Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2021)Volume 10, Issue 1
Affirmation of variability among Ocimum basilicum L. genotypes is quite sure through sole morphological description. Furthermore, chemical print enhances confidence about the presence of varieties within genotypes. Characterizing the chemical compositions of essential oils for morphologically classified Ocimum basilicum genotypes was an aim for this work. Hydro distillation of the fresh leaves of Ocimum basilicum genotypes were acquired an essential oil yield of the range 0.09-0.26%. Totally, 36 compounds were identified by GC-MS analysis of Ocimum basilicum essential oil. Thus, a major compound of Bisabolene (45.79% and 43.92%), Eugenol (13.96% and 12.08%) and Estragole (13.35% and 10.15%) were characterized for 01WOL and 02WOL genotypes of Ocimum basilicum respectively. The genotype 03HAD was abundant in Bisabolene (27.22%), Geraniol (10.05%), Estragole (8.72%) and Citral (8.52%). Genotypes of 04HAD, 05KAM, and 06WON were majorly constituted with Methyl cinnamate (51.12-58.05%) and β-Linalool (28.71-32.56%). The six genotypes were characterized as three chemotypes of distinctive constituent, of which Bisabolene and Eugenol (01WOL, 02WOL); Citral and Geraniol (03HAD) and Methyl cinnamate and β-Linalool (04HAD, 05KAM, 06WON). Therefore, consumption of Ocimum basilicum is guided through purposive interests of consumers as of the distinctive flavor character of Ocimum basilicum genotypes.
Chemotype; Ethiopia; Genotype; Ocimum basilicum
Ocimum basilicum L. (Sweet Basil) belongs to the family Lamiaceae and the genus Ocimum which comprises about 68 to 129 species which is widespread over Asia, Africa and Central and Southern America [1,2]. In particular, Ocimum basilicum is characterized by a large diversity among its genotypes from various aroma types, leaf size and shape, leaf and stem color, inflorescence color and structure, grow habit and seeds morphology [3,4]. Besides, the ease of crosspollination has given rise to a large number of species, subspecies, varieties and forms [5]. Thus, the taxonomy of Ocimum bacilicum is further complicated by the existence of numerous variety, cultivars and chemotypes within the species that do not differ significantly in morphology [6,7]. The essential oils of Ocimum basilicum have had a main constituent of Linalool, 1,8, cineol, eugenol, methyl cinnamate, camphor, methyl eugenol, methyl chavicol, β-elemene, βocimene, camphene, carvacrol, α-bergamotene, α-cadinol and geranial [8].
The fresh leaves of Sweet Basil have a strong and characteristic aroma which have used for flavor of food, dental and oral products, in fragrance and traditional rituals and medicines [9]. Ocimum basilicum is widely cultivated in Ethiopia for the purpose of flavor of milk products, sauce, and a spice ingredient of red pepper flour ‘Berbere’. It is locally known as ‘Besobila’. In Northern Ethiopia, it is common practice to cultivate by intercropping with red pepper and also called ‘Zika qibe’, which indicates the flavor additives of Ghee (processed butter). Ocimum basilicum is also widely utilized in Southern Ethiopia as flavor of milk product through strip addition of the leaves and rinsing of the container by the leaves.
The variability of 28 accessions of Ethiopian Ocimum basilicum was studied by Egata et al. [10] and latter classified as 6 genotypes through morphological markers. Thus, the classification of genotypes only by morphological features becomes difficult due to anthropogenic interference with selection, cultivation, and hybridization [11]. Further, chemical characterization can be used to separate the accessions based on the presence or concentration of specific compounds and to determine the intrinsic variability or variability among accessions of the same species hybridization [11]. Therefore, this work had an aim to characterize six genotypes of Ethiopian Ocimum basilicum via chemotypically, which have maintained in Wendo Genet Agricultural Research Center (Figure 1).
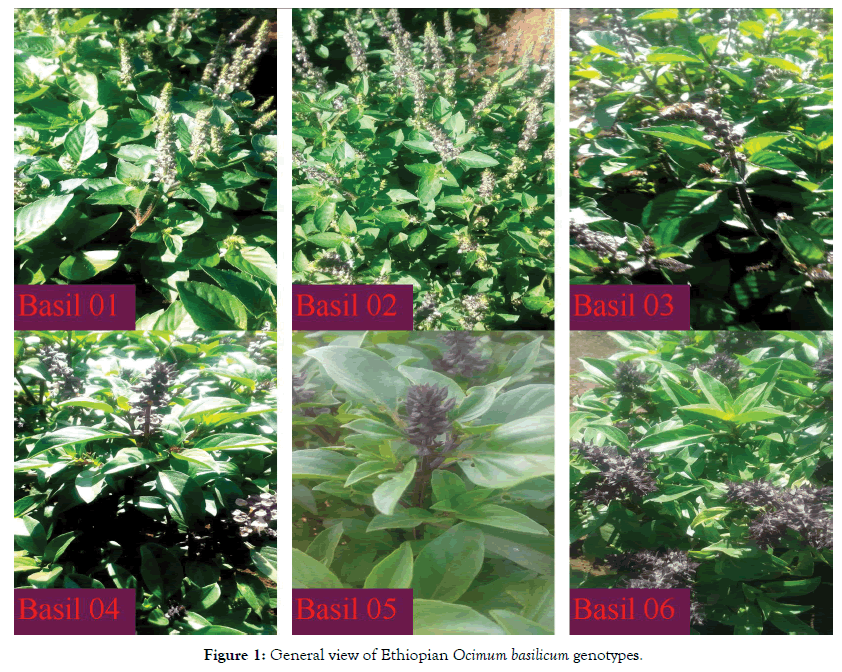
Figure 1: General view of Ethiopian Ocimum basilicum genotypes.
Sample preparation
The matured leaves (after blooming of inflorescence) of all the Ocimum basilicum genotypes (01WOL, 02WOL, 03HAD, 04HAD, 05KAM and 06WON) were harvested from Wendo Genet Agricultural Research Center experimental field (1800 m a.s.l., N 07° 05' 576", E 038° 38' 015") in June, 2019. The leaves and inflorescence were striped from the stem and subjected to extract.
Extraction of essential oil
The fresh leaves (200 g) were distilled through hydro distillation by using Clevenger type apparatus for 3 hours and collected the essential oil after drying with anhydrous Na2SO4. The extraction was replicates three times for each genotype sample. The analysis of variance and comparison of mean yield of essential oil between genotypes were performed through statistical analysis software (SAS, version 9.1) with completely randomized design sampling procedure. The yields of the essential oil were calculated based on the formula described as below;:
Oil yield (v/w (%)) = Amount of Distilled oil (mL) × 100/ Amount of fresh distilled leaf (g)
Chemical composition analysis of essential oil
Gas Chromatography - Mass Spectrometry (GC-MS) (Agilent model 7820 A) equipped with ALS was used to determine the chemical composition profile of the essential oil. Solutions of essential oils (0.2%) were prepared by dissolving 10 μL essential oil within 5 mL N-Hexane. The instrument was conditioned with a split/ split less injector mode, MS detector (5975), and HP-5 SM capillary column (0.25 mm i.d. × 30 m × 0.25 μm film thickness). Injector was operated on a split ratio of 1:10 with an injection volumes were 1 μL and injector temperature was set at 250°C. The MSD interface temperature was set 260°C. Helium was used as carrier gas and controlled in constant flow mode at a linear velocity of 36.6 cm/ sec. The oven temperature was programmed to started at 60°C, which was held for 1 minute and ramped at 5°C/min to 80°C with 3 min holding; next to the temperature was ramped at 4°C/ min to 180°C, which was held for 3 min. Finally, the temperature was raised at 25°C/min to 300°C withheld for 6 min. The MSD was operated on scan mode in 40-500 m/z range, with ion source and transfer line temperatures held at 230°C and 260°C, respectively. The solvent delay time was 3.4 min and taken 46.8 min for total run (Figure 2).
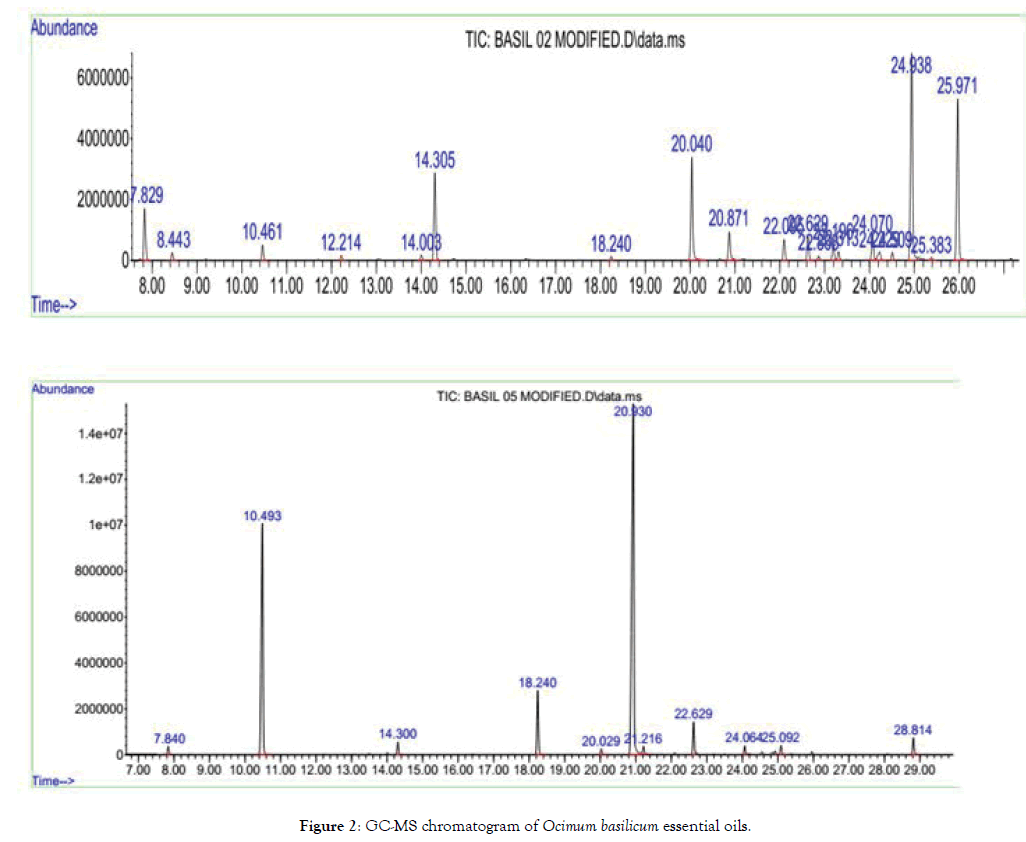
Figure 2: GC-MS chromatogram of Ocimum basilicum essential oils.
Based on ANOVA test, the essential oil yields of Ocimum basilicum were significantly influenced by genotypes at probability level less than 0.05 (Table 1). Ocimum basilicum genotype (06WON) was superior in essential oil yield (0.26% and 2.06%) from fresh and dried leaves respectively. Other genotypes (04HAD and 05KAM) were yielded comparable essential oil content (0.22% and 0.19%) from fresh leaves respectively. The remain three genotypes (01WOL, 02WOL and 03HAD) have acquired lower essential oil yield (0.12%, 0.09% and 0.1%) from fresh leaves respectively (Table 2). These results were in agreement with the work done by Amal et al. [12], which had an essential oil yield of 0.1 - 0.4% from the matured leaves of Saudi Arabia accessions, and a range of 0.29% to 0.33% for flowers and 0.32% to 0.48% for fresh leaves of Ocimum basilicum accessions from Sudan [13]. Similar results were obtained from Poland origin Ocimum basilicum var. with an essential oil yield varied from 0.75% to 1.89% from dried herb [14].
| Source of variation | DF | Essential oil yield (% v/w fresh base) | Essential oil yield (% v/w dry base) |
|---|---|---|---|
| Genotypes | 5 | 0.01450667**** | 0.83679222**** |
| Error | 12 | 0.00093889 | 0.04880000 |
| CV | - | 18.75998 | 18.83624 |
Table 1: Influence of genotypes on mean square of essential oil yield of Ocimum basilicum from the analysis of variance.
| Genotypes | Essential oil yield (% v/w fresh base) | Essential oil yield (% v/w dry base) |
|---|---|---|
| 01WOL | 0.11667c | 0.8167c |
| 02WOL | 0.09333c | 0.6800c |
| 03HAD | 0.10000c | 0.7600c |
| 04HAD | 0.21667ab | 1.3933b |
| 05KAM | 0.19333b | 1.3300b |
| 06WON | 0.26000a | 2.0567a |
| LSD at p < 0.05 | 0.0545 | 0.393 |
Table 2: Influence of genotypes on the mean of essential oil yield of Ocimum basilicum L.
The chemotypic characterizations of six morphologically classified Ocimum basilicum genotypes were determined through its essential oil chemical composition analysis. The chemical composition analysis by GC-MS resulted that a total of 36 identified compounds (Table 3). Thus, a major compound of Bisabolene (45.79% and 43.92%), Eugenol (13.96% and 12.08%) and Estragole (13.35% and 10.15%) were characterized for 01WOL and 02WOL genotypes of Ocimum basilicum respectively. The genotype 03HAD was abundant in Bisabolene (27.22%), Geraniol (10.05%), Estragole (8.72%) and Citral (8.52%). Genotypes of 04HAD, 05KAM, and 06WON were majorly constituted with Methyl cinnamate (51.12-58.05%) and β-Linalool (28.71-32.56%). Besides, some compounds were appeared as a minor constituent for all genotypes such as; Eucalyptol, α-Bergamotene, β-Copaene, Bergamotol and γ-Muurolene. These findings were in accordance with the chemical composition profiles of Ocimum basilicum collections which were determined from different origins of the world. Nurzyńska- Wierdak [14] characterized that, three chemotypes of different of Ocimum basilicum L. varieties with primary component of Citral (‘Lime’, ‘Lemon’ and var. citriodorum), E-methyl cinnamate/ linalool (‘Licorice’, var. cinnamon) and methyl chavicol (‘Tai’) from Poland origin. Carovic´-Stanko et al. [15] also reported that five chemotypes of Ocimum basilicum L. with linalool (58.79-66.40%), linalool (30.49%)/eugenol (19.57%), linalool (46.16%) /(Z)-methyl cinnamate (18.75%), estragol (47.52%) /linalool (20.82% ), and estragol (78.20-94.57%) from Croatia collections (Figure 3).
| Peak No. | RT (min) | MW (g/mol) | MF | Compound Name | Relative concentration (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 01WOL | 02WOL | 03HAD | 04HAD | 05KAM | 06WON | |||||
| 1 | 5.144 | 136.125 | C10H16 | α-Pinene | - | - | 0.19 | - | - | - |
| 2 | 6.15 | 136.125 | C10H16 | β-Pinene | - | - | 0.19 | - | - | - |
| 3 | 7.739 | 136.125 | C10H16 | D-Limonene | - | - | 0.25 | - | - | - |
| 4 | 7.829 | 154.136 | C10H18O | Eucalyptol | 7.55 | 6 | 5.22 | 2.17 | 1.01 | 1.57 |
| 5 | 8.443 | 136.125 | C10H16 | β-Ocimene | 1.85 | 1.17 | 0.6 | - | - | - |
| 6 | 9.195 | 154.136 | C10H18O | γ-Terpinene | - | 0.33 | 0.24 | - | - | - |
| 7 | 10.455 | 154.136 | C10H18O | β-Linalool | 0.84 | 2.13 | 2.8 | 32.56 | 28.71 | 30.28 |
| 8 | 12.214 | 152.12 | C10H16O | (-)-Camphor | - | 0.8 | 1.11 | - | - | - |
| 9 | 13.029 | 154.136 | C10H18O | Isoborneol | - | 0.27 | 0.37 | - | - | - |
| 10 | 13.495 | 154.136 | C10H18O | Terpinen-4-ol | - | - | 0.31 | - | - | - |
| 11 | 14.003 | 154.136 | C10H18O | α-Terpineol | 0.32 | 0.91 | 0.8 | - | - | - |
| 12 | 14.305 | 148.089 | C10H12O | Estragole (methylchavicol) | 13.35 | 10.15 | 8.72 | 0.98 | 1.46 | 2.02 |
| 13 | 14.723 | 150.104 | C10H14O | D-Verbenone | - | 0.49 | 0.52 | - | - | - |
| 14 | 15.428 | 154.136 | C10H18O | cis-Geraniol (Nerol) | - | - | 3.61 | - | - | - |
| 15 | 15.91 | 152.12 | C10H16O | β-Citral (Neral) | - | - | 3.02 | - | - | - |
| 16 | 16.407 | 154.136 | C10H18O | trans-Geraniol | - | 0.27 | 6.44 | 0.51 | - | - |
| 17 | 17 | 152.12 | C10H16O | α-Citral (Geranial) | - | - | 5.5 | - | - | - |
| 18 | 17.551 | 196.146 | C12H20O2 | Bornyl acetate | - | - | 0.21 | - | - | - |
| 19 | 18.234 | 162.068 | C10H10O2 | (Z)-Methyl cinnamate | - | 0.55 | 0.63 | 8.06 | 6.66 | 8.28 |
| 20 | 20.029 | 164.084 | C10H12O2 | Eugenol | 13.96 | 12.08 | 7.4 | - | 0.92 | 1.86 |
| 21 | 20.871 | 162.068 | C10H10O2 | (E)-Methyl cinnamate | 3.81 | 3.83 | 4.64 | 43.06 | 51.39 | 47.86 |
| 22 | 21.21 | 204.188 | C15H24 | (-)-β -Elemene | - | - | 0.46 | 0.68 | 0.95 | - |
| 23 | 22.095 | 204.188 | C15H24 | Caryophyllene | 1.89 | 2.67 | 2.24 | 0.9 | - | - |
| 24 | 22.624 | 204.188 | C15H24 | (E)- α-Bergamotene | 2.21 | 2.98 | 2.06 | 3.82 | 3.5 | 3.27 |
| 25 | 22.868 | 204.188 | C15H24 | (E)-β-Famesene | - | 0.68 | 0.51 | - | - | - |
| 26 | 23.191 | 204.188 | C15H24 | Humulene | 1.41 | 1.97 | 0.98 | - | - | - |
| 27 | 23.307 | 204.188 | C15H24 | (Z)- β-Farnesene | 0.74 | 1.28 | 0.5 | - | - | - |
| 28 | 24.07 | 204.188 | C15H24 | β-Copaene | 6.06 | 3.1 | 2.97 | 1.3 | 1.06 | 0.98 |
| 29 | 24.229 | 204.188 | C15H24 | Alloaromadendrene | 0.62 | 1.82 | 6.13 | - | - | - |
| 30 | 24.509 | 204.188 | C15H24 | Bergamotol | 0.61 | 1.05 | 2.77 | 0.54 | 0.52 | - |
| 31 | 24.933 | 204.188 | C15H24 | β-Bisabolene | 23.24 | 25.16 | 14.61 | - | 0.53 | - |
| 32 | 25.092 | 204.188 | C15H24 | γ-Muurolene | - | 0.26 | 0.44 | 1.42 | 1.15 | 1.04 |
| 33 | 25.383 | 204.188 | C15H24 | α.-Copaene | - | 0.46 | 0.38 | - | - | - |
| 34 | 25.965 | 204.188 | C15H24 | (Z)-α.-Bisabolene | 21.55 | 18.76 | 12.61 | - | - | - |
| 35 | 27.162 | 220.183 | C15H24O | Caryophyllene oxide | - | 0.4 | - | - | - | - |
| 36 | 28.814 | 222.000 | C15H26O | tau.-Cadinol | - | - | - | 3.99 | 2.15 | 2.83 |
Table 3: Chemical composition of Ocimum basilicum L. genotypes.
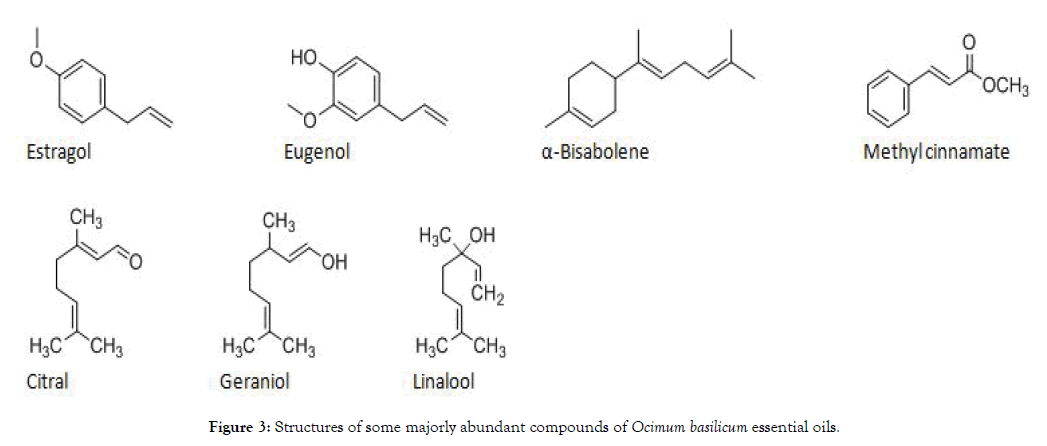
Figure 3: Structures of some majorly abundant compounds of Ocimum basilicum essential oils.
Based on the unique compound composition of the Ocimum basilicum L. essential oils, genotypes 01WOL and 02WOL are identified as Bisabolene and Eugenol chemotype and have an odor of cloves and spicy pungent taste. This character is favored as to use as spices ingredient of red pepper flour, ghee and sauce. The genotype 03HAD was chemotyped as Citral and Geraniol with having citrus odor and taste. These constituents privileged to use as salads and cooking vegetables. The remaining three genotypes 04HAD, 05KAM and 06WON were characterized as Methyl cinnamate and β-Linalool riches which have an odor of balsamic with reminiscent of cinnamon and strawberry. This characteristic is governed to use in flavor of cheese and milk product either by rinsing or strip addition of the leaves.
Considering the mean yield of essential oils from Ocimum basilicum genotypes, they had scored to three significantly different values. Furthermore, the six genotypes were characterized as three chemotypes of distinctive constituent, of which Bisabolene and Eugenol (01WOL, 02WOL); Citral and Geraniol (03HAD) and Methyl cinnamate and β-Linalool (04HAD, 05KAM, 06WON). Consumption of Ocimum basilicum is guided through purposive interest of consumer as of the distinctive flavor character of Ocimum basilicum genotypes. Overall Ethiopian genotypes of Ocimum basilicum have a good yield of essential oil with typical chemical print.
I am gratefully acknowledged Wendo Genet Agricultural Research Center for financial support.
Citation: Abdo BM, Geja W, Sisay W (2021) Chemotypic Characterization of Ocimum basilicum L. Essential Oils for Ethiopian Genotypes. Med Aromat Plants (Los Angeles) 10: 366.
Received: 21-Dec-2020 Accepted: 02-Jan-2021 Published: 08-Mar-2021 , DOI: 10.35248/2167-0412.21.10.366
Copyright: © 2021 Abdo BM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.