Journal of Cell Signaling
Open Access
ISSN: 2576-1471
ISSN: 2576-1471
Commentary - (2019)Volume 4, Issue 3
In chronic infection, T-cell exhaustion due to sustained programmed cell death (PD)-1 expression on cytotoxic T lymphocytes (CTLs) is one of the major issues leading to ineffective virus elimination. Programmed cell death ligand-1 (PD-L1) pathway blockade has been found to restore the function of exhausted CTLs during chronic infection. Understanding mechanisms involved in the conversion of CTL exhaustion is essential to improve disease therapy and vaccine protocols. In this commentary, we focus on the mechanism by which triggering CD40 signal alone can convert CTL exhaustion and synergize with PD-1 checkpoint blockade in rescuing exhausted CTLs.
T-cell exhaustion; Programmed cell death ligand-1; CD40; Chronic infection; mTORC1
Generation of an effective cytotoxic T lymphocyte (CTL) immune response against foreign antigens requires the engagement of antigen-specific T-cell receptors (TCRs) by antigenic peptide-major histocompatibility complex (pMHC) on antigen-presenting cells (APCs). The outcome of this interaction is influenced not only by cytokine milieu but also by the balance between positive (e.g., CD28) and negative (e.g., CTLA-4) co-stimulations. Generally, CTL response during infection develops in three phases: expansion, contraction, and memory formation [1,2]. In acute viral infection (e.g., murine lymphocytic choriomeningitis virus (LCMV) Armstrong strain), the virus is cleared 8 days post-infection; however, in chronic infection (e.g., LCMV clone 13 strain, hepatitis B virus, and human immunodeficiency virus type-1 (HIV-1)) the virus persist for several months [3]. In murine chronic infection, memory CTLs (mCTLs) express multiple inhibitory signals, such as programmed cell death protein-1 (PD-1), lymphocyte-activation gene-3 (LAG3), and T-cell Ig and mucin-3 (TIM3), due to lingering T-cell stimulation by persistent viral pathogens [4]. As a result, CTLs gradually lose effector functions and exhibit varying degrees of functional impairment, such as defects in cell proliferation, secretion of effector T-cell cytokines such as IFN- γ, and cytolytic activity (Figure 1). Thus, CTL exhaustion is one of the major barriers to efficient elimination of viruses in chronic infections [4].
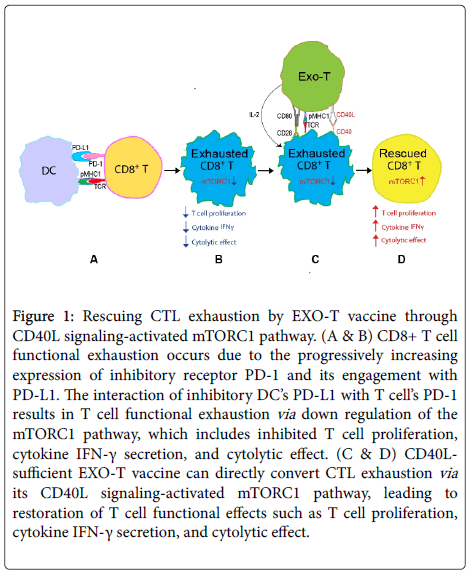
Figure 1. Rescuing CTL exhaustion by EXO-T vaccine through CD40L signaling-activated mTORC1 pathway. (A & B) CD8+ T-cell functional exhaustion occurs due to the progressively increasing expression of inhibitory receptor PD-1 and its engagement with PD-L1. The interaction of inhibitory DC’s PD-L1 with T-cell’s PD-1 results in T-cell functional exhaustion via down regulation of the mTORC1 pathway, which includes inhibited T-cell proliferation, cytokine IFN-γ secretion, and cytolytic effect. (C & D) CD40Lsufficient EXO-T vaccine can directly convert CTL exhaustion via its CD40L signaling-activated mTORC1 pathway, leading to restoration of T-cell functional effects such as T-cell proliferation, cytokine IFN-γ secretion, and cytolytic effect.
PD-1, an inhibitory receptor that belongs to an extended CD28/ CTLA-4 costimulatory family, was found in T, B, and myeloid cells following cell activation [5]. In mice, PD-1 was also highly expressed on exhausted CD8+ CTLs during LCMV chronic infection, but absent on functional CTLs in acute LCMV infection [4]. CTL exhaustion featuring cellular functional defects in cell proliferation, production of IFN-γ, and cytolytic activity is the main reason for ineffective virus elimination in chronic infectious diseases [6].
PD-1 ligand-1 (PD-L1), the ligand of PD-1, was found to express in normal mouse splenocytes [7] as well as in dendritic cells (DCs) in chronic infection [8]. In human and mice, it was reported that triggering PD-1 on CTLs by PD-L1 reduces T-cell functions by inhibiting TCR signaling by recruiting phosphatases [9,10] and decreasing phosphoinositide 3-kinase (PI3K)/Akt/mTORC1 and Ras- ERK (extracellular signal-regulated kinase) pathways [11-13], thus leading to CTL functional exhaustion. The in vivo administration of antibodies interferes with the interaction of PD-1 with PD-L1, thus disrupting ensuing inhibitory signals and resulting in rescued CTL exhaustion and enhanced T-cell responses (Figure 1) [5]. An enhanced conversional effect on CTL exhaustion was observed when a dual blockade of PD-1 with TIM3 or LAG3 was administered to treat chronic infection in mice [14,15].
A later study suggested that PD-1 blockade-mediated rescue of CTL exhaustion markedly depends on the CD40L signal in chronically infected mice [16]. Bhadra et al. demonstrated that blockade of the CD40-CD40L pathway using anti-CD40L antibody (Ab) abrogated the ameliorative effects of anti-PD-L1 treatment on exhausted CD8+ T-cells in a murine Toxoplasma model [16]. These authors also identified that, among a panel of costimulatory molecules, CD40 was highly upregulated on CD8+ T-cells in chronically infected mice [16]. CD40 is a member of the tumour necrosis factor receptor (TNFR) superfamily that is broadly expressed by immune cells, in particular DCs, and plays an important role in stimulation of CTL responses and protective immunity against pathogens via DC licensing [17]. Direct CD40- CD40L interaction is necessary for effective DC licensing [18]. Licensed DCs up regulate cytokines such as IL-12, costimulatory molecules such as CD80 and CD86, adhesion molecules such as CD54, and an array of other TNFR superfamily members that engage their receptors on T-cells [19]. These licensed DCs then became capable of priming CD8+ T-cell responses [20]. These studies suggest that blockade of the CD40-CD40L pathway in vivo using anti-CD40L Ab by Bhadra et al. [16] possibly interfered with CD40L signaling on DCs. Findings reported by Isogawa et al. also support this assumption; these authors found that CD40-mediated activation of myeloid DCs could rescue CTL exhaustion of PD-1-inhibited CD8+ T-cells in hepatitis B virus (HBV) transgenic mice whose livers produce infectious viral particles that lead to chronic infection [21]. Rescue of exhausted CTLs by PD-1 targeted therapies was recently demonstrated to be CD28 dependent in human and mice [22]. The dependence of PD-1 blockade upon CD40L and CD28 signaling indicates conversion of CTL exhaustion by PD-1 blockade in chronic infection in an indirect manner.
In murine model, we previously reported that antigen ovalbumin (OVA)-specific DC (DCova)-released exosomes (EXOOVA) expressing exosomal pMHC-I complexes and costimulatory molecule CD80 can be loaded onto polyclonal CD4+ T-cells to generate OVA-specific EXO-T vaccines that are capable of directly stimulating effective CTL and memory CTL responses independent of host CD4+ T-cell help and DCs via its signaling of exosomal pMHC-I complexes, exosomal CD80 costimulation, and CD4+ T-cell’s CD40L and IL-2 cytokine [17]. Our lab [23] and others [24] showed that CD40 signaling is delivered directly to CD8+ T-cells for effective CTL and memory CTL responses. We were interested in examining if the CD40L-sufficient EXO-T vaccine can convert CTL exhaustion in chronic infection. Therefore, we assessed the therapeutic effect of the EXO-T vaccine in our recently developed chronic infection model by infection of C57BL/6 mice with OVA-expressing adenovirus (AdVova), in which CTLs expressing PD-1, LAG-3, and CD40 showed functional exhaustion [25]. Interestingly, we found that the CD40L-sufficient EXO-T vaccine could directly convert CTL exhaustion during chronic infection despite the expression of inhibitory receptors PD-1 and LAG-3 by CTLs (Figure 1), which is distinctive from PD-1 blockade’s effect in an indirect manner [25]. We further examined the molecular mechanism for CD40 signaling-mediated rescue of CTL exhaustion. We, for the first time, demonstrated that the CD40L/IL-2 expressing EXO-T vaccine converted CTL exhaustion via activation of PI3K and mTORC1-regulated S6 kinase (S6K) and eukaryotic initiation factor 4E (EIF4E) (Figure 1), leading to up regulation of T-bet, which controls T-cell activation and increases expression of Ki67, a protein associated with cell cycle progression [25]. We found that CD40L signaling of EXO-T vaccine plays a major role in direct conversion of CTL exhaustion, while EXO-T’s IL-2 is also involved in its conversional effect [26] but to a much lesser extent than CD40L signaling [25]. Importantly, our HIV-1 Gag-specific EXO-T vaccine also induced Gag-specific therapeutic immunity in chronic infection [25]. The significant effect of CD40L signaling in rescuing CTL exhaustion has recently been confirmed using agonist CD40 (anti- CD40 Ab, clone FGK4.5 purified from ascites of hybridoma cell lines) treatment [6]. We demonstrated that agonist CD40 treatment alone could directly convert CTL exhaustion and that agonist CD40 treatment in conjunction with the PD-1 blockade (anti-mouse PD-L1 Ab, clone 10F.9G2 from BioXCell Inc, West Lebonan) has a synergistic effect for conversion of CTL exhaustion in chronic infection in mice [6]. The unique feature of CD40 agonist in direct conversion of CTL exhaustion in chronic infection is also distinctive from other costimulatory (41BB, OX40, and CD27) signaling. Administration of anti-41BB, anti-OX40, and anti-CD27 Abs alone did not induce any conversional effect for CTL exhaustion but did enhance PD-1 blockade’s effect on rescuing exhausted CTLs in chronic infection [26,27]. Taken together, our data suggest that CD40 signaling can directly convert CTL exhaustion in chronic infection via activation of the PI3K/Akt/mTORC1 pathway, and CD40 agonist-activated mTORC1 signaling synergizes with PD-1 checkpoint blockade to counter T-cell exhaustion in chronic infection. Over the past decade, several agonist CD40 antibodies have been developed that demonstrated considerable clinical efficacy yet can also exert adverse effects such as cytokine release syndrome and hepatotoxicity in the clinic [28]. However, new research for improving the efficacy and safety of agonistic anti-CD40 antibody are essential [29].
Recent advances in immunology suggest that T-cell immunity is largely dictated by the metabolic program [30]. T-cells with high mTORC1 activity have demonstrated elevated glycolytic flux and generated T-cell populations with enhanced effector capacity in mice [31]. Because mTORC1 influences T-cell metabolic capacity, especially glycolysis [31], and activated T-cells rely on glucose metabolism for enhanced proliferation and effector function [32,33], it will be interesting to investigate the role of CD40 signaling in conversion of CTL exhaustion through mTORC1-mediated metabolic switching. Although details of the mechanism by which CD40L signaling synergizes with PD-1 blockade to rescue CTL exhaustion remain to be established, CD40 agonist clearly plays an important role distinctive from PD-1 blockade in conversion of CTL exhaustion in chronic infection.
Citation: Ara A, Xiang J (2019) CD40 Agonist-activated mTORC1 Signaling Synergizes with PD-1 Checkpoint Blockade to Counter T-cell Exhaustion in Chronic Infection. J Cell Signal 4: 202. doi: 10.35248/ 2576-1471.19.4.202
Received: 27-Mar-2019 Accepted: 10-Apr-2019 Published: 17-Apr-2019 , DOI: 10.35248/2576-1471.19.4.202
Copyright: © 2019 Ara A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.