International Journal of Physical Medicine & Rehabilitation
Open Access
ISSN: 2329-9096
ISSN: 2329-9096
Mini Review - (2021)Volume 9, Issue 5
For a century, it was believed that neuron membrane transmits electric field, that pulse is the information content. Everything inside remain silent. Nerve spike is the foundation of artificial intelligence, neuro medicine, and neuroscience. In recent years, it has been shown using state-of-the-art technology that filaments fire, regulate spike time modulation precisely, redefining cognition. The results are going to change neuroscience fundamentally; it would change the fundamentals of artificial intelligence and brain science.
Hodgkin huxley paradigm; Microtubule; Neurophysiology; Nerve spike; Electromagnetic resonance
1907, Lapicque proposed that an electric field passes through the membrane and transmits a signal. A snake curve was used back then to depict how linear flat current receives a sudden peak [1]. That very picture remained for more than 115 years in the textbooks. Despite so many discoveries in neuroscience, it is surprising that no one pointed out that the membrane has a cylindrical shape. A dot spike cannot propagate through a cylindrical surface. It would instantly dissipate. A nerve spike should look like a ring, encompassing the diameter of a cylindrical axon or Dendron [2]. However, this subtle change would have remarkable implications. Holding the circular shape of an electric field is not easy, especially on an organic surface. Here we would explain how neuroscience could redefine itself, just trying to answer this question.
Neuroscience is one field where time to time, rigorous, bold, insightful experiments were performed, reported, but those studies hardly made an inroad to mainstream neuroscience. In the 1920s [3], it was shown that neurons communicate and fire even if we block transmission between two neighboring neurons.
There is a non-physical communication between neurons, but it was ignored. In the 1960s, when Hodgkin and Huxley were melting the filaments inside a neuron and still generating the neuron burst, one important question they did not take into account . Could one regulate the time gap between spikes without filaments? In the brain's cognitive process, sub threshold communication that modulates the time gap between spikes holds the key to information processing [4]. The membrane's ability to modulate time was assigned to the density of ion channels. Such speculations were debated because neurons would fail to process a new pattern of spike time gaps before adjusting density. If a neuron waits until the density of ion channels modifies and makes itself fit, till then (~20 minutes), the cognitive function would collapse. Many discrepancies were noted, but no efforts were made to resolve them. In the 1990s, reports started pouring in that electromagnetic bursts or electric field disbalance in the environment causes a firing [5]. Those reports remained outside the model of a neuron. It was not surprising because tons of works made in the 1990s correcting the Hodgkin and Huxley model with better equations were ignored simply because it was difficult to model neural networks with complex equations. When computing power amplified, they remained ignored. We should note the final discovery that finding the grid-like network of actin and beta-spectrin just below the neuron membrane [6]. The grid is directly connected to the membrane. Why is it out there bridging the membrane and the filamentary bundles in a neuron?
The list is endless, but the mother of all concerns is probably the simplest question ever asked in neuroscience. How does a nerve spike look like in reality? The answer is out there. It is a 2D electric field perturbation. In Figure 1, we have compared the shape of a nerve spike, perception vs. reality.
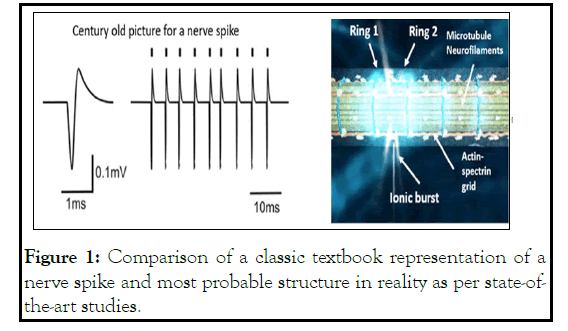
Figure 1: Comparison of a classic textbook representation of a nerve spike and most probable structure in reality as per state-ofthe- art studies.
It is not a simple difference. All the ion channels in that circular area should be activated at a time. In this circular area, polarization, depolarization for all ion channels should happen together. That is easy to presume, difficult is to explain the mechanism, and how membrane could control all ion channels in a ring shape and propagate the ring linearly along the membrane.
There is another problem. In the history of neuroscience, whenever new and unique features were discovered, neuroscientists kept referring to the density of ion channels and attributed it to everything. In the 1960s, branch selection became an exciting research problem [7]. Certain neural branches are selected when nerve spike transports. Initially, it was attributed to the change in diameters of the branches and then to the density of states. Surprisingly, synaptic junctions switch on and off, synchronize in a group; burst is not random and not homogeneously transported through all junctions. For a certain period, a group of junctions turns active, and then for a particular period turns silent and then active again. Again, complex selection and deselection of groups were attributed to the density of ion channels. If we list the entire different things ion channels have to do, it is a historic burden.
So, if we do not stop here, we will see the rise of another attribution for the circular shape. A ring-shaped area should have an equal density of Na, K, and Caion channels. The demand is contradictory to branch selection. However, there is another concern. In current CMOS-based computer chips, very fast processor clocks group to create longer and slower clocks. There is no science known where a milliseconds clock controls time gaps of milliseconds. We need at least the microsecond’s clocks that precisely control the ion channels, not making milliseconds error. Neuroscience literature is rich in addressing events operating in milliseconds, nanoseconds, picoseconds to femtosecond domains, as reports on corresponding ac frequencies are abundant. There is a missing time domain, microseconds. So, it was essential to bridge this gap while assigning a backup clock to the streams of milliseconds pulses governing the key cognition signals. A suitable component did not exist in the literature.
Therefore, around 2008, investigations started on microtubules to find that it stores information, reversibly [8,9]. If microsecond’s pulses are sent through a single microtubule, the transmitted electric field across a single microtubule amplifies 104 times depending on the time width of pulses and the time gap between pulses. It is difficult to assume that time modulation could be tested by sweeping the frequencies of ac signals we shine on microtubules using an antenna. Frequencies that cause bursts are resonance frequencies. Quantum tunneling images reveal how proteins look when they resonantly vibrate [10]. All the magic frequencies where the microtubule bursts microsecond signals revealed that peaks nearby in the transmission spectrum form triplet of triplet groups, and shockingly the grouping of resonance frequencies is scale-free [11]. A single protein, a protein-made filament, and whole neuron cell groups preferred frequencies similarly.
Moreover, each peak is associated with a phase. While passing through a microtubule, the ac signal shifts its phase by nπ/4. The sum of phase shifts of a set of resonance frequencies in a group is 360, so it is not an accident. Microsecond bursts integrate time to form a new slower clock, just what we require to support the millisecond pulses. Synthesizing clocks, integrating the clocks, and building 3D clock assembly are fundamental properties of all major filaments and associated proteins, neurons [12-14]. The immediate concern is, if microtubule-like filaments are controlling the precise timing of ion spikes, are microsecond bursts of filaments correlated with the milliseconds' bursts of neurons?
The correlations are reported [15,16]. However, new observation required newly invented tools. The patch-clamp where a metal probe was inserted inside a glass tube to neutralize ions of cell fluids compared to external culture media, claimed for single ion channel measurement. However, glass tube opening is 20-30 nm wide, difficult to probe a single ion channel 0.4nm wide. So, we used a coaxial probe where the central Pt electrode has an atomic sharp needle covered by the insulated glass [17,18]. The outer layer has a metal Au layer, which ensures local thermal, electromagnetic, electric noises are filtered out. The most exciting part of this study is to recognize a particular component deep inside a living cell. Every biomaterial has a unique set of resonance frequencies or a band; at least all documented proteins and filaments resonate at particular frequencies [11,19,20]. When the probe is inserted into the cell, at contact, a band is used as a signature to recognize the element. Resonance frequency means a burst of electromagnetic signal when the sample is remotely shined with an ac signal of a particular frequency. Thus, the coaxial probe read ultrafast signals deep inside the axon or Dendron or neural branches, while a patchclamp measured the classic nerve-based ionic bursts.
Simultaneous measurements of signals from membrane and filaments, transmitting signals at two distinct time domains, clarify several neuroscience mysteries. Ionic transmissions of membranes are resonated at milliseconds, and filamentary dipoles resonate at microseconds. They feed each other together. Filaments deep inside a neuron acquire signals of suitable frequencies from thermal noise, filter out the microseconds bursts and send it like an antenna. Since they are not metals, absorption, emission, and reflection of electromagnetic signal turns interesting since all three parts play governing roles in energy transfer. So, filamentary bundles do not see neuron membranes or any other components in a neural network. They see only filamentary bundles of similar geometry so that there is a resonant energy transfer. So, the role of synaptic junctions turns insignificant. We cannot see this new circuit using an optical microscope [20]. We need a dielectric microscope where remotely one could image those filaments which are resonating across the network. These hidden circuits operating in the microsecond time domain are very different from the circuit we see under the optical microscope.
Thus, these filaments are noise-driven. They do not need signals. Using energy from the noise, they create a superposition of many integrated circuits which remain silent in the background. It will be very interesting to see how these circuits govern the plasticity of a neural network when a brain learns. Therefore, E. Katz [3] might not have blocked electromagnetic signals in the 1920s. Once we know that filaments of distant, isolated neurons are connected, electromagnetic signals of a set of frequencies could arrive and resonate, build and hold electric potential. Sustaining it is important. Imagine a component beneath the neuron membrane at the filamentary core, holds the potential 103 times faster than the ionic potential build-up. Now, these memorized potentials would homogeneously activate the membrane all around [21,22].
For this reason, it was repeatedly observed that filaments fire 250 microseconds earlier than ionic nerve impulses [15]. So, at least four filamentary bursts are there to regulate the time width of an ionic burst. If two filamentary bursts are used to set an ionic burst's upper- and lower-time limits, two other filamentary bursts ensure peak formation in an ionic burst. Thus, one primitive concern regarding time gap regulation for a stream of nerve spikes is resolved. The second and the most important part is shaping the circular nerve spike.
Then we need to bring into another great observation of neuroscience, 200 nm X200 nm grids made of actin filaments and beta spectrin just beneath the neuron, separating the membrane from the filamentary core [6]. Using a dielectric resonance microscope, it is easy to image filamentary part live as an alternative to STORM selectively. Simultaneously we can measure signal transmission through the grid layer, only to find that it integrates the micro seconds clocks or bursts [19,20]. It was reported that a pair circular ring made of proteins separated by a 200nm gap across the neural branches activated one after another. The pair of rings was imaged live in a dielectric resonance microscope. The ring of an electromagnetic field is a vortex, and 8-10nm below the membrane, the pair of rings can activate all the ion channels located between rings holding a homogeneous potential distribution in the entire region.
Now, we come to the final part of the unique adventure through neurons. Monochromatic polarized lasers were shined on a neuron, and helical assemblies changed the photon's angular momentum in various ways. Holographic projection is deconvoluted from the hologram. One could identify the exact ring of light comes from a particular component. Using an external antenna, shine resonance frequency signals remotely on the neuron cell; the exact component would brighten the light ring. So, the 3D clock assembly of a neuron is now converted into an optical hologram to see what is happening inside a neuron, live.
Thus, we saw that three new technologies, coaxial atom probe, dielectric resonance microscopy, and remote microwave inputoptical vortex output, can reveal neuroscience that was hinted at many times for the last 100 years but was never integrated into a model.
Authors acknowledge the Asian office of Aerospace R&D (AOARD) a part of the United States Air Force (USAF), for the Grant no.FA2386-16-1-0003 (2016-2019) on the electromagnetic resonance-based communication and intelligence of biomaterials.
Declare conflicts of interest or state "The authors declare that they have no competing financial and non-financial interests."
Citation: Ghosh S, Singh P, Saxena K, Sahoo P, Bandyopadhyay A (2021) Century-Old Picture of a Nerve Spike is wrong: Filaments Fire, Not Just Membrane. Int J Phys Med Rehabil. 9:612.
Received: 13-Sep-2021 Accepted: 27-Sep-2021 Published: 04-Oct-2021 , DOI: 10.35248/2329-9096.21.9.612
Copyright: © 2021 Ghosh S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.