
| F | MD | 77 | lower back | years | severe | Y | Y | 1 wk to 1 month |
minor | mild | opioid | Y | N | Y | N | N | N | ||||
| M | JF | 72 | lower back | 4 weeks | moderate | Y | Y | > 3 | major | none | Y | N | Y | Y | N | Y | N | ||||
| F | MD | 77 | lower back | years | severe | Y | Y | 1 wk to 1 month |
minor | mild | opioid | Y | N | Y | N | N | N | ||||
| F | PS | 66 | arm | one day | moderate, severe |
Y | Y | > 3 months |
major | off meds | Aleve | Y | N | N | N | N | N | N | |||
| M | JB | 68 | upper and lower back,leg |
3 years | moderate, severe | N | > 1 week | none | no change | Aleve | possibly | N | Y | N | Y | Y | Y | N | |||
| F | JS | 81 | lower back and sciatica |
years | very severe | N | none | none | no | Aleve | possibly | N | N | N | Y | Y | Y | N | |||
| M | NM | 68 | head, neck, lower back |
16 months | severe | N | none | none | none | Aleve | no | N | N | N | N | N | N | N | |||
| M | EM | 64 | low back | months | moderate | N | none | none | mild | Tylenol | no | N | N | N | Y | N | N | N | |||
| M | MK | 57 | neck, low back, arm | years | mild, moderate, severe |
N | none | ||||||||||||||
| F | MZ | 77 | low back | 2 years | severe | N | none | none | none | Tylenol | strong | N | N | Y | N | N | N | followed by surgery |
|||
| F | MS | 67 | lower back | years | severe | N | none | none | none | possibly | N | N | N | Y | Y | Y | followed by surgery |
Table 1: Full data set.
Of the respondents, 35 (79.5%) reported one or more years of pain duration, 7 (15.9%) reported months of pain and 2 (4.5%) reported weeks of less pain duration. Of those reporting one or more years of pain, 16 (42.8%) reported complete relief of pain (86.6% of which reported relief of pain for greater than 3 months) and 31.4% reported moderate relief of pain (45.5% of which reported pain relief for greater than 3 months). Of those with one or more years of pain 26(74.3%) reported moderate or complete relief of pain of which 51.4% for greater than 3 months. Of the patients with prior back surgery 3 of the 7(42%) had durable relief or pain. The number of patients with prior unsuccessful treatments was 41(93.2%). It is notable that 23(52.3%) of the patients had undergone prior epidural injections. The average duration of pain when specified was 6.6 years and the longest was >20 years. A single treated patient, not in this survey, reported near complete pain relief and return of function after 35 years.
Of the 36 patients who noted pain relief, 25 (69.4.%) had relief for >3 month, 6 (18.6%) had relief for 1-3 months, 4 (11%) had relief for 1-4 weeks and 1 had relief for <1 week. Patients with pain relief for one to three months are suspect to have gone back to full activity too quickly before the treated muscle had fully recovered.
There was a strong tendency for treated patients to refer others for this treatment. On the survey 17 (38.6%) had already referred, and additional 16 (36.4%) would strongly consider referring and an additional 4 would possibly consider referring while 3 stated that they would not refer. In total 33 (75%) had referred or would strongly consider referring.
On the question of impact on overall health, wellbeing, or ability to function 25 (56.8%) noted a major impact, 6 (13.6%) noted a minor impact with the remaining 12 noting none or not reporting. A total of 70.4% noted a minor or major impact on health, wellbeing,or ability to function. Of the 44 patients reporting, 13 were taking opioid medications, 7 of which came off all medications following the procedure, 1 had a moderate and an additional 5 had a mild reduction in opioid use. A total of 19 (50%) of the 38 patients taking medications came off all pain medications following the procedure. Six patients had reported no use of pain medication prior to the procedure. Of patients who underwent epidural injections 12 had complete relief for >3 months, 4 had moderate relief for >3 months and 2 had partial relief for >3 moths. An additional 3 had moderate relief for 1 to 4 weeks. Overall, 19 (82.6%) of the 23 patients who had undergone prior epidural injections had some degree of longlasting relief and 16 (69.5%) had complete or moderate long-lasting relief.
There were 5 treated patients with no pain relief with one injection. Two additional patients were injected twice without relief and subsequently required back surgery. All other patients had pain relief at one or more sites of the 12 (27.2%) patients that had prior back surgery, 3 had complete relief for >3 months, 2 had moderate relief for 1-4 weeks and one had partial relief for <1 week (Figure 2).
Figure 2: A: Stacked bars describing the number of patients reporting significant relief of pain (blue) or no relief of pain (orange) following treatment. A significant difference can be detected between CMECD®® and prior treatments (Fishers’ exact test for contingency tables). B: As reported by most patients, CMECD®® had a positive impact on their overall health and led to a reduced use of pain medications.

Despite the relatively small data set, there is evidence of statistical significance of pain relief with the CMECD® procedure. It also can be noted from the statistical evaluation that sex, age, and duration of pain are not contributing variables. The fact that success of the procedure was not affected by the duration of the chronic pain supports the use of the procedure for individuals for whom there was little hope of success given their duration of their chronic pain (Table 1).
The procedures that generated the results were from a single solo medical practice not primarily involved in pain management or physical rehabilitation is no longer active. As a result, the data collection is complete and cannot be extended as would be preferential for reporting of an experimental procedure. However, despite the obvious shortcomings of the reported data, there is enough information to demonstrate enough cause and effect that it would be negligent not to report it to the medical and scientific community. The current need for treatment of pain without the use of opioid drugs necessitates seeking potential treatment options and for that reason such a treatment option is herein presented.
The successful outcomes of those treated and reported upon notwithstanding, the scientific import of this study is the discovery that SEA is both the presenting and responsible agent for chronic muscle spasm and knowledge of which provides potential treatment pathways. Prior reporting of an ischemic model of chronic muscle spasm provides additional potential pathways for treatment (Tables 2-4) [9].
| Number of CMECD®-treated patients | 44 |
| Males | 25 |
| Females | 19 |
| Mean Age and range | 66.2 (34-94) years |
| Average duration of pain and range | 5.85 years (1month–15years) |
| Number of patients who had prior treatments | 36 |
Note: 35 (79.5%) reported one or more years of pain duration; 23 (52.3%) of the patients had undergone prior epidural injections;12 (27.2%) patients that had prior back surgery;13 (29.5%) were taking opioid medications
Table 2: Demographic and clinical data from the returned questionnaires.
| Pain in percentage | Duration |
|---|---|
| Of those reporting one or more years of pain:15 (42.8%) reported complete relief of pain | (86.6% of which were >3 months) |
| 31.4% reported moderate relief of pain | (45.5% of which were >than 3 months) |
| 26 (74.3%) reported moderate or complete relief of which | 51.4% were >3 months |
| Of the 36 patients who noted pain relief: 25 (69.4.%) had relief for | >3 month |
| Of the 23 patients who underwent prior epidural injections: | |
| 12 had complete relief, 4 had moderate relief and 2 had partial relief for | >3 months |
| 16 (69.5%) had complete or moderate long-lasting relief | |
| 12 (27.2%) patients had prior back surgery, 3 of which had complete relief for | >3 months |
Note: Of the 13 patients taking opioid medications, 7 of which came off all medications; 19 (50%) of the 38 patients taking medications came off all pain medications; 25 (56.8%) noted a major impact on health, wellbeing or ability to function; 37 (70.4%) noted a minor or major impact on health, wellbeing or ability to function; 33 (75%) had referred or would strongly consider referring others for CMECD®
Table 3: Clinical Outcomes of pain relief with the CMECD® procedure.
| Pain relief | Sex | Age | Duration of pain prior to treatment | |||
|---|---|---|---|---|---|---|
| M | F | Under 65 | Over 65 | Years | Months | |
| Complete | 14 | 7 | 11 | 10 | 15 | 6 |
| Partial | 7 | 9 | 6 | 10 | 14 | 2 |
| None | 4 | 3 | 2 | 5 | 5 | 2 |
| p | 0.3783 | 0.4621 | 0.473 | |||
Table 4: Number of patients reporting their level of pain relief following CMECD®, stratified according to ‘sex’,‘age’ and ‘duration of pain prior to treatment.
However, statistics do not tell the whole story and individual reports of long-term outcomes should be given consideration in valuation of this procedure. A selection of patient and staff attestations shown below should provide the believability that statistics always seems to lack. Moreover, the addition of real-life circumstances adds another dimension to what otherwise is mere outcome data. Survey results indicate the length of time that relief was sustained but not the life impact of that relief.
Shown below are a number of self-reporting examples of what should be considered as valuable as pure digital data in a scientific inquire of correlation and causation.As may be seen below, some of the patients had a life altering change. Not all patients had dramatic results, but as an experimental treatment is undertaken, that would have been unexpected. With more experience in the suitability of injection sites, the proportion of successful results did improve and would be expected to improve further.
Patient and staff attestations
July 9, 2012: I am an active runner for many years and have done damage to my hip. The pain was so severe that I had to stop running. I lived with this for a few years and one day you men mentioned the treatment you offer. I tried it and it worked! The pain is gone and I am back to running 10 miles per day again.
July 6, 2012: I have had severe back pain for over 15 years. The injections have relieved this pain 100%. I feel like a new person. I used to wake up with major back pain and could barely get out of bed. Now with your treatments, I can jump out of bed and have zero pain! I feel young and vibrant again…it’s simply amazing. For 15 years I could not raise my arm above my head. It forced me to give up golf and working out. Now after the treatments, I go to the gym every day and freely lift weights and exercise without restriction. I am happy to say my handicap is back to 10.
April 18, 2012: Thank you for being the only doctor to analyze my case. I have been in pain for 12 months after chemo and after you injected me, I was on the dance floor. No pain enjoying the life I was made to have. You are a wonderful doctor and made my pain go away (PS. I’m only 22). Thanks for studying my case with the chemo’s aftereffects.
August 17, 2012: I experience extreme back pain. I tried all home remedies to alleviate the pain including but not limited to: aspirin, muscle relaxants, heating pads. I went to see Dr. Coletti on 2/8/12. Dr. Coletti proceeded to give me injections to the painful areas. These injections completely cure the pain and it currently has not returned.
June 20, 2012: (Hospital CEO) My pain is gone at the injection site. I continue to have numbness in my toes, but I am able to wake up in the morning without any stiffness at all, which is really wonderful. Also, I played golf yesterday and usually when I have finished my back will tighten up and I will need to stretch out before doing anything else…not the case though I feel great.
April 27, 2015: For the past 15 years I have been suffering from extreme neck and back pain with tremendous headaches. All the doctors, who have been many, have said the conditions were cause of arthritis and that nothing could be done...on April 8, 2015 I received 4 injections in my neck area and on April 23, 2015 one injection. These injections stopped the chronic muscle spasms in my neck and upper back, which resulted in no more neck pain or headaches. After 15 years of suffering this treatment was like a miracle.
August 21, 2016: Thank you for the opportunity to share my experience and significant life changing results that I have experienced from your work. As you know, I had tried many methods of pain relief for my lower back pain. This pain prevented me from walking even a quarter of a block. I was unable to enjoy basic activities such as walking my dog, strolling in the evening on the beach, and even walking around the hospital where I was employed as a chief operating officer.
As a board-certified Nurse, I am very aware of treatment options available and actually tried numerous methods of pain relief to include injections, massage, acupuncture, and daily multiple dosing of Motrin. None of these gave me anything but some minor relief that was temporary in nature. Your treatment that you provided me on two occasions was successful in eliminating all of my lower back pain. I no long am in need of any other type of treatment or even intermittent relief from medications. I can’t thank you enough for what you have done for me. (Note: as of January 2022, there was no recurrence of back pain)
On November 16, 2015: I walked into Coletti’s office with extreme pain in both legs, in the hamstring area. After the injections I walked out with no pain. It is now August 23, 2016 and I have not experienced the pain since the injection.
August 15, 2016: I developed a very bad case of plantar fasciitis into the fall of 2012. I saw an orthopedic surgeon who specialized in podiatry and was sent to physical therapy. When that failed to work, I was given 3 cortisone injections over a 5 month period. No relief. It was suggested that I have surgery, I declined. I was put on light duty and wore a boot for 2 months. This offered some relief but as soon as the boot came off and I resumed normal activity. I was again in excruciating pain. This pain which went on in first one, then both feet and ankle was intolerable and life limiting. The only thing I could do was get through a day of work, rest my feet at night and repeat. I had to stop all forms of physical activity, I couldn't even take my dog for a short walk or ride a stationary bike. Massage and chiropractor helped a little but after standing all day a work (I am an x-ray tech in a Cardiac Cath Lab) my feet were destroyed. I tried different shoes and orthopedic insoles. Anti inflammatories and tramadol only kept me from wanting to cut my feet off. While describing this pain to a co-worker Dr. Coletti overheard. He explained a procedure he could do that might help. After 3 years of misery, I was willing to anything. One visit to his office, a few injections in my calf (anterior and posterior), one week of leg achiness and my foot/ankle pain was 50% better. Within a month it had improved by 75%. Three months later the pain was almost gone. This was not a 100% cure my feet will hurt after strenuous physical activity or a really bad day at work but so markedly that I tell friends and co-workers that Dr. Coletti saved my life. Before the injection I was in constant pain, miserable and could do nothing and go nowhere without knowing I was going to be in agony. Now I do anything I want. To NOT learn this technique and dismiss it is a huge mistake for the medical community. I thank Dr. Coletti every time I can go for a walk or finish a day of work and I am not in tears from pain.
August 15, 2016: Rising from a seated position was extremely painful. It was difficult to walk after being seated. I can now stand without pain in my back. I still have difficulty walking, however it is because my left knee needs to be replaced. PS Thank you Dr. Coletti.
August 2016: Coletti’s treatment of my sciatica and lower back pain was not only easy but thorough. He injected me at the site of the horrible pain with little discomfort. After the injection I could not feel any pain and walked out of his office as though there never was a problem. Prior to Coletti’s treatment, I had to crawl to the bathroom at night. I highly recommend his technique and treatment to all. No more drugs. Today I am without any pain.
August 2016: Interventional Health has allowed me to continue working daily and so far has avoided back-spine surgery that was scheduled one year ago. Note: recurrence with reinjection in 6/19 and 4/21. Still working as an engine mechanic with heavy lifting and no back surgery as of 3/22.)
March 2016, Staff note: I had the pleasure of working with Dr. Coletti and seeing firsthand the miracles that walked out of our office after the injections. We had patients walk in with a cane or walker and leave with the cane over their shoulder or someone taking their walker out for them. The greatest was hearing the feedback of how positive the long-term affect was and best of all no more pain meds. The success rate was high. Seeing these patients struggle to get out of their chairs and walk down the hallway and walk out a different person and pain free was amazing. Dr. Coletti has created miracles here for patients who had given up hope and he was their last stop.
The key feature as noted in this survey is that, except as noted above and in the tables, patients were given only a single treatment that had a long-lasting result. A single practitioner could generally perform patient evaluation and treatment including history taking, physical exam, explanation of the procedure, obtaining consent, injection of the discovered site of chronic spasm based upon EMG evaluation and post treatment evaluation within a one-hour patient office visit.
Additional treatments were only given to treat additional sites. Repeat injections to a given site were rare and only with a subsequent repeat overuse injury at months to years following the initial treatment. Diagnostic tools in the search for a cause of chronic pain potentially caused by chronic muscle spasm consists of both clinical and technical elements.
These include identification of palpable spasm and tenderness on physical examination and the finding of SEA on EMG of the muscle. Used together these are good prospective tools in this endeavor. Numeric outcome data and personal reporting play an important role in the acceptance of any newly developed treatment that has the potential to positively impact an individual’s health and function.
In the situation where the data set is limited, statistical evaluation for retrospective diagnosis and putative correlation of chronic pain and chronic muscle spasm is limited. In evaluating the proposed causation of chronic pain from chronic muscle spasm, self-reporting by the subjects provides a deeper understanding of a positive outcome and its impact on wellness. Confirmation of individual outcomes in a self-reporting format can provide the necessary “proof” of the etiology of the presenting complaint of chronic pain as resulted from chronic muscle spasm.
This article has sought to present adequate information for understanding and subsequently undertaking use of the CMECD® procedure to treat patients with chronic pain caused by chronic muscle spasm. Additional technical information can be obtained on the physician teaching website, CMECD®.info and will be available in the soon to be released book “Chronic Muscle Spasm and Pain - Discoveries in the Etiology, Identification and Treatment of Chronic Muscle Spasm and Resultant Chronic Pain” by this author. In conclusion, the treatment of chronic pain caused by chronic muscle spasm can successfully be treated with long lasting results with use of the CMECD® procedure. Patient survey data reached statistical significance with a confidence p value of <0.01 regarding relief of pain with the CMECD® procedure. The identified lack of correlation between success of treatment and duration of pain supports the use of the CMECD® procedure on patients relegated to chronic pain treated only with chronic opioid use. With this information provided in this article, the online sources and ultimately the upcoming book, it is hoped that a classical international clinical trial could be designed and implemented leading ultimately to full acceptance and use of this new clinical tool for relief of chronic pain.
The author drafted, wrote, revised and provided final approval of the typescript.
This research received no external funding.
The study was conducted in accordance with the Declaration of Helsinki, ethical review and approval was waived for this study because the protocol use FDA approved drugs for the study population.
The authors declare no conflict of interest.
The author would like to thank prof. Diego Guidolin, Dept. of Neuroscience, University of Padova (Italy) for discussion and data processing support. The author would also like to thank Ugo Carraro, Editor-in-Chief, European Journal of Translational Myology (EJTM), Department of Biomedical Sciences, University of Padua (Italy) for his support and encouragement to write this article.
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef]
[CrossRef]
[CrossRef]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef]
[CrossRef]
[CrossRef]
Citation: Coletti RH (2022) Chronic Muscle Spasm Induced Chronic Pain Treated with the CMECD® Procedure. Int J Phys Med Rehabil. 10:626.
Received: 08-Mar-2022, Manuscript No. JPMR -22-16177; Editor assigned: 10-Mar-2022, Pre QC No. JPMR -22-16177 (PQ); Reviewed: 24-Mar-2022, QC No. JPMR B-22-16177; Revised: 28-Mar-2022, Manuscript No. JPMR -22-16177 (R); Published: 04-Apr-2022 , DOI: 10.35248/2329-9096.22.10.626
Copyright: © 2022 Coletti RH. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.