
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research Article - (2016) Volume 7, Issue 4
The Jarisch-Herxheimer reaction (JHR) is a transient immunological response that may occur after the initiation of treatment for any type of viral, bacterial or fungal infection such as syphilis, lime disease or Candida albicans. The reaction is similar to the systemic inflammatory response syndrome (SIRS) and characterized by shaking, joint pain, muscle aching, chills, fever, headache, sore throat, malaise, myalgia, tachycardia as well as exacerbation of existing cutaneous lesions such as worsening inflammatory reaction at the sites of localized infection. The reaction generally occurs six to eight hours after treatment beginning with penicillin or other antibiotics and it may be easily confused with a SIRS. The intention of this definition is to outline a clinical response to a nonspecific insult of either infectious or noninfectious origin. The mechanism underlying patho-physiological response remains elusive for modern medicine since it was described over a century ago. An increase in the incidence of JHR may be expected among patients co-infected with HIV and other infectious diseases including HCV and HCB. In this paper we collected a number of patients underwent autologous peripheral blood stem cell transplantation that developed JHR during the first phase of the treatment. As this matter has received very limited attention in recent researches and clinical approaches apart from brief remarks in manuals, we felt significant to provide an overview of its various attributes including the current concepts in pathophysiology and management during clinical stem cell treatment and not only following conventional antibiotic, antifungal or antiviral treatment.
Keywords: Tachycardia; Transplantation; Hypersensitivity; Tuberculosis; Immunity response
The JHR is a transient inflammatory condition that may occur hours following the treatment for bacterial, viral or fungal infections [1,2]. Clinically, JHR manifests as an unexpected worsening of symptoms and exacerbation of cutaneous lesions that tend to resolve spontaneously within few days from the onset of the condition. However, JHR's pathogenesis still remains unclear whilst histopathologically rarely reported [1,2]. Prevalence, clinical manifestations and outcome of JHR have been well studied in syphilis [3], Lyme disease [4], tick-born relapsing fever [5] and louseborn relapsing fever [6-8]. Few hypotheses have been proposed to explain the JHR especially in the field of infective disease as syphilis, candida or HIV secondary infection. The adopted treatment induces the release of lipoproteins, cytokines, and immune complex [9]. Chia et al., found that more than half of HIV-infected patients with both primary and secondary syphilis developed JHR, something similar previously reported in the literature HIV infection epidemic [2,10-12]. Of note, the risk of JHR was seen strictly correlated to RPR level, as the former titer increased proportionally with the inflammatory response; this event was explained by the presence of higher load of spirochetes in the early stages of syphilis which in turn raises the risk of JHR during bactericidal treatment [2]. Hypersensitivity has also been suggested to contribute to JHR as the reaction takes place with the same severity among patients with different stages of infection, even though late latent infection such as Syphilis, EBV, Candida, Tuberculosis, Endocarditis, Lyme disease or Influenza has been considered to contain fewer spirochetesor toxins that can be triggered by the treatment [2,13-17]. However, a clear idea about JHR during stem cell therapy is something that still lacking within scientific literature. In order to quantify the frequency and the impact of JHR as the consequence of either stem cell or conventional drug therapy, we systematically reviewed the published literature and put it in a broader perspective by identifying the data gaps that should be addressed. Herein, we report 16 cases on a total of 116 treated patients in total, with JHR like symptoms who presented few days after commencing autologous PB-SCs therapy. Their main complaints were low grade fever malaise, mild dizziness, gingival erythema, buccal eruptions, minimal erythema, joints and muscle aching. A complete CBC which included inflammatory markers and immunity, TNF alpha, IFN gamma, IL2-6-17a, RF, CRP was performed and though not uniformly confirmed a sub-acute look like infection reaction, JHR/SIRS patterns. Results mild to sensitive neutrophilia and lymphopenia/lymphocytosis. In addition, within this 12 patients 1patient presented positive for Influenza b virus and a spongiotic dermatitis, 1patient was positive for Burkholderia mallei and 2 patients were IgG positive for Toxoplasma gondi . The spirochetes were found in blood and sputum, all apparently in the latent stage. Burkholderia mallei and Toxoplasma gondii DNA were detected by nested polymerase chain reaction (RT-PCR). In all cases stem cell treatment was discontinued. These signs and symptoms were resolved within a few days. Liberation of endotoxin-like materials (eg, lipoproteins) from degenerating spirochetes and concomitant cytokine production is the suspected cause of JHR and supported by the finding of lesional spirochetes. Alternatively, a reversal reaction with a delayed-type hypersensitivity reaction is also a plausible cause based on spirochetes found in the lymphocytic spongiotic dermatitis as per our case reported above.
Patients
We enrolled all participants during 2014-2016. Eligible participants were total 116 (N=116), of different ages both male and females, with diverse condition having chosen stem cell therapy rather than conventional treatment (for reasons unrelated to the study), so it was possible to assess the association between the treatment and related changes without potentially confounding treatments. There were not specific inclusion criteria the protocol for this study was approved by the Tan Tao University Institutional Review Board. All participants were provided the informed consent and signed accordingly.
Interventions
Participants did undergo autologous PB-SCs intervention. A physician was on site during procedures. Of note, few patients, 16 in total, experienced symptoms related to JHR either during the course of therapy or delayed after stem cell injection one month c.ca (Table 1). Blood test were performed and results showed few abnormalities in markers related to immunity response, the main changes have been seen in level of WBC, Neutrophils, Lymphocytes, Eosinophils, IL6, TNF gamma and CRP. Patients could request the availability of an external physician by telephone at these times, as needed.
| Patient# | NEU% | LYM% | CRP | TNFalpha | IL-6 | Reaction on PB-SCs | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (45-70) | (20-45) | (<5 mg/L) | (<8.1 pg/ml) | (<7 pg/ml) | |||||||
| Before | After | Before | After | Before | After | Before | After | Before | After | _ | |
| 1 | -- | Off | Off | -- | -- | Off | -- | -- | -- | -- | Yes |
| 2 | Off | Off | -- | Off | Off | Off | -- | -- | -- | -- | Yes |
| 3 | -- | Off | -- | Off | -- | Off | -- | -- | -- | -- | Yes |
| 4 | -- | Off | -- | Off | -- | -- | -- | -- | -- | -- | Yes |
| 5 | -- | Off | -- | Off | -- | -- | -- | -- | -- | -- | Yes |
| 6 | -- | -- | -- | -- | -- | Off | -- | Off | -- | -- | Yes |
| 7 | Off | Off | Off | Off | -- | Off | -- | Off | -- | -- | Yes |
| 8 | -- | Off | -- | Off | -- | -- | -- | -- | -- | Off | Yes |
| 9 | -- | Off | -- | Off | -- | Off | -- | Off | -- | Off | Yes |
| 10 | -- | Off | -- | Off | -- | -- | -- | -- | Off | -- | Yes |
| 11 | -- | -- | -- | -- | -- | -- | -- | Off | -- | -- | Yes |
| 12 | -- | Off | -- | Off | -- | -- | Off | Off | -- | -- | Yes |
| 13 | -- | -- | -- | Off | -- | -- | -- | -- | -- | -- | Yes |
| 14 | -- | -- | -- | -- | -- | Off | -- | Off | -- | Off | Yes |
| 15 | -- | -- | -- | -- | -- | -- | -- | Off | -- | -- | Yes |
| 16 | Off | Off | Off | Off | -- | Off | -- | -- | -- | Off | Yes |
Table1: Herxheimer reaction on PB-SC on a group of 16 patients out of 116 patients.Symbol “—“in cells denotes the level of the respective inflammatory value within the normal range, while cells which contain “Off” denote the abnormal level of the respective marker. Deviation of any of the factors off the normal range indicates a reaction to the treatment.
Peripheral blood stem cell isolation
Mononucleated cells were isolated from consented patient’s peripheral blood according to the guidelines of Helsinki Declaration. Mononucleated cells were isolated by density gradient centrifugation using Ficoll-Paque™ PLUS (GE Healthcare, Uppsala, Sweden). A total of 10 blood samples (35 ml each) were carefully layered 1:2 on Ficoll- Paque and centrifuged at 300 g for 20 min at 20°C. The mononucleated cell layer, 4×107, at the plasma-Ficoll interface, were aspired and was washed three times with phosphate buffered saline and cultured in 25T flasks with free serum medium containing 2% (v/v) penicillinstreptomycin at 37°C in a humidified atmosphere containing 5% CO2 for a period of 7.3 days. Suspension and adherent mononucleated cells were cultured in free serum medium (FSM-Life, Technology-CTSTMStemProR, Canada). For both suspension and adherent mononucleated cells, the trypan blue exclusion assay was used to observe the proliferation of the cells. Before each transfusion samples were collected to be analyzed for bacteria, fungal and viral contamination. Cells were cultured for 7.3 day average, subsequently suspension and adherent cells were collected and injected to patient.
Original data
Table 1 was made from the original study, totally, 12 patients on a total of 166 patient treated by PB-SCs. The tables include the following immunity parameters:
White blood cell, Neutrophils, Lymphocytes, Monocytes, C-reactive protein, Interleukin 6, Tumor Necrosis factor alpha, Rheumatoid factor.
Data were organized by categories and presented in its new view in Table 1. As is evident some categories contain few information and cannot be per se’statistically analyzed. For this reason only two categories, Neutrophils and Lymphocytes, contain reasonable volume of data. These categories contain data for 11 patients.
Organized and informative data
Table 2 shows NEU and LYM measurements for 11 patients before and during the treatment. The table also shows:
• The difference between the measurements “before” (X1), “during” (X2), and their difference (ΔX = X1 – X2).
• the mean values on each sample, before, during, and the difference,
• standard deviation on each sample (s),
• SE (sampling error, s2/n).
| # | Gender | Age | NEU% (40-74) | LYM% (19-44) | ||||
|---|---|---|---|---|---|---|---|---|
| 40 | 74 | 19 | 44 | |||||
| Before (X1) | During | Difference | Before | During | Difference | |||
| (X2) | (ΔX=X1-X2) | (X1) | (X2) | (ΔX=X1-X2) | ||||
| 1 | M | 60 | 46.8 | 90 | -43.2 | 47.2 | 6 | 41.2 |
| 2 | F | 43 | 54.5 | 55.5 | -1 | 36.2 | 3.8 | 32.4 |
| 3 | M | 60 | 73.1 | 80.7 | -7.6 | 24.1 | 7.8 | 16.3 |
| 4 | M | 46 | 76.1 | 83.3 | -7.2 | 21.1 | 6.22 | 14.88 |
| 5 | M | 75 | 64.8 | 73.2 | -8.4 | 29.8 | 15.1 | 14.7 |
| 6 | F | 48 | 67 | 75.2 | -8.2 | 1 | 13.6 | -12.6 |
| 7 | M | 55 | 68 | 69.6 | -1.6 | 22.5 | 15.4 | 7.1 |
| 8 | M | 55 | 33.2 | 30.4 | 2.8 | 53.3 | 58 | -4.7 |
| 9 | M | 60 | 64.4 | 85 | -20.6 | 27.5 | 17 | 10.5 |
| 10 | F | 83 | 70 | 75.1 | -5.1 | 19.8 | 21.1 | -1.3 |
| 11 | M | 64 | 40 | 47 | -7 | 48.6 | 45 | 3.6 |
| Mean | 60 | 59.8091 | 69.5455 | -9.73636 | 30.1 | 19.0018 | 11.098 | |
| Variance | 143 | 200.855 | 328.497 | 158.165 | 234.462 | 295.155 | 245.169 | |
| St.Dev | 11.9 | 14.1723 | 18.1245 | 12.5763 | 15.3122 | 17.1801 | 15.6579 | |
| n = | 11 | |||||||
| d.f. = n-1 = | 10 | |||||||
| SE = | 3.97699 | 4.95145 | ||||||
| t = | -2.56766 | 2.35078 | ||||||
| P-Value | 0.028003 | 0.040585 | ||||||
| Stat. Significance | 5% | error | Significant | Significant | ||||
| Conf.Interval | 95% | -18.2 – -1.29 | 0.57 – 21.62 | |||||
Table 2: Data for NEU and LYM (11 patients). Results are significant for 5% level of error. It means that with the given level of sampling error (5%) the null hypothesis is rejected and the alternative hypothesis is accepted. The alternative hypothesis states, that the difference between measurements of NEU and LYM “before” and “during” the treatment are statistically significant.
Statistical analysis of herxheimer data
The data presented in Table 2 has the following features:
• Data in a “before” and a “during” samples are paired, i.e. related one-to-one.
• Both samples, “before” and “during” have the same size and this size is small.
• The standard deviation on the population is unknown.
Inflammatory markers results after peripheral blood stem cell injection
We conducted measurements of most common pro-inflammatory markers Neutrophils, Lymphocytes, CRP, RF, TNF alpha, INF gamma, IL-6 on a sample of 116 patients before and after treatment with PBSCs. A group of 16 patients exposed a reaction (mild to strong) by showing abnormal level of those markers as shown in Table 1.
The available data does not provide the information to conclude about cause and effect due to lack of a control group also, data “during” treatment does not imply final results.
Under the given circumstances, we can only test whether the samples “before” and “during” represent the same population for both measurements, NEU and LYM. The hypothesis is formulated as the mean value on the population of the differences between measurements “before” and “during” has the mean value equal zero. It means that
H0: μ (ΔX = X1 – X2) = 0 (1)
The alternative hypothesis says that if H0 is rejected then the measurements “before” and “after” represent populations with different mean values.
HA: μ (ΔX = X1 – X2) ≠ 0 (2)
Due to the small sample size and unknown standard deviation on the population, we used the dependent t-test for paired samples,
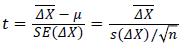 (3)
(3)
Where n is the sample size (n = 11 in this case),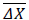 is the mean on the sample of the differences between “before” and “during”, s (ΔX) is the standard deviation on the sample of differences. The t-test should be performed with the degree of freedom, d.f., calculated as
is the mean on the sample of the differences between “before” and “during”, s (ΔX) is the standard deviation on the sample of differences. The t-test should be performed with the degree of freedom, d.f., calculated as
d.f.=n=1 (4)
where n = n1 = n2 is the sample size (n = 11 in this case),
It is well established that clinical exacerbation of JRH such as fever, headache, malaise, sweating aching joints, skin reaction have been reported during or after the treatment of infection caused by different pathogens as in syphilis, leptospiral infection, tuberculosis, candida and Lyme disease [1,2,18]. Though the causes are not defined we have the proposed mechanisms including endotoxin release from the death of agents, delayed hypersensitivity, and decreased suppressor mechanisms.
In this paper we describe a JHR in about 10% of patients treated for different condition which experienced a JHR manifesting chills, fever, muscle pains, rapid heartbeat, and slight dropping of blood pressure. These symptoms usually were present for several hours to few days, and required little more than aspirin and rest. Yet part of group participants showed a late reaction, beginning days after the start of therapy. Some of these patients were likely to be suffering from the side effects of their prescribed medication such as the case of the female patient with multiple sclerosis (MS). It is also safe to assume that stem cells treatment involves temporary worsening will lead some people to neglect other illnesses, such could be the cases of the presence of previous undetected infection as the case of Toxoplasmosis gondii , Influenza b, EBV and Bulkhoderia mallei . Clinical symptoms for example could be either silent or manifest in a way that might be confused with a different condition. The fact that few infectious agents were detected could be related to the immune-modulatory activity of stem cells which lead to a dominant immune response against an infection as it was reported in our precedent case study on HIV and Toxoplasmosis infection [19-21]. There were no fatalities strictly related to the therapy, though constitutional symptoms were in some cases severe during JHR. Plasma Neutrophils, Lymphocytes, Monocytes, TNF, IL-6, RF and CRP were raised in several patients on follow-up and were occurring in transient form coincidental with observed pathophysiological changes of JHR.
A small group of 16 patients out of 116 total patients have developed a distinct abnormal reaction related to JHR either during the course of therapy or within one month after stem cell injection. Such reaction occurs quite seldom and represents a special case. For this reason, we processed these 12 patients as a separate sample.
On this special sample, we may assert that yet we can’t statistically assert any cause and effect relationship, we may eventually state that with statistical error of 5%, the measurements of Neutrophils and Lymphocytes are statistically significant before and after the PB-SCs treatment. We also observed some abnormal reaction by elevation of plasma WBC, Monocytes, TNF, IL-6, RF, and CRP concentrations, but the data is scarce, so no statistical analysis was possible. Consequently, we cannot claim statistically significant differences in the “before” and “after” measurements on the entire pool of patients or PB-SCs treatment as a cause of the effect, we may eventually assert that something at clinical level has happened.
Although these data can be statistically non relevant due to small number of participants, on a larger scale a“10%”would certainly acquire a more solid relevance on clinical application, which is something that physicians and medical scientists should be aware of. To conclude, the intent of this report was to draw attention “emotionlessly” to responses may happen during and after the in vivo procedure with stem cells in order to promote a better and safer method for the future.