
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research - (2023)Volume 14, Issue 6
Introduction: The Cuban population has a high proportion of older adults and faces age-related changes in the immune system, known as immunosenescence. Natural Killer T (NKT) cells and Natural Killer (NK) cells play an important role in innate immunity and modulating adaptive immune responses. Their diminished function in older adults contributes to increased susceptibility to infectious diseases, tumors and autoimmune diseases in this population group.
Objective: To characterize distribution of NKT and NK cells in Cuban older adults.
Method: A cross-sectional study was carried out in 30 Cuban older adults, considering age, sex and malignant neoplasm comorbidity as factors of influence on the values of NK and NKT. A linear regression model was used to analyze the data, as well as a two-tailed Mann-Whitney U test to compare independent samples. In addition, odds ratios were used as measures of effect. NK cells and NKT lymphocytes were quantified in peripheral blood using flow cytometry.
Results: In the studied populations of NK and NKT cells, age and sex did not show any significant differences. However, most cases exhibited values above the normal reference ranges, with the exception of one female patient. While no significant differences were found in the comparisons by sex and age, higher values of NK and NKT cells were observed in the group under 80 years old and in males. The adequate NK cell numbers in Peripheral Blood (PB) might be a protective factor against malignant neoplasms.
Conclusion: NK and NKT cells play a fundamental role in the regulation of immune response and directly influence the impairment of immune response in older adults, age and sex showed no significant impact on NKT and NK cell counts and percentages. Nevertheless, the presence of adequate NK cell percentages might be a protective factor against malignant neoplasms.
Natural killer; Natural killer t; Malignant neoplasm; Immunosenescence; Innate immunity; Flow cytometry
The innate and adaptive components of the immune system undergo significant age-related changes known as immunosenescence. These changes include the state of dysregulation and decreased immune response, contributing to morbidity and mortality due to increased incidence or reactivation of infectious diseases, autoimmunity and cancer. The immune response of T cells is most affected by this natural process, although age-related alterations in phenotype and function have been demonstrated in other immune cell populations [1-4].
NKT cells and NK cells are cellular subpopulations that are part of innate immunity. NKT lymphocytes recognize lipid or glycolipid antigens presented by Cluster of Differentiation 1d (CD1d), a non-classical Major Histocompatibility Complex (MHC) molecule. NKT cells represent a very small proportion of total T lymphocytes in PB, although they constitute a larger number in other organs such as the liver, lungs, thymus and spleen [5,6]. NKT cells play an important role in regulating multiple immune responses, including microbial infection, autoimmunity and cancer [7-9]. Even in a steady state, cytokine production by NKT cells influences the basal state and function of other immune cells, such as dendritic cells, CD8+ T cells and NK cells [10].
NKT cells have been found in the thymus, liver, spleen and bone marrow. While the liver could be a potential site for extrathymic development of T cells, evidence suggests that the thymus is the main organ responsible for the development of most NKT cells [11].
Increased NKT cell activity has been found in the pathogenesis of certain autoimmune diseases, allergies and atherosclerosis [12]. Additionally, research on the antitumor function of NKT cells has shown them as a therapeutic alternative against certain types of cancer [8].
On the other hand, NK cells are a population of heterogeneous lymphocytes that act against a wide variety of infections and tumors. The role played by NK cells in defense against viruses is due to both their cytotoxic capacity and the cytokines they produce, particularly Interferon (IFN) production. The decline in NK cell functionality, specifically their cytotoxicity, in the elderly is associated with a higher incidence of infectious diseases in this age group [13-15].
NK cells are derived from pluripotent hematopoietic stem cells. T and NK cell lineages share a common precursor that expresses Fc Gamma Receptors (FcγRIII). While T cell progenitors transfer to the thymus for differentiation and maturation, NK cells can develop independently of the thymus and proliferate in the bone marrow under the stimulation of growth factors. In NK cells, Immunoglobulin (Ig) or T Cell Receptor (TCR) rearrangements do not occur, so neither Ig nor the TCR/CD3 complex are expressed on the cell surface. Mature NK and NKT cells are characterized by the expression of markers such as CD56, a neural cell adhesion molecule isoform [13,14].
The Cuban population is one of the most aged in Latin America, with approximately 20.1% being over 60 years old and a life expectancy of 78.45 years [1]. Therefore, the present research aims to characterize the distribution of NKT and NK cells in older adults and their relation to age and sex.
Study design and subjects
A cross-sectional study was conducted as part of the clinical trial “Evaluation of the Efficacy and Safety of a New Dosage Regimen of BIOMODULINA T® for the prevention of infections, Including COVID-19, in older adults in Cuba” (Public registration code: http://registroclinico.sld.cu/ensayos/RPCEC00000319-Sp). The sample consisted of 30 randomly selected subjects from the “Alfredo Gomez Gendra” Elderly Home. The study included subjects aged 60 years and older, who provided written consent to participate and both sexes were included. Older adults receiving immunomodulatory treatments in the previous two months, with acute allergic states, a history of severe allergic reactions or uncontrolled intercurrent disease were excluded from the study. The comorbidities existing in the patients are declared (Table 1).
| Comorbidity | Total (n=30) |
|---|---|
| Cardiovascular diseases | 25 |
| Dementia | 16 |
| Type II diabetes mellitus | 9 |
| Chronic obstructive pulmonary disease and cerebrovascular diseases | 8 |
| Malignant neoplasms | 4 |
| Skin infections | 5 |
| Urinary tract infections and bronchial asthma | 2 |
Table 1: Distribution of comorbidities in the older adults.
Sample collection and processing
Approximately 2 mL of PB was extracted from each patient using Ethylenediamine tetraacetic acid (EDTA) tubes through venipuncture. All samples were processed and analyzed at the immunology laboratory of the Institute of Hematology and Immunology. Fifty microliters of each sample were taken and incubated in 15 mL tubes for 10 minutes at 4°C, protected from light, with the following fluorochrome-conjugated monoclonal antibodies: anti-CD45 Fluorescein Isothiocyanate (FITC), anti- CD56 Phyco-Erythrin (PE) and anti-CD3 Phycoerythrin Cyanine 5 (PC5) (MACS®, Miltenyi Biotec, Germany). Subsequently, red blood cell lysis was performed using a lysing solution for 10 minutes at room temperature. The cells were washed twice with 0.9% sodium chloride and centrifuged for 10 minutes at 300 g.
A Beckman Coulter Gallios® 8-color flow cytometer (Beckman Coulter®, USA) was used for sample reading, with a minimum of 100,000 acquired events. The data obtained were analyzed using Kaluza Analysis® software version 1.2 for Microsoft®.
The NKT lymphocyte population was defined by the immunophenotype CD45+/CD3+/CD56+ and the NK population by CD45+/CD3-/CD56+. The analysis strategy for NKT cells, where the dot plot over the total labeled cells allows for the separation of singlets; individual cells passing through the laser and presenting uniform height, Forward Scatter Height (FSC-H) and area, Forward Scatter Area (FSC-A) signals that the cytometer registers as an event, discarding cell aggregates (Figure 1A). Then, a dot plot was created using the pan-leukocyte antigen CD45+ expression (Figure 1B). Subsequently, the T lymphocyte population was defined using the CD3+ antigen for NKT cells and CD3- for NK cells (Figure 1C). Finally, both subpopulations were defined using another dot plot with the CD56+ antigen (Figure 1D). The results were expressed as relative values (% of cells) and absolute values (cells/μL). The absolute values of both cellular subpopulations were calculated according to the following formula;
Absolute count (cells/µL) = Lymphocyte count (number of cells/ µL) in blood count x % of the specific cellular subpopulation of interest ÷ 100
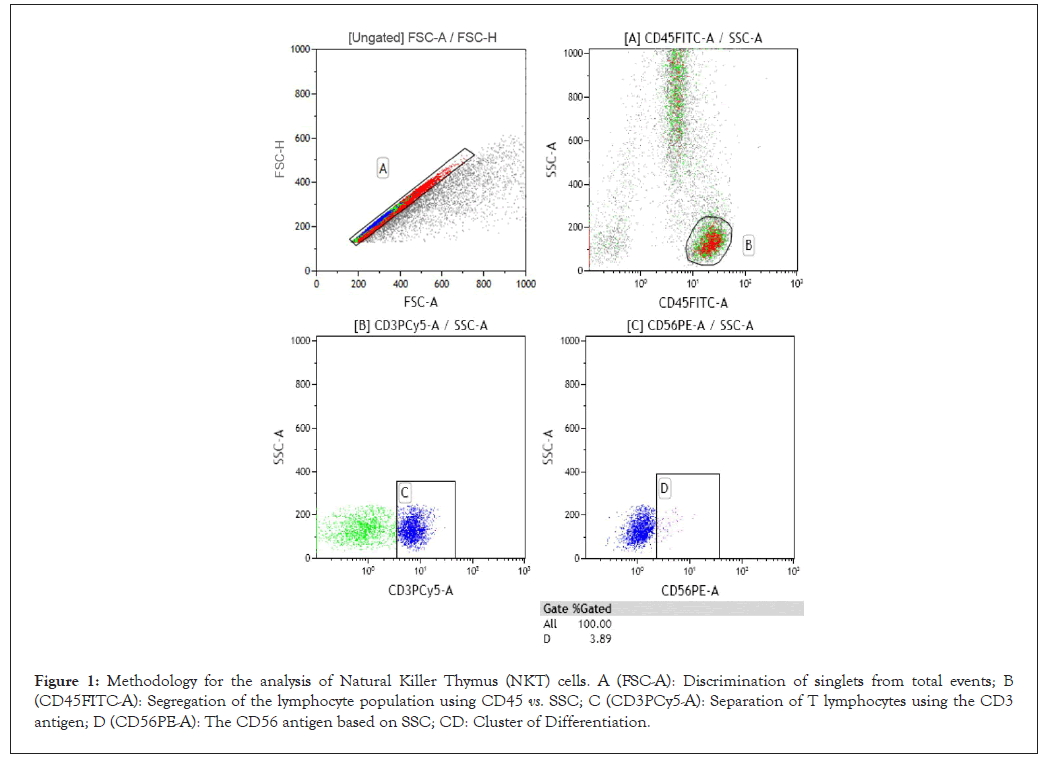
Figure 1: Methodology for the analysis of Natural Killer Thymus (NKT) cells. A (FSC-A): Discrimination of singlets from total events; B (CD45FITC-A): Segregation of the lymphocyte population using CD45 vs. SSC; C (CD3PCy5-A): Separation of T lymphocytes using the CD3 antigen; D (CD56PE-A): The CD56 antigen based on SSC; CD: Cluster of Differentiation.
Statistical analysis
The sample was stratified by sex and age into two groups: under 80 years old and 80 years old or older. Reference value ranges obtained from studies conducted on healthy Cuban adults were used. The Shapiro-Wilk test was used to assess the normality of the distribution of variable values. Descriptive statistics, including mean, median, Standard Deviation (SD) and 2.5th-97.5th percentile range were evaluated due to the variable data skewness. A linear regression model was used to assess the effect of age and the two-tailed Mann-Whitney U test for independent samples was used to evaluate the effect of sex. The significance threshold was set at p ≤ 0.05. The data were processed using Graph-Pad Prism software version 9.5.0. The Odd Ratio (OR) and their respective Confidence Interval (CI) with p ≤ 0.05 were used to determine the effect of malignant neoplasms on the relative and absolute values of NKT and NK.
Ethics
The study was approved by the Research Ethics Committees of the Institute of Hematology and Immunology and Ageing and Health Research Center and the principles of the Declaration of Helsinki were applied in all processes [16].
No effect of age or sex on the evaluated populations was found; the p-values and r² values were not statistically significant. Anormal values above the upper limit of the reference value ranges were found in all cases, except for one 78-year-old female patient, where only NK cells were below the normal values for her age and sex. NKT cells were anormal in 90.5% of the relative values and 81.0% of the absolute values in females (n=21), while in males (n=9), all values fell within the reference range. On the other hand, NK cells in females showed alterations in 42.9% of the relative values and 76.0% of the absolute values, with the latter being normal in males, although the relative values were anormal in 77.8% of males. Regarding age, NKT cells were normal in the percentage values, but 6.7% of the total patients had anormal absolute values. In NK cells evaluated with respect to age, 3.3% of the total had both absolute and relative values outside the range of normal values.
The comparisons made between the groups stratified by sex and age did not yield statistically significant results in any case. However, it is worth noting that NK cells in both distributions showed higher absolute and relative values in the group under 80 years old and in the male group. The mean of the relative and absolute values of NKT cells was also higher in the group under 80 years old and in the male group, only the absolute values were higher (Tables 2-4). The comparison according to neoplasia comorbidity showed that patients without neoplasia had considerably higher numbers of NK cells in PB compared to those who did have neoplasia, while the NKT cells were reduced but not significantly (Table 5).
| NK (CD45+CD3-CD56+) | NKT (CD45+CD3+CD56+) | |||||
|---|---|---|---|---|---|---|
| Median | Mean (SD) | 2.5th-97.5th Percentile | Median | Mean (SD) | 2.5th-97.5th Percentile | |
| % | 11.93 | 14.4 (7.6) | 9.1-19.1 | 5.2 | 6.8 (4.3) | 4.0-10.5 |
| cells/μL | 195.6 | 253.6 (211.0) | 136.5-292.0 | 80 | 126.4 (122.4) | 56.9-122.7 |
Note: SD: Standard deviation; NKT: Natural killer T cells; NK: Natural killer cells; CD: Cluster of differentiation.
Table 2: Absolute and relative values for total NK and NKT cells (n=30).
| < 80 years (n=17) 56.7% of Total | ≥ 80 years (n=13) 43.3% of Total | ||||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | 2.5th-97.5th Percentile | Median | Mean (SD) | 2.5th-97.5th Percentile | Median | p-value | |
| CD3+CD56+ (%) (17) | 7.0 (4.0) | 4.2-10.5 | 5.9 | 6.649 (4.719) | 3.1-10.5 | 5 | 0.5634ns |
| CD3+CD56+ (cells/μL) (17) | 140.0 (143.0) | 54.8-127.1 | 87.9 | 108.6 (91.4) | 55.4-148.4 | 72.7 | 0.4570ns |
| CD3-CD56+ (%) (17) | 15.16 (7.779) | 10.28-20.24 | 13.27 | 13.46 (7.438) | 8.270-18.97 | 10.44 | 0.3851ns |
| CD3-CD56+ (cells/μL) (17) | 281.2 (251.5) | 139.1-317.6 | 218 | 217.5 (144.1) | 120.0-274.4 | 173.1 | 0.4083ns |
Note: SD: Standard deviation; n: Sample size; ns: Not significant; NKT: Natural killer T cells; NK: Natural killer cells; CD: Cluster of differentiation.
Table 3: Absolute and relative values of NKT and NK cells by age groups.
| Male (n=9) 30% of Total | Female (n=21) 70% of Total | ||||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | 2.5th-97.5th percentile | Median | Mean (SD) | 2.5th-97.5th percentile | Median | p-value | |
| CD3+CD56+ (%) (15) | 6.3 (3.5) | 3.1-10.5 | 3.7 | 7.1 (4.6) | 4.3-10.5 | 7.7 | 0.6567ns |
| CD3+CD56+ (cells/μL) (15) | 151.6 (160.7) | 57.9-211.9 | 72.6 | 115.7 (104.9) | 51.0-116.6 | 80.3 | 0.8243ns |
| CD3-CD56+ (%) (16) | 16.8 (7.1) | 10.8-25.0 | 13.3 | 13.4 (7.7) | 8.7-18.5 | 11.7 | 0.1635ns |
| CD3-CD56+ (cells/μL) (16) | 389.4 (321.2) | 153.3-504.4 | 218 | 195.4 (106.7) | 109.5-258.6 | 193.1 | 0.0697ns |
Note: SD: Standard deviation; n: sample size; ns: Not significant; NKT: Natural killer T cells; NK: Natural killer cells; CD: Cluster of differentiation.
Table 4: Absolute and relative values of NKT and NK cells by sex.
| Neoplasia (n=4) 13.3% of Total | No neoplasia (n=26) 86.7% of Total | |||||
|---|---|---|---|---|---|---|
| Median | CI | Median | CI | OR [CI] | p-value | |
| CD3+CD56+ (%) | 10.6 | 9.0-11.6 | 5.1 | 3.9-7.4 | 1.19 [1.51-0.95]ns | 0.100ns |
| CD3+CD56+ (cells/μL) | 166.2 | 121.8-209.8 | 76 | 53.9-111.3 | 1.00 [1.01-1.00]ns | 0.088ns |
| CD3-CD56+ (%) | 5.5 | 3.1-8.3 | 13.5 | 10.3-19.6 | 0.50 [1.06-0.24]* | 0.010* |
| CD3-CD56+ (cells/μL) | 95.9 | 70.8-114.1 | 208.1 | 165.7-312.2 | 0.96 [1.00-0.93]* | 0.005* |
Note: CI: Confidence interval; OR: Odds ratio; ns: Not significant; NKT: Natural killer T cells; NK: Natural killer cells; CD: Cluster of differentiation.
Table 5: Distribution of relative and absolute values of NKT and NK according to the neoplasia comorbidity.
Linear regression analyses to assess the effect of age did not yield r² values close to unity, with statistically non-significant p-values for the evaluated cellular populations (data not shown). These findings are consistent with the results reported by Villegas et al., [17] regarding NKT cells and by Kokuina et al., [18] for NK cells in two studies conducted on a Cuban adult population.
There was not influence of sex on the values of NKT cells in older adults. Rojas-Pandales et al., [12] also did not find a sex effect on NK and NKT values in Colombian individuals, although it is worth mentioning that they only studied subjects between 18 and 58 years of age. However, Villegas et al., [17] found a sex effect on the NKT population, with a slightly higher number in males. On the other hand, a study by Apoil et al., [19] showed a sex effect on NK cells in a French adult population, with significantly higher values in males, but no age effect was observed for this subpopulation.
Only 30% of the evaluated older adults were male, which results in an unbalanced representation of this sex and the sample size was much smaller compared to the aforementioned studies. These factors may explain why these effects were not observed in the presented results. However, in the comparison of absolute values of NK cells in males, the p-value was close to 0.05 (p=0.0697) and the mean was higher in both absolute and relative values, indicating that a larger sample size might reveal the sex effect.
Generally, women exhibit stronger humoral and cellular immune responses than men, linked to higher antibody levels and immune mediator production. Sex chromosome genes and sex hormones like estrogen, progesterone and androgens influence immune responses, with estrogen enhancing both cell-mediated and humoral immunity, while androgens suppress Type 1 T Helper (Th1) cell differentiation and reduce Interferon‐Gamma (IFN-γ) production. Nutritional and microbiome differences also impact immune function between sexes [20-23]. Taking into account that women participating in this study are over 60 years of age, the influence on the cellular immune response associated with the presence of estrogens would not be as much as significant.
Aging leads to decreased lymphocyte concentration and function. T lymphocyte decline is mainly due to thymic involution. Conversely, the increase of NK absolute and relative values in PB due to successive antigenic challenges is an evidence of the immunosenescence process. Subpopulation balance changes, with fewer naive lymphocytes and more memory, exhausted and senescent phenotypes accumulate. Clonal diversity decreases and oligo clonal populations expand, contributing to immunosenescence [4,13-15,24].
The values outside the range of normal values were found only above the upper limit of each reference value range for both NK cells and NKT cells. The high data dispersion observed in NKT cells, could be related to previous exposures to stimulating agents. The means of the absolute values for NK and NKT cells were higher in the group under 80 years old and in the male group. However, specifically for NKT cells, the Standard Deviation (SD) was higher than the mean in both distributions, indicating a high dispersion among the data. NKT cells accumulate in PB as their affinity antigens are encountered, so the inter-individual variability found may be directly related to the number of previous exposures to agents capable of stimulating NKT cells [17]. Additionally, the expansion of this population has been reported in pathological conditions such as sarcoidosis, allergies, various types of cancer and Human Immunodeficiency Viruses (HIV) or Human Cytomegalovirus (HCMV) infection [17,19]. Apoil et al., [19] found in the aforementioned study that HCMV seropositivity was associated with an increased frequency of CD56+ T cells. García et al., [25] observed a high prevalence of HCMV infection in a group of Cuban adults aged 18 to 102 years. Considering this, it is possible that HCMV infection contributes to the increase in the NKT cell population in these groups.
The accumulation of terminally differentiated NK cells (CD56dim: with Natural Killer Group 2D (NKG2C+), Cluster of Differentiation 57-antigen (CD57+) or Fc-tetrameric Receptor Complex (FcεRIγ-)) in PB can be caused by factors such as advanced age, chronic antigenic exposure following HCMV infection or sex chromosome genes, as it has been found that males have higher values of these cell populations. Some sex differences in the maturation and differentiation process of NK cells persist in elderly females, where a higher NK CD56bright/CD56dim ratio has been reported compared to elderly males [19,26,27].
The 13.33% of the patients had malignant neoplasms and within this percentage, there was one patient with relative and absolute values below the normal reference range, but only for NK cells. Among the most studied effector immune cells with biological significance in cancer are NK cells as part of innate immunity, further research into lymphocyte immunophenotypes has led to the discovery of new subpopulations with important roles in cancer biology, such as NKT cells. The effects of immunosenescence manifest rapidly in cancer and are associated with a worse prognosis, then with less immunosurveillance support, cancer incidence considerably rises with age [4,7,13-15].
The comparison according to neoplasia comorbidity showed that patients with neoplasia had considerably lower numbers of NK cells in PB compared to those with no neoplasia, while the NKT cells were reduced but not significantly. The OR indicates that adequate numbers of NK cells in PB might be a protective factor against malignant neoplasms. Arango et al., [26] evaluated lymphocyte subpopulations in Cuban cancer patients and found that with increasing age, there is a tendency for a decrease in T, B and NK lymphocyte populations and patients with solid tumors showed the lowest values. Wang et al., [28] demonstrate that age is one of the variables that most influences immunosenescence and they conclude that T cells, NK cells and NKT cells are gradually affected in cancer.
NK cells play important roles in the antitumor immunity in the absence of sensitization, which contributed to the prevention of cancer invasion and metastasis and finally prevented the early dissemination of cancer cells. It is known that terminally differentiated NK cells increase in frequency in cancer patients, whereas NK CD56 bright cells are in an exhausted state, which refers to the exhaustion or dysfunction caused by immunosuppression in the tumor microenvironment and tumor evasion mechanisms that result in a decreased ability of NK cells to effectively recognize and eliminate tumor cells. In this sense, NKT cells play an important supporting role, as they not only exert immune surveillance against cancer but also help restore effector function and the recognition and elimination capacity of cancer cells by NK cells. NKT cells reverse NK cell exhaustion through cytokine production, expression of co-stimulatory molecules and their immune regulatory activity, reducing immunosuppression in the tumor microenvironment [25,26].
However, previous studies have shown certain associations between sex and these cells, although the unbalanced representation of sexes in this study may have affected the results. Further research with larger and balanced samples is recommended to obtain more solid conclusions.
NK and NKT cells play a fundamental role in the regulation of immune response and directly influence the impairment of immune response in older adults. This impairment contributes to increased susceptibility to infections, tumors and autoimmune diseases in this population group. In this study, no significant influence of age and sex was found on the values of NKT and NK cells in peripheral blood of older adults. However, adequate numbers of circulant NK cells could be a protective factor against malignant neoplasms.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Ramos EH, Suarez VM, Hernandez IC, Zamora MCR, Guerra LPH, Marrero YT, et al (2023) Characterization of Natural Cytotoxic T Lymphocytes and Natural Killer Cells in Cuban Older Adults. J Clin Cell Immunol. 14:704.
Received: 28-Oct-2023, Manuscript No. JCCI-23-28034; Editor assigned: 30-Oct-2023, Pre QC No. JCCI-23-28034 (PQ); Reviewed: 13-Nov-2023, QC No. JCCI-23-28034; Revised: 20-Nov-2023, Manuscript No. JCCI-23-28034 (R); Published: 27-Nov-2023 , DOI: 10.35248/2155-9899.23.14.704
Copyright: © 2023 Ramos EH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.