Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Research Article - (2015) Volume 4, Issue 4
Novel, chemoselective, non-hazardous and mild reaction protocol was developed for an efficient reduction of nitroarenes to aromatic amines using “iron activated water”. Water function as a terminal hydrogen source without any external catalyst, acid, salts or base and in the absence of a solvent. In the course of the reaction, zero valent iron was oxidized to magnetite.
Keywords: Water-iron; Hydrogenation; Reduction; Nitroarenes; Aromatic amines
The reduction of nitroarenes is an important process as the products, aromatic amines carrying chloro-, carbonyl, cyano, etc. groups are important intermediates in the synthesis of chemicals such as antioxidants, dyes, pigments, photographic, pharmaceutical and agricultural materials [1-3]. Several reviews and book chapters have covered the continuous progress in this field of reduction of nitroarenes to aromatic amines [4-7]. Aromatic amines can be produced from the corresponding nitroarenes by catalytic hydrogenation [8-15]. Catalytic hydrogenation is a clean and convenient method, but when other reducible groups are present in the molecule, it is difficult to reduce the nitro group selectively in a catalytic hydrogenation. Alternative to catalytic hydrogenation; the catalytic transfer hydrogenation (CTH) has been also used for nitro reductions in which alcohols, hydrocarbons, hydrazines, organic acids and their salts etc. are employed as hydrogen source with a wide range of metal-based catalysts [16-23]. The nitroarenes reduction using reducing metals such as zinc, tin and iron has been reported in presence of an acid [24,25] or salts [26,27]. However, notable disadvantages to these methods include high reaction temperatures, incompatibility of acid-sensitive functional groups, lack the chemoselectivity over other functional groups and reduction of aromatic nitro compounds often yield a mixture of products [28]. The notable applications of in-situ generated carbonic acids (from CO2 and water) in conjunction with Fe/Zn as reducing metals have been recently demonstrated in the reduction of nitroarenes [29,30]. However necessary requirement of CO2 pressure to activate iron through in-situ generated carbonic acid (using water) and high reaction temperature (120°C) (to achieve high yields) were drawbacks of these methods [29,30]. An efficient catalytic reduction of water for generation of hydrogen is one of the most challenging transformations in chemistry [31]. Recently our research group has demonstrated that water activated through reducing metals (Zn, Mg) act as green source of hydrogen including for hydrogen transfer reactions [32,33]. Poliakoff and Boix [34] have tried the reduction of nitroarenes using a metallic reducing reagent directly in pure water at 250°C. The yield of aniline was only 10% using iron powder under the given reaction conditions. Wang et al. [35] applied nano-sized activated metallic iron powder for the reduction of nitroarenes directly in water, and good reaction yield can be achieved at 210°C. However, nano-sized metallic iron powder is expensive and also high reaction temperature is a limiting factor [35]. Ranu et al. [36] developed elegant method for reduction of nitroarenes to aromatic amines using pre-synthesized iron nanoparticle. However, such pre-requisite synthesis of iron nanoparticle (using iron sulfate as iron precursor, sodium borohydride as reducing agent and citric acid as stabilizing agent) imposes additional chemicals and their cost, post processing problems and chemical wastes in the process. In above cases [34-36], an efficient reduction of nitroarenes using commercial metallic iron was in-effective. Moreover the search for new, mild, and selective reduction methods for nitro compounds to amines is still an active area of research. Herein, we are pleased to disclose a novel and mild reaction protocol for reduction of nitroarenes using cheap, non-hazardous, abundant, and eco-friendly “water-iron” pair as hydrogen donor (Scheme 1) without any external catalysts or additives. stoichiometric of the reaction can be formulated as follows (Scheme 1).

General
Chemicals were purchased from commercial firms [Nitroarenes purchased from Sigma Aldrich and iron powder (about 90 mesh) from BDH Chemicals] and used without further purification. Reactions were performed in a 30 Cm or 20 Cm pressure glass tube with closing cap. GC analyses were performed using Focus GC from Thermo Electron Corporation, equipped with low polarity ZB-5 column. GCMS analyses were performed using Trace 1300 Gas Chromatograph model from Thermo Scientific, equipped with the Rxi-1ms (crossbond 100% dimethyl polysiloxane) column. 1H-NMR spectra were recorded with Bruker DRX-400 instrument in CDCl3. XRD measurements were performed on a D8 Advance diffractometer (Bruker AXS, Karlsruhe, Germany).
Experimental procedure for nitroarenes hydrogenations reactions
In a typical reaction; 0.23 g (4 mmol) iron powder, water (10 mL) and 0.14 g p-nitrotoluene (1 mmol) was placed in a pressure glass tube equipped with a magnetic stirrer. The tube was sealed and heated with stirring for 29 hours at 50°C. At the end of reaction, the reaction glass tube was allowed to come at room temperature. The product p-toludine was extracted with diethylether (15 × 3=45 mL) followed by filtration using Whatman paper, dried with magnesium sulphate and analyzed by GC. The GC analysis shows >99.9% of p-nitrotoluene conversion to p-toludine. The residue after solvent (diethylether) evaporation affords the desired p-toludine product of good purity (Isolated yield=90%). The isolated product was further characterized by NMR (1H).
In a typical example a mixture of 1 mmol p-nitrotoluene (0.14 g), iron powder (4 mmol, 0.22 g), and 10 ml of water was placed in a pressure glass tube equipped with a magnetic stirrer. The tube was sealed and heated with stirring at 50°C for 29 hours. After cooling the reaction mixture was found to contain 0.1 g of p-toludine and >99% yield (based on GC area) and 0.28 g (crude weight) of Fe3O4. p-Nitrotoluene was selected as model substrate for optimization study in the present work (Table 1). Initially reaction temperature was optimized while other reaction parameters [4 equivalent of iron, water (10 mL), 29 h] kept constant (Table 1). The reduction of p-nitrotoluene was carried out at room temperature (RT) showed 64% conversion of p-nitrotoluene to p-toludine (Table 1, entry 1). In the next step reaction temperatures were increased from RT to 50°C (Table 1, entries 2-3); at 50°C (Table 1, entry 3) the quantitative and selective conversion (>99%) of p-nitrotoluene to p-toludine was observed. Further study for increase of reaction temperature upto 100°C reveals that, the reaction was selective below 80°C temperature (Table 1, entry 5) while at 100°C product selectivity was slightly decreased to 98% (Table 1, entry 6). It should be noted that the selective reduction of a nitro group to corresponding amine is a difficult task because reduction of aromatic nitro compounds often stops at an intermediate stage, producing hydroxylamines, hydrazines and azoarenes as side products [37]. Next various amount of iron powder from 4 equivalent to 2 equivalent (Table 1, entries 3, 8-9) were tested. The 4 equivalent of iron was sufficient for quantitative and selective reduction of p-nitrotoluene 1 to p-toludine 2 (Table 1, entry 3). Using 3.0 and 2.0 equivalent of iron, incomplete reduction of p-nitrotoluene was observed (Table 1, entries 8-9). In the complete absence of iron (Table 1, entry 11) or water (Table 1, entry 12), a neglible or no conversion of p-nitrotoluene to p-toludine was observed. The decrease of reaction time to less than 29 h resulted into incomplete p-nitrotoluene conversion (Table 1, entry 4). An effective stirring was found critical for the reaction to achieve quantitative conversion of nitroarenes. Finally 4 equivalent of iron, 50°C reaction temperature and 29 h reaction time set as optimum reaction parameters to achieve desired conversion and selectivity for nitro reduction under given conditions (Table 1, entry 3). Under the optimized reaction conditions (Table 1, entry 3), we performed hydrogenation of nitroarenes with diverse substituent groups. Importantly, the present reaction protocol was found to be a highly active and almost exclusively selective for the hydrogenation of substituted nitroarenes. Apart from p- nitrotoluene (Table 2, entry 1), other substituted nitrobenzenes having electrondonor or electron-withdrawing groups were also furnished with better to excellent yields (Table 2). Notably, halogen-substituted nitroarenes proceeded smoothly to the respective haloaromatic amines without any dehelogenation (Table 2, entries 4-6). Moreover, present reaction system also showed remarkable chemoselectivity in the hydrogenation of the challenging substrates bearing other easily reducible functional groups. The reducible functional groups in aromatic nitro substrates such as ether (Table 2, entries 7-8), nitrile (Table 2, entry 9), alkene (Table 2, entry 10), and ester (Table 2, entry 11) remained unaffected, thus giving the corresponding amines selectively. Moreover, bicyclic 2-nitronaphthalene also successfully reduced to corresponding 2-aminonaphthalene (Table 2, entry 12). We assert that the described system is composed of consecutive steps of hydrogen generation followed by hydrogenation rather than direct transfer hydrogenation (TH) from water to nitroarenes. This is supported by experimental observations. Under closed vessel conditions p-nitrotoluene was quantitatively converted to p-toludine (Table 1; entry 3). However; the reaction in an open tube showed only 41% conversion of p-nitrotoluene to p-toludine (Table 1, entry 12). In addition we found that iron is readily oxidized to magnetite even in the absence of p-nitrotoluene. The amount of hydrogen evolved from iron oxidation only with pure water was measured (for example; 2.9 mmol of hydrogen was evolved starting from 5 mmol of iron (0) powder under given conditions) [38]. It is well known fact that dissolved CO2 may accelerate the water-iron reaction to generate the hydrogen [29,30]. To exclude the presence of CO2 in water; we have used degassed distilled water (water was purged with nitrogen for 5 minutes before use) for p-nitrotoluene reduction under optimized reaction condition (Table 1, entry X). Here also reaction resulted into similar output (>99% conversion and selectivity) and rule out the role of dissolved CO2 in the activation of water-iron to generate hydrogen. To detect the reaction intermediates we have performed GC-MS analysis of reaction mixture after 6 h. However p-toludine was only product observed. Based on the literature [36,37], we hypothesize that the reduction of aryl nitro compounds could have preceded via -N=O, -NHOH, -N=N(O)- as transient intermediates to provide the NH2 product.
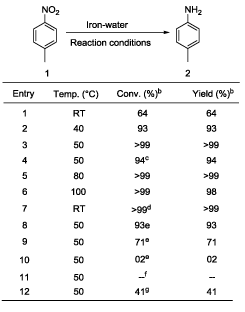 |
| aReaction conditions: p-nitrotoluene 1 (1 mmol), iron metal powder, water,29 h bConversion and product 2 yield based on GC area. cReaction run for 27 h dReaction run for 60 h e3 eqv., 2 eqv. and no iron used in the entries 8, 9 and 10 respectively fNo water used gOpen atmosphere reaction. NR=No reaction. RT=Room temperature |
Table 1: Optimization of various reaction parametersa.
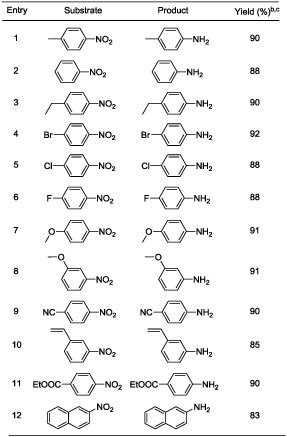 |
| aReaction conditions: Substrate (1-3 mmol), Iron powder (4 eqv.), water (10 mL), 50°C (oil bath), 29 h bConversion, yield and selectivity (for desired product to other products) was >99% (based on GC area) in all the entries cIsolated yield |
Table 2: Oxidation of various naphthalene derivativesa.
In conclusion; novel, non-hazardous and mild process was developed for an efficient and chemoselective reduction of nitroarenes to aromatic amines using simple commerical metalic iron as reducing species and water as terminal hydrogen source. The straight forward operation, use of inexpensive and benign reagents as hydrogen donor (iron and water), high yields of aromatic amines and, above all, the unique chemoselectivity over a wide range of functional groups make this procedure an obvious choice for reduction of aromatic nitro compounds. No acid or base additives, salts were added neither salts generated during the current nitroarenes reduction. The end product magnetite generated in the reaction is useful commercial material and can be easily separable from reaction using external magnet. Therefore technicaly end aqueous effluent contains only pure water, and present system free from hazrordous waste generation. From the environmental point of view this novel methodology could be the economical, efficient and waste-free approch towards reduction of nitroarenes.
We are thankful to Elad Meller for his help for GC-MS analysis.