Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2023)Volume 13, Issue 4
Rationale: Nanotechnology is the study of manipulation of material at nanoscale. At nanoscale particles exhibit unique properties like, sensor, catalytic, and antibacterial. Copper Nanoparticles (Cu-NPs) were synthesized using Brassica oleracea L. (Cabbage) extract in the current examination through an ecological green and straightforward method.
Rationale: Nanotechnology is the study of manipulation of material at nanoscale. At nanoscale particles exhibit unique properties like, sensor, catalytic, and antibacterial. Copper Nanoparticles (Cu-NPs) were synthesized using Brassica oleracea L. (Cabbage) extract in the current examination through an ecological green and straightforward method.
Results: temperature, time, concentration, and pH, A UV-visible spectrophotometer confirmed the formation of copper nanoparticles. Obtained spectra showed the absorption peak at 550 nm. UV-visible spectrophotometer, Energy Dispersive X-Ray spectroscopy (EDX), Fourier infrared spectroscopy (FTIR), and Scanning Electron Microscopy (SEM) was utilized to characterize the produced Cu-NPs. SEM results showed the synthesized Cu-NPs have an average size and shape. FTIR results committed the existence of functional groups at different positions. EDX spectra showed the elemental composition of extract and synthesized Cu- NPs. The biosynthesized Cu-NPs is also used as an antibacterial agent against selected pathogens like Escherichia coli, Gram-negative Pseudomonas spp, and Bacillus spp.
Discussion: The biosynthesized Cu-NPs used as a catalyst in the reduction of 4-nitrophenol into 4-aminophenol by utilizing sodium borohydride in a liquid solution and showed tremendous results. Results revealed that the Brassica oleracea L. (cabbage) extract is effective for the synthesis of copper nanoparticles and biosynthesized copper nanoparticles used as an antibacterial agent for Bacillus spp. and a catalyst in the organic transformation of 4-Nitrophenol.
Ecological green; Brassica oleracea L; Copper; Nanoparticles; Antioxidant system; Catalytic activity
Nanotechnology is the study of a nanomaterial on a 10-9 scale. The nanoparticles have gained huge interest because of their bundles of applications. Because of their unique chemical and physical properties at the nanoscale, nanoparticles have been used in many narrative performance applications. Nanoparticles have been synthesized by various methods, including the Physical, Chemical, and green routes, but the green approach has more than a few benefits over physical and chemical processes like uncomplicated, cost-effective use of nontoxic chemicals and required low temperature [1]. Various papers have been published on the ecological synthesis of copper nanoparticles through the utility of plant essence, for instance; Pineapple, Ginger, Guava [2], Lemon, Punica granatum Peel, Aloe Vera flower [3], Plantago asiatica leaf and many more. A bundle of research has been reported that copper nanoparticles are promising antimicrobial agents that show the novel performance against different microorganisms’ species [4]. A recent study revealed that the green synthesis of copper nanoparticles as an active antimicrobial agent is efficient. Various metallic nanoparticles have been used as antimicrobial agents but, copper nanoparticles are promising and revealed novel performance against multiple pathogens. Textile industries effluent contains various organic dyes which can be harmful to environment and living things. Dyes wastewater should be treated before drain outside the industry to minimize the risk. Various methods have been studied for efficient removal of these toxic dyes from industrial wastewater unfortunately, commercial methods are not reliable because of their cost and toxicological behaviour, among them catalytic method suggested by researchers for efficient removal which would be cheapest and ecological in nature. For the two decades, among the noble metals, the copper nanoparticle has been gaining curiosity due to its broad range of applications like; electrical, anti-fungal, antibacterial, optical, and catalytic functions [5]. CuNPs have measured suitable catalysts in various organic transformation reactions due to cost-effective and natural abundance compared to Ag, Pt, and Au. 4-Nitrophenol is a toxic substance that exists in agricultural waste and industries. There are several methods to reduce 4-Nitrophenol, for instance, Chemical Oxidation [6], Photocatalytic [7], and Fenton Oxidation reaction [8]. Using NaBH4 to reduce 4-Nitrophenol into 4-Aminophenol is the most promising method. Ag [9], Au, Pt [10], and Cu metals reported the reduction of 4-Nitrophenol with different morphology. Several factors affect the catalytic activity, for instance, degree of dispersion, size, and shape. In this paper, the CuNPs were synthesized through the green method by Brassica oleracea L, (Cabbage) extract and characterized by UV- visible spectrophotometer, EDX (Energy Dispersive X-Ray), SEM (Scanning Electron Microscopy), and FTIR (Fourier Transform Infrared Spectroscopy). The synthesized CuNPs were utilized as an antibacterial agent against selected pathogens and a catalyst for the reduction of 4-Nitrophenol to 4-Aminophenol by using NaBH4.
Chemicals
Green cabbage (Brassica oleracea) was purchased from a supermarket. Copper acetate, Sodium Hydroxide, Hydrochloric acid, sodium borate, and 4-Nitrophenol were purchased from Sigma-Aldrich (Pakistan) in analytical grade and used without any further purification. Throughout the experiment, Milli Q water was used.
Preparation of extract
The Inner part of Brassica oleracea L. was washed with distilled water three times after chopping into small pieces and dried at room temperature for three days. After that, the oven was used for further drying at 100℃ for 30 minutes by 15 minutes gap. Then, the dry chopped cabbage was crushed with mortar and pestle and converted into fine powder. 5 g of powder was dissolved into 100 mL of Milli Q water in 250 mL of conical flask under continuous stir at the hot plate for 30 minutes. The Whatman’s filter paper was used for the filtration process and collected into a clean conical flask and stored for further experiments.
Synthesis of green-CuNPs
For the CuNPs synthesis, 100 mL of (0.25-2 mM) copper acetate solution was poured into a conical flask and put that flask onto a hot plate with magnetism. 10 ml of the extract was added drop wise under continuous stir at 30-60℃ for approximately 30-150 minutes. The colour of solution was changed from Pale blue to bluish green that visually confirmed the formation. The solution was periodically examined using a UV-visible spectrophotometer in the range of 300-500 nm for the confirmation of formation.
Optimization of parameters
Few fundamental parameters were optimized to evaluate their effect on synthesis like time (30-150 min), concentration (0.25 mM-2 mM), pH (4-10), and temperature (20℃-60℃). UV-visible spectrophotometer was used to evaluate the changes. To maintain the pH of reaction medium, 0.1 M NaOH and 0.1 M HCl were used and monitored with pH meter.
Purification of CuNPs
After the effective formation of CuNPs, the solution was placed into a China dish, evaporated the solvent at low flame, and then dried in the oven. The nanoparticles were then crushed into powder using a mortar and pestle and collected in a clean, sealed jar for further testing. That container was kept in a dark spot.
Characterization of CuNPs
Several techniques were used to characterize the synthesized CuNPs. To monitor the formation and reduction process of CuNPs, UV-visible spectrophotometer (UV-526) with a quartz cell has 1 cm length used periodically. The spectra were observed over a range of 300-500 nm. Scanning Electron Microscopy (SEM- JEOL/EOJSM-6490) was utilized to characterize the morphology of synthesized CuNPs at an accelerating voltage of 20 Kv. To examine the elemental composition of synthesized CuNPs (EDX-Spectrometer JAGUAR S6) was used. Fourier-Transform Infrared spectroscopy (FTIR Spectrometer 100-PERKINELMER, USA) was used to obtain the Infrared spectrum of emission or absorption of the sample. The Catalytic activity of CuNPs was monitored through a UV-visible spectrophotometer (UV-526) in the range of 310-550 nm. The zone of inhibition of each pathogen was measured by the antibiotic zone scale.
Antibacterial activity
The antibacterial potential of synthesized CuNPs was studied against Pseudomonas (ATCC 27583), Bacillus spp. (ATCC 6633) and Escherichia coli (ATCC 25922) pathogens through a well diffusion method. MHA (Muller-Hinton Agar) media was used to observe the antibacterial activity. The bacteria were grown in nutrient broth for 24 hours and then stored for further investigation. Nutrient’s agar plates were set, solidified, and antiseptic. After solidification, 100 uL from each culture was taken from the nutrient broth and spread into separate Petri dishes by sterile glass rod for preparing the bacterial lawns. The solution of 10 mg/mL of CuNPs was prepared and used for further experiments. Copper nanoparticles were loaded at the required volume of 6 uL, 10 uL, 14 uL, and 18 uL on respective discs. The sterile disc diameter of 3 mm was placed on these plates and incubated for 24 hours at 37℃.
Catalytic activity
The catalytic property of CuNPs was observed by analysing the kinetics of reducing 4-nitrophenol by sodium borohydride. In the typical reaction, 40 uL of 0.01 M aqueous solution of 4-nitrophenol was mixed with 4 mL of DI water and 0.01 g of CuNPs. Ina further step, 6 ml freshly produced sodium borohydride solution (0.1 M) was added. Then, the reaction was monitored using UV-spectrophotometer with the passage of time; the solution colour changed from yellow to colourless which indicated the reduction of 4-Nitrophenol into 4-Aminophenol.
Biosynthesis of copper nanoparticles
100 mL of 2.5 mM copper acetate solution was prepared freshly in conical flask, that flask was put on hot plate under continuous stir condition for 150 minutes at 60℃. 15 mL of extract was added drop wise. The pH of solution was manipulated with 0.1 HCl and fixed it at pH 4. The colour of solution was changed from pale blue to bluish green which confirmed the visual confirmation of formation. The reduction process of copper acetate to copper nanoparticles was monitored by recording the absorption spectrum of the reaction after diluting a small part of the sample with Milli q water in a quartz cuvette of 1 cm. CuNPs have shown the Surface Plasmon Resonance (SPR) peak in the range of 400-800 nm. The absorbance peak has been observed at 550 nm that confirmed the formation of copper nanoparticles as shown in Figure 1A.
Optimization of synthesis parameters
There are several fundamental parameters those effects on the synthesis of nanoparticles. In the current study, few parameters were optimized like Time, Concentration, pH, and Temperature to evaluate their effect on copper nanoparticles synthesis. To monitor the changes, UV-visible spectrophotometer was used.
Optimization of time
Time is fundamental parameter that could affect the morphology of nanoparticles. 100 ml of 2 mm copper acetate solution was taken with 15 mL of extract at 60℃ under continuous stir condition at pH 4. The solution was periodically monitored with UV-visible spectrophotometer by the function of time. The spectra show the high absorption peak at 150 minutes that revealed the optimum time for time for synthesis as shown in Figure 1B.
Optimization of concentration of metal ions
To evaluate the effect of concentration on synthesis, the solution was taken in different concentration (0.5 mM, 1 mM, 1.5 mM. 2 mM, 2.5 mM) and monitored with UV-visible spectrophotometer. The spectra shows that the absorbance peak increased when the concentration increase as shown in Figure 1C. The high absorbance peak was observed for 2.5 mM which is the optimum concentration for synthesis.
Optimization of pH
Effect of pH was evaluated on synthesis at different pH (4, 6, 8, 10). The pH of solution was manipulated and fixes with help of 0.1 M HCl and 0.1 M NaOH. The pH meter was used for monitoring the pH and changes were observed through UV- visible spectrophotometer. The Obtained spectra showed that the maximum formation of nanoparticles observed in acidic medium while minimum in basic medium. The absorbance peak observed at pH 4 that confirmed the optimum pH for synthesis as shown in Figure 1D.
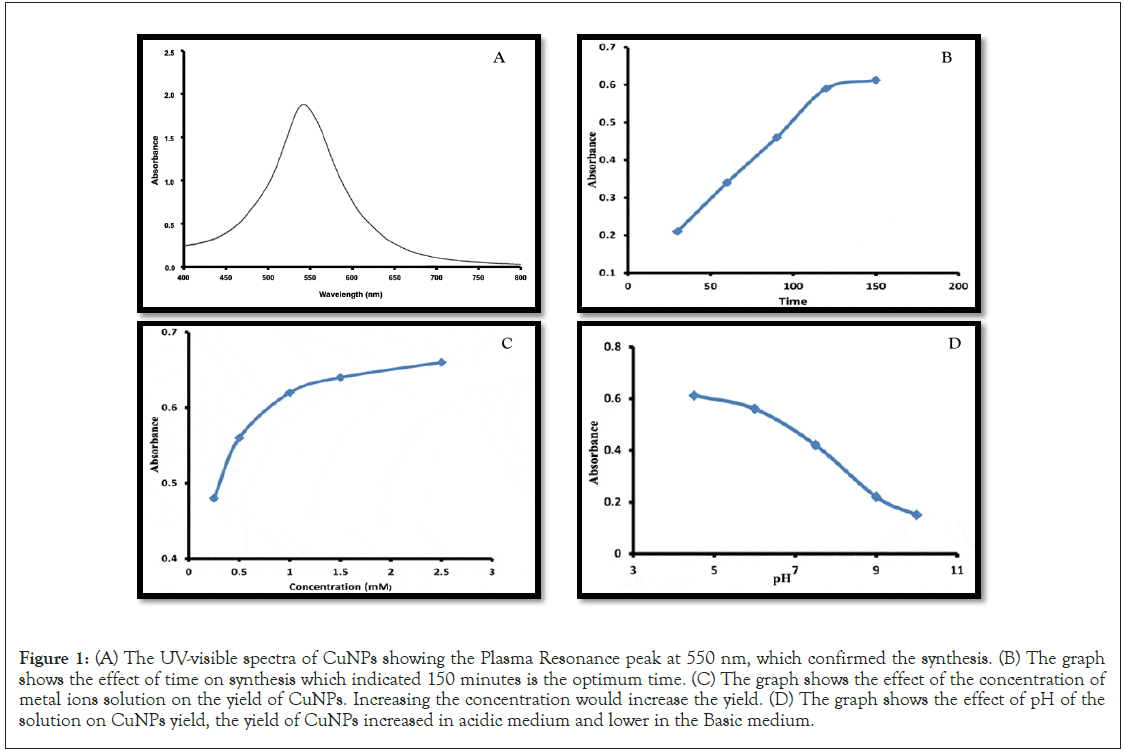
Figure 1: (A) The UV-visible spectra of CuNPs showing the Plasma Resonance peak at 550 nm, which confirmed the synthesis. (B) The graph shows the effect of time on synthesis which indicated 150 minutes is the optimum time. (C) The graph shows the effect of the concentration of metal ions solution on the yield of CuNPs. Increasing the concentration would increase the yield. (D) The graph shows the effect of pH of the solution on CuNPs yield, the yield of CuNPs increased in acidic medium and lower in the Basic medium.
Optimization of temperature
The synthesis was conducted at different temperature (20℃, 30℃, 40℃, 50℃, and 60℃) to measure its effect on synthesis. A UV spectrum has been conducted at each temperature. The absorbance peak increase when the temperature increases as shown in Figure 2A. The results showed that 60℃ is the optimum temperature for synthesis.
FTIR studies
Fourier-Transform Infrared spectroscopy (FTIR Spectrometer-100 PERKINELMER, USA) was used to classify the probable bio-molecules for stabilization and proper capping of copper nanoparticles. The FTIR spectra of ecologically synthesized copper nanoparticles are shown in Figure 2B. At the wave number of 2918 cm-1 secondary amine was located, and at 3228 cm-1 asymmetric stretching of C-H, C-N group, and hydroxyl groups of alcohols are located. A slight variation in the peak position showed that some metabolites, flavonoids, tannins, phenols, and alkaloids are rich in cabbage extract that can produce CuNPs.
EDX studies
The elemental composition of Brassica oleracea was found by Energy Dispersive X-Ray spectroscopy (EDX-JAGUAR S6). The synthesized CuNPs obtained spectra are shown in Figure 2C. The EDX examination of BO-CuNPs shows as an intense signal at 1-8 kev, indicating the presence of copper acetate nanoparticles. The CuNPs peak was observed, indicating the synthesis of BO- CuNPs formed. Furthermore, EDX analysis shows that Cu is present on the surface of cabbage (Brassica oleracea) with other elements as sulphur, carbon, oxygen, etc.
Morphological studies
Scanning Electron Microscopy (SEM-JEOL/EOJSM-6490) was utilized to reveal the morphological data of extract and synthesized CuNPs at an accelerating voltage of 20 kv. The size of CuNPs was 70.2 nm with a mostly spherical shape. CuNPs were observed at a resolution of 200X and 150X, as shown in Figure 2D.
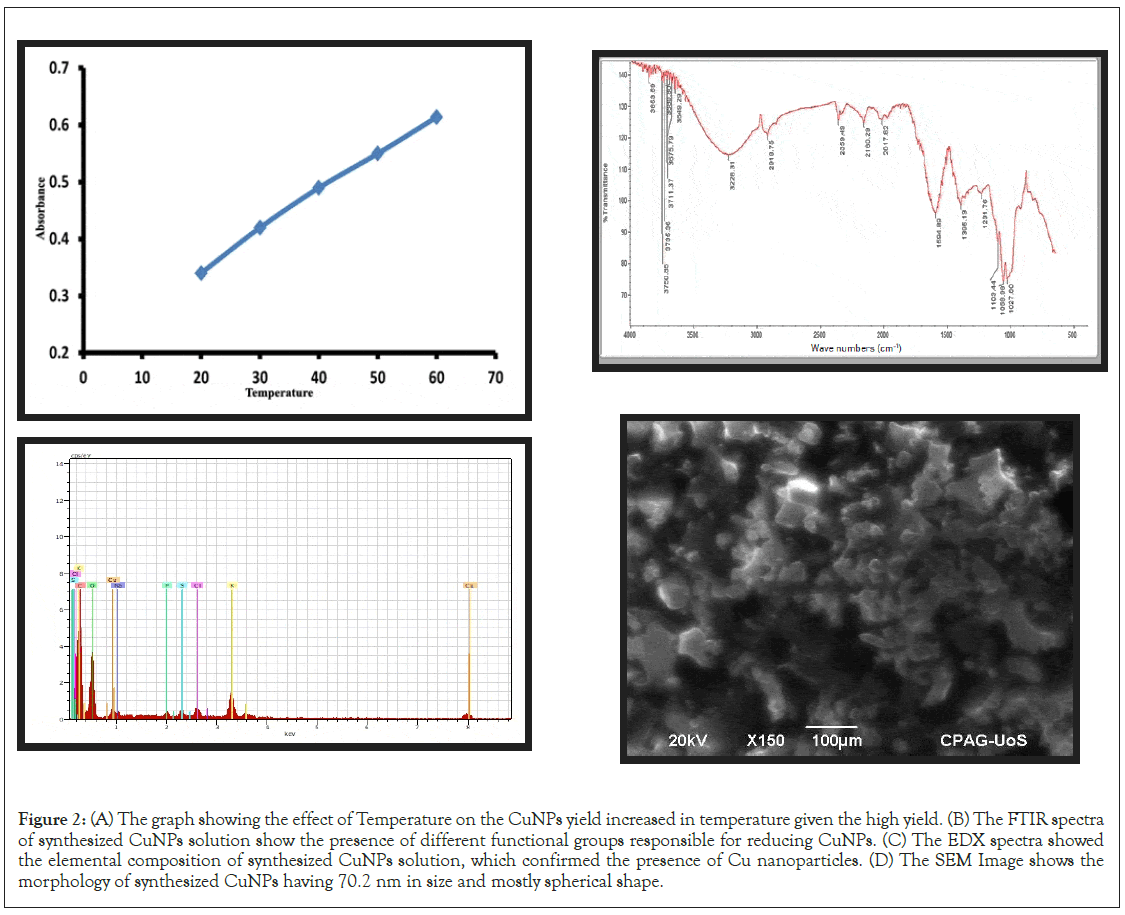
Figure 2: (A) The graph showing the effect of Temperature on the CuNPs yield increased in temperature given the high yield. (B) The FTIR spectra of synthesized CuNPs solution show the presence of different functional groups responsible for reducing CuNPs. (C) The EDX spectra showed the elemental composition of synthesized CuNPs solution, which confirmed the presence of Cu nanoparticles. (D) The SEM Image shows the morphology of synthesized CuNPs having 70.2 nm in size and mostly spherical shape.
Antibacterial activity
Various nanoparticles have been reported as antibacterial agents [11,12]. But copper nanoparticles have potent antimicrobial activity and can reduce the concentration of bacteria by 99.9%. The biosynthesized CuNPs have revealed the main antibacterial activity against Pseudomonas(ATCC 27583), Bacillus spp. (ATCC 6633) and Escherichia coli (ATCC 25922) test pathogens. The variation in the zone of inhibition at various pathogenic bacteria is depicted in (Table 1). Antibacterial studies have shown that the Bacillus spp. has additional strong and effective bacterial activity and potentially eliminated the zone of inhibition of 14 mm at 18 uL of CuNPs volume, as shown in Figure 3A. The results are comparable with other reported literature. This work is ecological, cheap, and exhibits efficient efficacy towards Bacillus spp as compared to other’s nanoparticles. As a result, biosynthesized CuNPs can be used as an ultramodern effective antimicrobial agent in the biomedical field, and their efficacy can be enhanced to reduce their size.
| CuNPs Volume (uL) |
E. Coli (Inhibition Zone) |
Pseudomonas spp. (Inhibition Zone) | Bacillus spp. (Inhibition Zone) |
|---|---|---|---|
| 6 uL | 6 mm | 10 mm | 12 mm |
| 10 uL | 7 mm | 10 mm | 12 mm |
| 14 uL | 9 mm | 11 mm | 13 mm |
| 18 uL | 10 mm | 12 mm | 14 mm |
Table1: Table exhibits the zone of inhibition of selected pathogen at the different volume of CuNPs; it clearly showed that CuNPs has high antibacterial activity against Bacillus spp. with a zone of inhibition of 14 mm at 18 uL.
Catalytic activity
For two decades, copper nanoparticles have been used as an effective catalyst in many organic transformations. The reaction was performed to determine the catalytic abilities of biogenic CuNPs by reducing 4-nitrophenol to 4-aminophenol with an excess of sodium borohydride. The reduction kinetics was studied by the spectroscopic method. The change in colour illustrates the production of 4-nitrophenolatcolor from light yellow to colourless. 4-nitrophenol displays an absorption peak at 317 nm in the acidic and neutral medium of the solution. But the absorption peak shifts to 400 nm by adding sodium borohydride as shown in Figure 3B, because sodium borohydride deprotonates the OH group of 4-nitrophenol and forms 4-nitrophenolate ions under the basic condition. CuNPs catalysed 4-nitrophenol to 4-aminophenol with sodium borohydride, commonly evolving the Langmuir-Hinshelwood mechanism. The rate of catalytic reduction was measured near 12 min, which showed the efficient potential of CuNPs as a catalyst. The present work was compared with recent literature and observed that various literature used harmful way to synthesize the CuNPs and used it for the reduction of 4-Nitrophenol hence, their reaction was very slow and took several minutes to complete [13-18]. The study revealed that copper nanoparticles are suitable for the fast and effective reduction of 4-Nitrophenol to 4-Aminophenol compared to other metallic nanoparticles. The reaction does not continue in the absence of a catalyst. CuNPs shows improved catalytic activity toward the reduction of 4-nitrophenol in the presence of sodium borohydride. It can be altered and fast by reducing the size of CuNPs.
Figure 3: (A) The Image shows the stain of Bacillus spp. Zone of inhibition of 14 mm at 18 uL of CuNPs volume. (B) The UV-visible spectra show the maximum absorbance at 400 nm, which confirms the transformation of 4-nitrophenol into 4-aminophenol.
In conclusion, nanoparticles of copper were prepared using Brassica oleracea L. (Cabbage) extract and optimized with various parameters. UV-visible spectroscopy, SEM, EDX, and FTIR were used to characterize the produced CuNPs. The biosynthesized CuNPs revealed the effective antibacterial activity against Bacillus spp. 18 uL CuNPs cleared 14 mm zone of inhibition of Bacillus spp. The biosynthesized CuNPs showed strong catalytic activity for the 4-nitrophenol’s reduction in a liquid solution using sodium borohydride. The calculated rate constant of reduction rate was 0.1753 min-1. It has been concluded that biosynthesized CuNPs using Brassica oleracea L. (cabbage) extract utilized in the industrial wastewater treatment as catalyst and antibacterial agent, which is cheapest, ecological, and efficient as compared to commercial methods.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Memon GZ, Zohra K, Sidhu AR, Chang SA (2023) Catalytic Reduction of 4-Nitrophenol to 4-Aminophenol Using Copper Nanoparticles on Brassica oleracea L: Environmental Green Method. J Phys Chem Biophys. 13:354.
Received: 19-May-2023, Manuscript No. JPCB-23-24303; Editor assigned: 22-May-2023, Pre QC No. JPCB-23-24303 (PQ); Reviewed: 05-Jun-2023, QC No. JPCB-23-24303; Revised: 12-Jun-2023, Manuscript No. JPCB-23-24303 (R); Published: 19-Jun-2023 , DOI: 10.35248/2161-0398.23.13.354
Copyright: © 2023 Memon GZ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.