Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2019)Volume 8, Issue 2
In this study, we characterized organic compounds from extracts of the herb Aframomum melegueta K. Schum using gas chromatography-mass spectrometry (GCMS) analysis. The influence of five different solvents, 10% sodium hydroxide (NaOH), methanol, ethanol, petroleum ether and n-hexane on the nature of extracted organic compounds and efficiency of extraction was evaluated. Results showed that organic compounds present in extracts of A. melegueta depends on the type of extraction solvents used and that only few compounds are similar in all the extracts analyzed by the GC-MS. Specifically, the polar solvents (10% NaOH, methanol and ethanol) were more effective for recovering phenolic compounds and organic acid esters. While the non-polar solvents (petroleum ether and n-hexane) helped to effectively, recover essential oil derivatives and cholesterols. We described in this paper the correlations among the structures of the most abundant compounds in all the extracts with their probable pharmacologic effects in living hosts. The findings of this study demonstrated that A. melegueta contains organic compounds, which may serve as new drug leads of natural products origin and make it employable in modern pharmacological practices.
Gas chromatography; Mass spectrometry; A. melegueta ; Cholesterols; Organic acid esters; Phenols
The herb Aframomum melegueta K.Schum is a tropical herbaceous herb of the family Zingiberaceae; it is commonly referred to as “Alligator Pepper” [1,2]. Several ongoing findings have confirmed this herb as a reservoir for potent phytochemicals of antimicrobial importance and the herb is of high research interest in southwestern Nigeria. Its seeds have pungent peppery taste due to aromatic ketones and phenolic derivatives while some other studies affirmed this herb may contain herbal drug leads [3,4]. Depending on the types of compounds desired, several studies recommend the use of extraction solvents such as methanol, ethanol, petroleum ether, n-hexane, and chloroform for recovery organic compounds from medicinal herbs in the tropics [5,6].
Holistic study of local herbs may involve identification and quantification of their organic compounds, to evaluate their medicinal potentials [7,8]. There is a growing interest for precise analyses on identification of active compounds present in local herbs, which necessitate structural elucidation studies on these herbs to provide scientific justifications for their pharmacologic effects in living hosts [8,9]. To achieve this holistic study of organic compounds present in herbs, scientists make use of analytical methods such as spectrophotometry, high performance liquid chromatography (HPLC), gas chromatography (GC) with flame ionization detection (FID) or gas chromatography- mass spectrometry (GC-MS) [7,8]. However, the GC-MS combines an ideal separation technique (GC) with stellar identification technique (MS), making GC-MS a useful technique for qualitative and quantitative analyses of volatile and semi-volatile organic compounds [7,8].
In this paper, we provided answers to impending research questions on organic compounds present in a local herb A. melegueta . We characterized the organic compounds from the herb Aframomum melegueta K. Schum using gas chromatography-mass spectrometry (GC-MS) analysis. The influence of five different solvents, 10% sodium hydroxide (NaOH), methanol, ethanol, petroleum ether and n-hexane on the nature of extracted organic compounds and efficiency of extraction was evaluated. We also described the correlations among the structures of the most abundant compounds in all the extracts with their probable pharmacologic effects in living hosts.
Collection and identification of test herb
We harvested the herb A. melegueta from its native region of Akure, Ondo State, Nigeria at 7°11’ N 7°12’ N/ 5°4’ E 5°9’ E coordinates. A botanist from the Department of Biological Sciences Herbarium, Federal University of Technology Akure, Nigeria identified the herb A. melegueta used in this study. The identification number assigned was FUTA/BIO/801.
Extraction solvents
The solvents used for extraction include methanol (Sigma Aldrich Grade A), ethanol (Fluka Sweden Grade A), 10% sodium hydroxide (NaOH) (Analar Grade A), petroleum ether (Poly-science Corporation, Evantson, Illinois) and n-hexane (Poly-science Corporation, Evantson, Illinois).
Preparation of herb extracts
We obtained the powdery form of the herb prior to the extraction process and adopted the methods of Chiejina and Ukeh [10] with some modifications. The total weight of the powdery form of A. melegueta was 500 g, from which we used a 100 g each for extraction with the 400 ml of each solvent (methanol, ethanol, n-hexane and petroleum ether) separately at a ratio of 1:4 (powder weight to solvent volume). We made the extraction at room temperature of in a Gallenhamp water bath coupled with orbital shaker for 72 h. We processed the resulting solution after extraction by filtration using Whatman filter paper 1 and obtained filtrates from each of the four solvents. We concentrated different extract filtrates for residues under vacuum at 40°C and weighed the residues obtained.
However, the authors adopted another protocol in to obtain the 10% NaOH extract of the herb [11,12]. As described by Karishma et al., we used a 20 g of the herb into 300 ml of the solvent 10% NaOH at a ratio of 1:15 (powder weight to solvent volume) [12,13]. We obtained the extract at a temperature of 61oC in a Gallenhamp water bath coupled with orbital shaker for 8 h. We processed the resulting viscous solution after the extraction by filtration using Whatman filter paper 1 and we prepared the filtrate separately in 10 ml test tubes. We neutralized the alkali (10% NaOH) in the filtrate with 10 ml each of 0.1 M hydrochloric acid (HCl), centrifuged at 3000 rpm for 10 min and tapped off the supernatant. We partially purified the residue obtained with ice cold 96% ethanol and centrifuged the mixture again at 3000 rpm for 5 min. Thereafter, we flushed the resulting residue aggregates with distilled water and centrifuged again at 3000 rpm for 5 mins to obtain our extract. The weights of extracts obtained from each solvent include 10.55 g for methanol, 8.25 g for ethanol, 4.65 g for petroleum ether, 5.65 g for n-hexane and 3.35 g for 10% NaOH respectively.
Derivatization procedure of herb extracts
The authors prepared a standard stock solution native to the Agilent Turbo Mass Spectrophotometer 7890 A GC with chemstation NIST for clarification of the extracts. We achieved this by adding 100 μl N, O-Bis (trimethylsilyl) trifluoroacetamide/trimethyl chlorosilane (BSTFA +TMCS) with 20 μl pyridine, we heated the mixture at 60°C for 8 min and added 100 μl of ethylbenzene and acetophenone (v/v) to the mixture [7,14]. We dissolved 1 g each of the five (5) extracts in the standard solution BSFTA+TMCS complex prepared using separate test tubes and added 50 μl acetonitrile. We then filtered each of the extract using 0.45 μ membrane filter into different Agilent 7890 A tube injection vials.
GC-MS conditions
The authors used Agilent Turbo Mass Spectrometer 7890 A GC with chemstation NIST for Gas Chromatography-Mass Spectrometry (GCMS) analysis. The column was Agilent Elite-5 capillary column measuring 30 m × 0.25 mm with film thickness of 0.25 millimeter (mm) and composed of 90% Dimethyl polysiloxane. The carrier gas used was Helium at a flow rate of 0.5 ml/min and we used a 1 μl sample injection volume with an equilibration time of 3 min. The inlet temperature was 250°C, oven temperature was initially at 110°C for 2 min but increased with a regulated rise to 280°C at a rate of 20°C/min for 9 min and the total run time was 38 min. The MS transfer line was at a temperature of 315°C and the source temperature was at 350°C. The septum purge flow was 3 ml/min and the total flow rate of the regulated carrier was 11.8 ml/min. The GC-MS was analyzed using electron impact ionization at 70 eV and data was evaluated using total ion count (TIC) for compound identification. We compared the spectrum of the compounds with database of known compounds stored in the GC-MS library. We used the Turbo-Mass-OCPTVSDemo SPL software of the NIST Agilent 7890 A MS library for measurement of peak areas, organic compound quantification and data processing. We also compared the spectrums of the organic components with the database of literature RI stored in NIMC database spectrum MS-NW-1522. We also juxtaposed the quantities of synonymous organic compounds detected with RI databases such as TSCA, RIECS, and IRDB.
The summary of the most abundant organic compounds characterized from each herb extract is represented in Table 1 while a typical ion chromatogram of organic compounds elucidated from the herb A. melegueta is in Figure 1. However, the supplementary file contains other necessary data informations, protocols and findings obtained in this work in figures two to six (Figures 1-5) and tables two to six (Tables 2-6) respectively. Furthermore, we used ChemDraw Ultra 8.0 to sketch and represent the organic compounds abundantly present in herb the extracts as additional information (Figures 6 and 7) in the supplementary file. The compounds identified from the 10% NaOH extract include conjugated phenols, organic acids and aromatic ketones. The identities of organic compounds present in 10% NaOH extract suggests that the extraction solvent (10% NaOH) is more suitable for recovering of phenols and organic acids compared to other polar solvents used in this study. The solvent 10% NaOH was better for extracting phenolic compounds from A. melegueta since phenols make up >70% of the total compounds by abundance from the extract. The caustic action of the alkali (NaOH) coupled with the polarity of the solvent (Water) and the extraction temperature maintained (61°C) enables the abundant liberation of phenols and organic acids forms characterized in the extract [12]. The most abundant compounds in the extract were benzaldehyde-3-hydroxy-4-methoxy and butan-2- one-4-(3-hydroxy-2-methoxyphenyl) (Table 1), and they are both phenols.
| Extraction media/ Most abundant compounds | Parameters for analysis | |||||
|---|---|---|---|---|---|---|
| Media | Compound designates | Compound name | % | Quantity (µg/g) | Retention time (RT) | Chemical formula |
| 10% NaOH | C1 | Butan-2-one-4-(3-hydroxy-2-methoxyphenyl) | 41.77 | 188.88 | 8.225 | C8H8O3 |
| C2 | Benzaldehyde-3-hydroxy-4-methoxy | 31.23 | 141.22 | 15.296 | C8H8O3 | |
| C3 | n-Hexadecanoic acid | 6.95 | 31.43 | 11.528 | C16H32O2 | |
| Ethanol | C1 | Corymbolone | 28.9 | 214.62 | 8.192 | C15H24O2 |
| C2 | γ-Sitosterol | 23.1 | 171.55 | 17.66 | C20H50O | |
| C3 | n-Hexadecanoic acid ethyl ester | 15.89 | 118 | 16.17 | C18H36O2 | |
| Methanol | C1 | 1, 4-Benzene diol-2-methoxy | 27.13 | 241.38 | 17.848 | C7H8O3 |
| C2 | Myrtenyl acetate | 21.22 | 16.268 | 188.8 | C12H18O2 | |
| C3 | 7-Oxo-2-oxa-7-thio-tricyclo [4.4.0.0(3, 8)] decan-4-ol | 16.12 | 143.42 | 8.22 | C8H12O3S | |
| Petroleum Ether | C1 | Naphthalene-2-methyl | 24.97 | 123.06 | 13.43 | C11H10 |
| C2 | γ-Muurolene | 15.85 | 78.11 | 11.448 | C15H24 | |
| C3 | Benzene (1-ethyl-1-propenyl) | 13.95 | 68.75 | 13.851 | C12H18O2 | |
| n-Hexane | C1 | Naphthalene-2-methyl | 11.89 | 61.64 | 15.4 | C11H10 |
| C2 | 1-H-indene, 2,3,dihydro 4,7,methyl | 11.07 | 57.4 | 16.344 | C11H14 | |
| C3 | Tetradecanoic acid ethyl ester | 9.04 | 46.87 | 16.915 | C16H32O2 | |
Table 1: The most abundant compounds in five extracts of the herb Aframomium melegueta .
| Compounds | RT | % Abundance | Quantity (μg/g) | Chemical Formula |
|---|---|---|---|---|
| Humulene | 4.245 | 0.42 | 1.90 | C15H24 |
| Isonicotinic acid | 5.570 | 0.29 | 1.31 | C6H5NO2 |
| Dodecanoic acid | 5.978 | 6.95 | 31.43 | C12H24O2 |
| Butan-2-one-4-(3-hydroxy-2-methoxyphenyl) | 8.225 | 41.77 | 188.88 | C8H8O3 |
| Hexahydro-3a-2(3H)-benzofuranone | 9.149 | 2.10 | 9.49 | C8H12O2 |
| n-Hexadecanoic acid | 11.528 | 6.95 | 31.43 | C16H32O2 |
| Caryophyllene | 14.002 | 6.95 | 31.43 | C15H24 |
| Benzaldehyde-3-hydroxy-4-methoxy | 15.296 | 31.23 | 141.22 | C8H8O3 |
| Spiro[5.6]dodecane-1,7-dione | 18.056 | 0.89 | 4.02 | C12H18O2 |
| Cis-Z-alpha-bisabolone epoxide | 18.999 | 0.50 | 2.26 | C15H24O |
| 1-Oxa-2-sila-5-boracyclopent-3-ene | 30.097 | 0.80 | 3.62 | C9H19BoSi |
| Other trace compounds | - | 1.15 | 5.20 | - |
| Total | 100 | 452.19 | ||
| Keys: RT- Retention time in min | ||||
Table 2: Organic compounds in 10% NaOH extract of A. melegueta .
| Compounds | RT | % Abundance | Quantity (μg/g) | Chemical Formula |
|---|---|---|---|---|
| 3,5-Heptadienal, 2-ethylidene-6-methyl | 8.069 | 4.88 | 43.42 | C10H14O |
| 7-Oxo-2-oxa-7-thio-tricyclo [4.4.0.0(3, 8)] decan-4-ol | 8.220 | 16.12 | 143.42 | C8H12O3S |
| Aromandendrene oxide-1 | 10.281 | 1.34 | 11.92 | C16H24O2 |
| Ethyl homovanillate | 11.476 | 0.28 | 2.49 | C11H14O4 |
| p-Fluorobenzaldehyde oxime | 15.112 | 2.76 | 24.56 | C7H6 FNO |
| 5, 8,11,14-Eicosatetraenoic acid, methyl ester | 15.291 | 10.68 | 95.02 | C21H34O2 |
| Myrtenyl acetate | 16.268 | 21.22 | 188.80 | C12H18O2 |
| Caryophyllene oxide | 16.772 | 6.97 | 62.02 | C15H24O |
| 1, 4-Benzene diol-2-methoxy | 17.848 | 27.13 | 241.38 | C7H8O3 |
| Sorbitol | 19.052 | 1.34 | 11.92 | C6H14O6 |
| Humulene | 20.823 | 1.92 | 17.08 | C15H24 |
| 3,4-Dimethoxyphenyl acetone | 30.102 | 0.44 | 3.91 | C11H14O3 |
| Other trace compounds | - | 4.92 | 43.77 | - |
| Total | 100 | 889.71 | ||
| Keys: RT- Retention time in min | ||||
Table 3: Organic compounds in methanol extract of A. melegueta .
| Compounds | RT | % Abundance | Quantity (μg/g) | Chemical Formula |
|---|---|---|---|---|
| Trans-beta-ionone | 7.495 | 1.58 | 11.73 | C13H20O |
| Humulene | 8.051 | 2.50 | 18.57 | C15H24 |
| 2(3H)-Furanone, 5-butyldihydro-4-methyl | 8.093 | 1.89 | 14.04 | C9H16O2 |
| Corymbolone | 8.192 | 28.9 | 214.62 | C15H24O2 |
| Pyridine, 2,6-dimethyl | 11.814 | 1.70 | 12.62 | C9H9NO4 |
| Cis-z-alpha-bisabolone epoxide | 14.275 | 1.67 | 12.40 | C15H24O |
| Caryophyllene Oxide | 15.069 | 2.10 | 15.60 | C15H24O |
| Ethyl oleate | 15.227 | 9.90 | 73.52 | C20H38O2 |
| n-Hexadecanoic acid ethyl ester | 16.170 | 15.89 | 118.00 | C18H36O2 |
| Linoleic acid ethyl ester | 16.701 | 3.61 | 26.81 | C20H36O2 |
| γ-Sitosterol | 17.660 | 23.10 | 171.55 | C20H50O |
| Aromandendrene oxide-1 | 18.974 | 1.14 | 8.47 | C16H24O2 |
| Alloaromandendrene | 30.071 | 1.09 | 8.09 | C16H24 |
| 1-Nortrachelogenin | 32.109 | 0.90 | 6.68 | C20H22O7 |
| Other trace compounds | - | 4.03 | 29.93 | - |
| Total | 100 | 742.64 | ||
| Keys: RT- Retention time in min | ||||
Table 4: Organic compounds in ethanol extract of A. melegueta .
| Compounds | RT | % Abundance | Quantity (μg/g) | Chemical Formula |
|---|---|---|---|---|
| Aromandendrene | 7.544 | 1.13 | 5.57 | C16H24 |
| γ-Muurolene | 11.448 | 15.85 | 78.11 | C15H24 |
| Adamantan-2-ol | 11.814 | 1.57 | 7.74 | C10H16O |
| Naphthalene-2-methyl | 13.430 | 24.97 | 123.06 | C11H10 |
| 9-Cyclohexylbicyclo (3.3.1) nonan-9-ol | 13.757 | 2.18 | 10.74 | C15H26O |
| Benzene (1-ethyl-1-propenyl) | 13.851 | 13.95 | 68.75 | C12H18O2 |
| 5-Hepten-3-yne-2-ol, 6-methyl-5 | 15.077 | 1.63 | 8.03 | C7H10O |
| Santolina triene | 15.195 | 1.34 | 6.60 | C10H16 |
| 2-Acetyl-5-trifluoroacetyl furane | 20.210 | 1.15 | 5.67 | C7H8O2 |
| 1-H-indene, 2,3, dihydro 4,7,methyl | 24.062 | 11.77 | 58.00 | C11H14 |
| Tetramethyl (z,z,z)- humulene | 25.043 | 1.82 | 8.97 | C15H24 |
| 3-Azabicyclo (3.2.2) nonane | 27.626 | 2.21 | 10.89 | C8H15N |
| n-Decanoic acid | 29.307 | 6.10 | 30.06 | C10H20O2 |
| 1, 4-Benzene thiol-2-methoxy | 30.013 | 9.03 | 44.50 | C7H8SO3 |
| Other trace compounds | - | 5.30 | 26.12 | - |
| Total | 100 | 492.83 | ||
| Keys: RT- Retention time in min | ||||
Table 5: Organic compounds in petroleum ether extract of A. melegueta
| Compounds | RT | % Abundance | Quantity (μg/g) | Chemical formula |
|---|---|---|---|---|
| Capsaicin | 4.377 | 2.16 | 11.20 | C18H27NO3 |
| Isolongifolene 9-hydroxy | 5.559 | 4.80 | 24.88 | C15H24O |
| Caryophyllene oxide | 5.981 | 7.01 | 36.34 | C10H16O |
| Oxirane, tetradecyl ledol | 7.513 | 4.69 | 24.30 | C11H10 |
| Santolina triene | 8.072 | 4.90 | 25.40 | C10H16 |
| Butan-2-one-4-(3-hydroxy-2-methoxyphenyl) | 8.222 | 4.95 | 25.66 | C11H14O3 |
| 3,7-Nonadien-2-ol | 11.594 | 4.20 | 21.77 | C11H20O |
| 2-Acetyl-5-trifluoroacetyl furane | 11.802 | 2.81 | 14.57 | C7H8O2 |
| Nerolidol-2 | 14.164 | 4.40 | 22.81 | C15H26O |
| 6-Dimethyl-5-hepten-1-ol | 14.302 | 3.08 | 15.97 | C9H18O |
| Naphthalene-2-methyl | 15.400 | 11.89 | 61.64 | C11H10 |
| 1-H-indene,2,3,dihydro 4,7,methyl | 16.344 | 11.07 | 57.40 | C11H14 |
| Tetradecanoic acid ethyl ester | 16.915 | 9.04 | 46.87 | C16H32O2 |
| Dodecanoic acid ethyl ester | 17.862 | 8.07 | 41.84 | C12H24O2 |
| 4, 8-Methanoazulen-9-ol | 19.926 | 3.51 | 18.20 | C11H8O |
| 4-Amino-2,3-xylenol | 20.840 | 1.59 | 8.24 | C8H11NO |
| Oleic acid | 29.341 | 2.41 | 12.49 | C18H34O2 |
| Ethanone, 1-(cyclohexen-1-yl) | 30.127 | 3.19 | 16.54 | C8H12O |
| Cyclotetracosane | 31.173 | 1.40 | 7.26 | C24H50 |
| Gingerol | 32.173 | 2.09 | 10.83 | C17H26O4 |
| Other trace compounds | - | 2.74 | 14.20 | - |
| Total | 100 | 518.42 | ||
| Keys: RT- Retention time in min | ||||
Table 6: Organic compounds in n-hexane extract of A. melegueta .
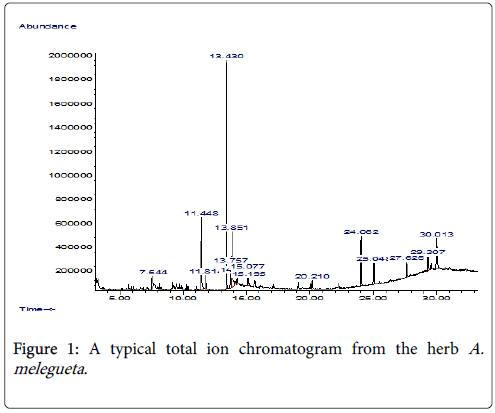
Figure 1: A typical total ion chromatogram from the herb A. melegueta .
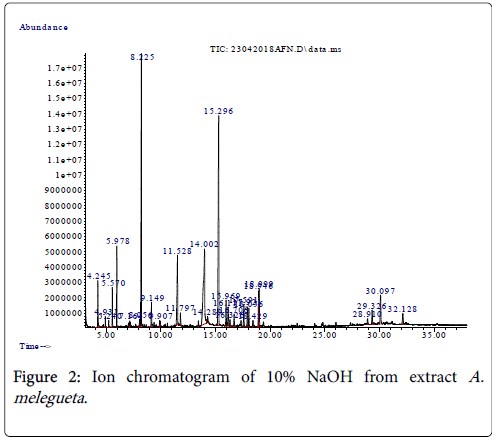
Figure 2: Ion chromatogram of 10% NaOH from extract A. melegueta .
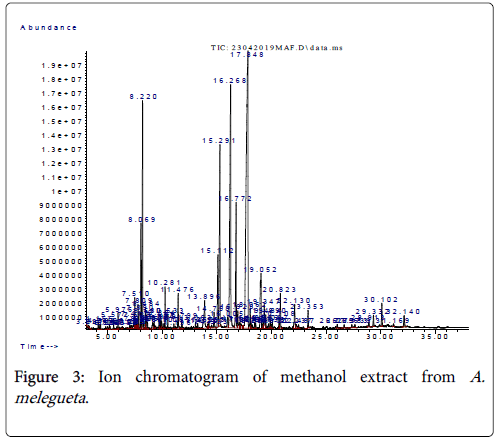
Figure 3: Ion chromatogram of methanol extract from A. melegueta .
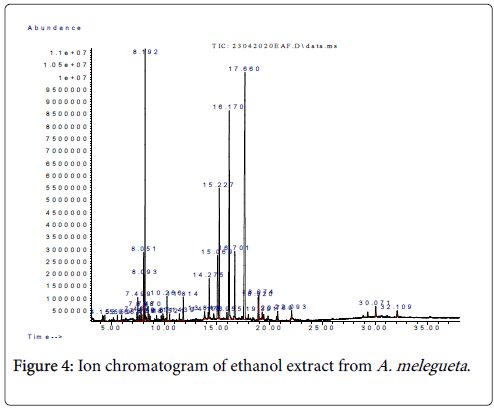
Figure 4: Ion chromatogram of ethanol extract from A. melegueta .
Figure 5: Ion chromatogram of petroleum ether extract from A. melegueta .
Figure 6: Ion chromatogram of n-hexane extract from A. melegueta .
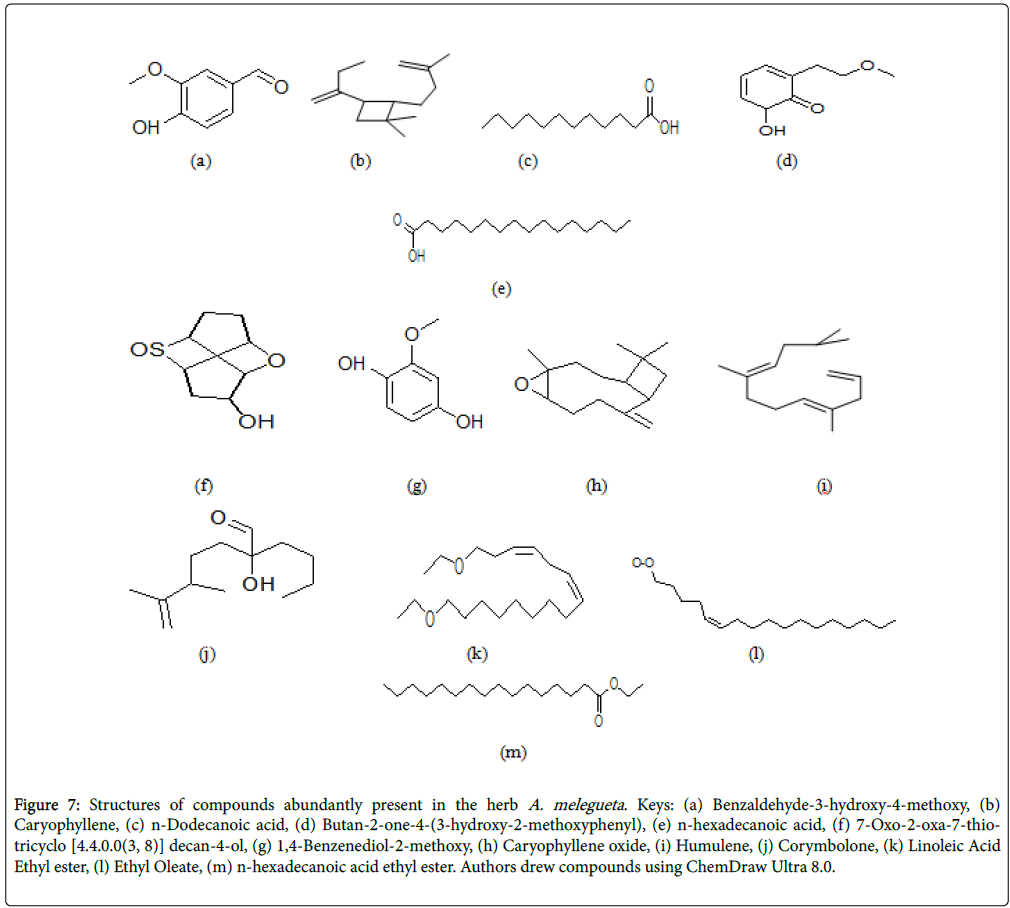
Figure 7: Structures of compounds abundantly present in the herb A. melegueta . Keys: (a) Benzaldehyde-3-hydroxy-4-methoxy, (b) Caryophyllene, (c) n-Dodecanoic acid, (d) Butan-2-one-4-(3-hydroxy-2-methoxyphenyl), (e) n-hexadecanoic acid, (f) 7-Oxo-2-oxa-7-thiotricyclo [4.4.0.0(3, 8)] decan-4-ol, (g) 1,4-Benzenediol-2-methoxy, (h) Caryophyllene oxide, (i) Humulene, (j) Corymbolone, (k) Linoleic Acid Ethyl ester, (l) Ethyl Oleate, (m) n-hexadecanoic acid ethyl ester. Authors drew compounds using ChemDraw Ultra 8.0.
As represented in Table 1, the phenol compounds benzaldehyde-3- hydroxy-4-methoxy (31.23% abundance) and butan-2-one-4-(3- hydroxy-2-methoxyphenyl) (41.77% abundance) both have phenol derivatives with -OH ends which affords them stellar antibacterial and antiviral properties [3,4]. The other compounds of lesser abundance in the 10% NaOH extract (≤ 10%) are dodecanoic acid and hexadecanoic acid. They are organic acid forms that exhibit high solubility in polar solvents; they also have affinity for alkaline mediums [11]. According to Li et al. [12], the two organic acids have inhibitory effects on aflatoxigenic molds and antibacterial effects on coliforms.
The organic compounds characterized in the methanol extract include branched chained thio-alkanols, organic acid esters and cyclic ketones. The most abundant compounds in the extract were 1,4- Benzene diol-2-methoxy and myrtenyl acetate (Table 1). The compounds 1,4-Benzene diol-2-methoxy (27.13% abundance) and myrtenyl acetate (21.22% abundance) possess medium polarity, hence it follows the two compounds have higher solubility in methanol than in water [6]. Furthermore, the studies of Chiejina and Ukeh, Thomas et al. [7,14] recently suggested that thio-alkanols and myrtenyl derivatives have some antimicrobial, anti-cholestrolic and antitumor properties. More interestingly, the compounds with lesser abundance (≤ 16.5%) such as 5,8,11,14-Eicosatetraenoic acid methyl ester and 7- Oxo-2-oxa-7-thio-tricyclo [4.4.0.0 (3,8)] decan-4-ol also have better solubility in methanol [9]. The other trace compounds in the methanol extract such as caryophyllene oxide and 3, 5-Heptadienal, 2- ethylidene-6-methyl- have been recently tested for antifungal and mild food preservative efficacy in the findings of Rani et al. and Rahman et al. [14,15]. Generally, we observed in this study that the solvent methanol fared less than the solvent 10% NaOH for extraction of phenolic compounds from the herb A. melegueta (this is because the phenol abundance was at <28% for methanol compared to >70% for 10% NaOH). However, methanol fared better well for extraction of medium polar compounds such as thio-alkanols, conjugated aldehydes and organic acid esters since they constitute >40% of all the compounds characterized from the methanol extract.
Moreover, the categories of compounds identified from the ethanol extract are organic acid esters, conjugated alkanols and branch chained cholesterols. The most abundant compounds in the ethanol extract were corymbolone and γ-sitosterol (Table 1). These two compounds have high molecular weights, indicating that the solvent ethanol has affinity for quaternary and heterocyclic compounds. This also points to the fact that ethanol has higher molecular density than methanol, hence, the ability to solubilize heavy weight compounds and their derivatives [6]. The findings in Rahman et al. and Isah et al. [15,16] suggest that quaternary ketones such as corymbolone (28.9% abundance) and heavy chained cholesterols such as γ-sitosterol (23.10%) might have inhibitory effects on many fungi of mycotic importance and antibacterial effects on faecal coliforms. The other compounds of lesser abundance in the methanol extract are nhexadecanoic acid ethyl ester, linoleic acid ethyl ester and ethyl oleate. However, the studies of Rani et al. and Isah et al. [14,16] described their pharmacologic effects. The study posits that many polymeric esters such as n-hexadecanoic acid ethyl ester and linoleic acid ethyl ester have lytic effects on bacterial skin pathogens while fatty derivatives like ethyl oleate possess mild antitumor effects against malignant growths. In this study, we discovered from the results that the solvent ethanol fared comparatively better than 10% NaOH and methanol for extraction of quaternary compounds and heavy weight organic acid esters from the herb A. melegueta .
Conversely, the most abundant compounds in the petroleum ether extract were naphthalene-2-methyl (24.97% abundance) and gammamuurolene (15.85% abundance) (Table 1). The compounds naphthalene-2-methyl and gamma-muurolene (which is an aromatic conjugate) are essential oil based derivatives. The herb A. melegueta contain essential oils, and the use of a non-polar solvent such as petroleum ether ensures the dissolution of a reasonable amount of the oils from the plant cell matrix [4]. More so, the petroleum ether extract also contain other compounds with <10% abundance such as 1, 4- benzene thiol-2-methoxy, benzene (1-ethyl-1-propenyl) and ndecanoic acid. These compounds and their derivatives possess antimicrobial and antiviral inhibitory potentials according to Isah et al. and Ekanem [16,17]. In summary, this study observed that solvent petroleum ether was best for extraction of compound groups such as hydrophobic aromatics and conjugated fatty acids as evidenced in the results since these compounds constitute >70% abundance in the petroleum ether extract. Although, this study also observed that while petroleum ether was able to recover more light-chained oil-derived compounds, the solvent n-hexane fared better for extracting more heavy chained polymeric hydrocarbons.
Finally, the compound with most abundance in the n-hexane extract was also naphthalene-2-methyl with 11.89% abundance. However, there were other compounds in the extracts with <10% abundance such as tetradecanoic acid ethyl ester, dodecanoic acid ethyl ester, 1-Hindene, 2,3,dihydro 4,7,methyl and caryophyllene oxide. All these compounds contained in the extract are also essential oil-derivatives used in treating wound infections of diabetic patients and they have lytic effects on cell walls of bacterial pathogens, which ultimately make them promising drug leads Isah et al. and Ekanem [16,17]. In this study, we observed that the n-Hexane extract also contained many other compound classes that are different from essential oil based derivatives such as polymeric fatty acids and aliphatic hydrocarbons. Interestingly, all these compounds constituted a combined majority of >88% abundance in the n-Hexane extract; this makes the solvent n- Hexane better suited for extraction of both essential oils and other quaternary hydrophobic compounds. In fact, the naphthalene-2- methyl was the only single compound with as high as 11.89% abundance as other single compounds had similar percentage abundances with as many as twenty (20) compounds elucidated from the n-Hexane extract; much more than the compounds found in all the first four solvents combined.
This study showed the presence of significant organic compounds in the herb A. melegueta and the quantities available through solvent extraction exhibit that the herb possesses overt pharmacologically active compounds. The study also showed extracts from the herb contain organic compounds of interest for further antimicrobial research and that the different extracts used in the study were effective for recovering different classes of compounds. This study has provided a broad study map for compounds present in the herb A. melegueta and thus, we recommend that scientists who want to perform subsequent further studies on this ensure that the choice of compounds desired play a pivotal role in selection of solvent choice employed for compound recovery.
There are no conflicts of interests.
The authors acknowledge Mr. Wale Sorungbe of the Department of Biological sciences, Federal University of Technology Akure, Ondo state, Nigeria for the identification of the test herb.
The authors received funding support from the Nigerian Tertiary Education Trust Fund (TETFund) for this research (Grant ID VCPU/ TETFund/155).
Citation: Oladunmoye MK (2019) Characterization of Organic Compounds in Aframomum melegueta K. Schum Using GC-MS. Med Aromat Plants (Los Angeles) 8: 331. doi: 10.35248/2167-0412.19.8.331
Received: 06-Mar-2019 Accepted: 19-Mar-2019 Published: 25-Mar-2019 , DOI: 10.35248/2167-0412.19.8.331
Copyright: © 2019 Oladunmoye MK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.