
Biochemistry & Pharmacology: Open Access
Open Access
ISSN: 2167-0501

ISSN: 2167-0501
Research Article - (2019)Volume 8, Issue 1
Osmotic fragility (OF) in red blood cells (RBCs) is a useful tool for evaluating the actions of various chemicals on the cell membrane in vitro. The effects of monocarboxylic and dicarboxylic acids on OF were evaluated in cattle RBCs. Isolated cattle RBCs were immersed in various carboxylic acids at 0-100 mM in a buffer solution for 1 hr and the 50% hemolysis was then determined by soaking in 0.1-0.8% NaCl solution. Although n-caprylic acid at 100 mM induced hemolysis, the other monocarboxylic acids possessing straight hydrocarbons did not affect OF. The dicarboxylic acids possessing straight hydrocarbons, except for glutaric acid, decreased OF in a dose-dependent manner. Some monocarboxylic acids with branched hydrocarbons tended to decrease OF, but these changes were not statistically significant. Although cyclopentanecarboxylic and cyclohexanecarboxylic acids at 100 mM decreased OF, other monocarboxylic acids with cyclic hydrocarbons did not affect OF. Among the dicarboxylic acids with cyclic hydrocarbons tested, only 1,2-cyclohexanedicarboxylic acid and phthalic acid with a benzene ring significantly decreased OF. There is no clear correlation between the effect of monocarboxylic or dicarboxylic acids on OF, and their partition coefficients. Thus, the partition coefficient is not a suitable parameter for explaining the effect of both groups of carboxylic acids on OF in cattle RBCs. With regard to the effect of monocarboxylic acids on OF, although an increase in OF was demonstrated in rat RBCs, no effect or rather a decrease in OF was demonstrated in cattle RBCs, similar to the results observed for guinea pig and sheep RBCs. With regard to the effect of dicarboxylic acids, decreases in OF were already demonstrated in rat, guinea pig and sheep RBCs. We have proposed that dicarboxylic acids exhibit a common stabilizing effect on the RBC membrane in various animals, which we termed a “wedge-like effect”. We clarified that cattle RBCs also show a similar OF response to dicarboxylic acids in this experiment.
Mammalian red blood cells (RBCs) have been used as a valuable tool, particularly as an experimental model for the cell membranes composed of a phospholipid bilayer, as RBCs possess a basic structure consisting of a cell membrane without a nucleus or complex metabolic system in the plasma. There have been many reports that resistance to osmotic pressure in RBCs is a useful indicator for evaluating the strength of the cell membrane. Osmotic fragility (OF) in RBCs is a valuable for expressing the robustness of membrane against osmotic pressure and is generally used for evaluating the interaction of various chemicals and the cell membrane in vitro, including general [1,2] and local anesthetics [3,4], some kinds of drugs [5,6] and toxins [7,8], inorganic [9,10] and organic compounds [11,12], as well as substances isolated from plants [13,14], and crude plant extracts [15,16]. Although the RBCs of various mammalian species were used in those experiments, it has not been considered if the differences in OF response are based on inter-species differences in RBC membrane characteristics.
Our series of experiments demonstrated the effects of carboxylic acids on OF in RBCs derived from various animal species. OF in rat RBCs was increased by the application of monocarboxylic acids possessing straight hydrocarbon chains from 0.1 to 100 mM for 1 hour [17-19]. Those increases were dependent on both dose and the length of the hydrocarbon chain. On the other hand, the same monocarboxylic acids with straight hydrocarbon chains did not have any effect on OF in guinea pig [19] or sheep RBCs [20]. Although a considerable number of monocarboxylic acids with branched or cyclic hydrocarbons, including a benzene nucleus, increased OF in rat RBCs [17], these monocarboxylic acids did not affect or rather decreased OF in guinea pig [19] and sheep RBCs [20]. Unlike monocarboxylic acids, most dicarboxylic acids possessing straight or cyclic hydrocarbon chain decreased OF dose-dependently, but the decrease in OF was not dependent on the length of the hydrocarbon chain in rat [18], guinea pig [19] and sheep RBCs [20].
Monocarboxylic acids are composed of a hydrophilic carboxylic group and a hydrophobic hydrocarbon, and their moieties are amphiphilic in nature. Amphiphilic compounds can act as a surfactant to directly affect artificial or biological membranes and perturb the phospholipid layer, resulting in lysis of the membrane [21-24]. The partition coefficient is a physicochemical parameter associated with the permeation of a chemical into an artificial or biological membrane [25]. As n-octanol is closer in nature to the phospholipid membrane than other non-polar organic solvents [26], the partition coefficient for octanol/water is generally used [27-29]. We have sought to clarify the relationship between the partition coefficients of monocarboxylic/dicarboxylic acids and the changes in OF induced by the application of these chemicals. At present, positive and statistically significant relationships are known to exist between the partition coefficient of monocarboxylic acids possessing straight and cyclic hydrocarbon chains, and the change in OF provoked by these acids in rat RBCs [30,31], but not in guinea pig [31] or sheep RBCs [20]. There is, however, no definite relationship between the partition coefficient of dicarboxylic acids and their effects on OF in rat [30,31], guinea pig [31] or sheep RBCs [20].
We have speculated that one cause of the inter-species differences in OF response in RBCs to monocarboxylic acids is due to differences in the fatty acid composition of the phospholipid layer in the RBC membrane [20,30,31]. On the other hand, the reason for the common OF response to dicarboxylic acids is assumed to be that the membrane resistance to osmotic pressure is strengthened by interaction between the conformation of the dicarboxylic acids and the structure of phospholipids close to the water/lipid layer, thus increasing the OF in the RBCs in various animal species [20,30,31].
The objectives of this experiment were to clarify the effects of the application of monocarboxylic and dicarboxylic acids on OF in cattle RBCs and to compare the OF responses to these chemicals with those in rat, guinea pig and sheep RBCs. The results of the present study allow us to the data on the OF response in cattle RBCs to the previous data obtained using the RBCs of different three mammalian species. The comparison among RBCs of different animal species may provide the key to clarifying the relationship between OF response to carboxylic acids and the composition of phospholipids, particularly the acyl-chain of fatty acids. In addition, we sought to analyze the relationship between the partition coefficient of carboxylic acids and their effect on OF by using cattle RBCs. This evaluation helps us to understand the relationships between the effects of carboxylic acids on OF response and the membrane characteristics of RBCs derived from different animal species. At the same time, we can confirm the validity about our hypotheses or action model, that we already proposed, for both carboxylic acids on the RBC membrane in various animal species.
Reagents
The biochemical grade carboxylic acids used in these experiments listed below were purchased from Tokyo Kasei Kogyo Co., Ltd (Tokyo, Japan) or Wako Pure chemical Co., Ltd. (Osaka, Japan): formic acid, acetic acid, propionic acid, n-butyric acid, n-valeric acid, n-caproic acid, n-enanthic acid, n-caprylic acid, isobutyric acid, isovaleric acid, 2-methyl-butyric acid, dimethyl-propionic acid, 2-methyl-n-valeric acid, 3-methyl-n-valeric acid, 4-methyl-n-valeric acid, 2-ethyl-nbutyric acid, 3,3-dimethyl-n-butyric acid, cyclopropanecarboxylic acid, cyclobutanecarboxylic acid, cyclopentanecarboxylic acid, cyclohexanecarboxylic acid, benzoic acid (benzenecarboxylic acid), oxalic acid, malonic acid, succinic acid, glutaric acid, adipic acid, pimelic acid, suberic acid, azelaic acid, and 1, 2-, 1, 3-, and 1, 4-cyclohexanedicarboxylic acids (cis- and trans- mixture), and 1, 2-, 1,3- and 1,4-benzenedicarboxylic acid (phthalic acid, isophthalic acid and terephthalic acid). All other reagents used in this study were of biochemical grade.
Preparation of cattle RBCs
Holstein female cattle aged 7 to 8 years old (n=6, body weight 400-450 kg) and fed in the Student-training Farm of Rakuno Gakuen University (Ebetsu, Hokkaido) were used for the experiments. Animals were allowed free access to concentrated feed and hay as well as tap water. Blood samples from the animals were collected by a veterinarian from Rakuno Gakuen University. The sampling and treatment of blood specimens were performed as follows. Blood samples (30 ml) were drawn by venipuncture from the left jugular vein into a heparinized test tube. The blood samples were carried to the laboratory of Hokkaido Bunkyo University and kept in a refrigerator at 4ºC for about 18 hours. Plasma and buffy coat were removed by centrifugation at 2000 g for 15 min (Model 2420, Kubota Inc., Tokyo, Japan) followed by aspiration of the upper layer. The crude RBCs thus obtained were then washed three times with two volumes of cold 0.9% NaCl solution. The resultant packed RBC suspension was kept in ice-cold water until subsequent use.
Experimental procedure
The experimental procedures followed those described in our previous report [20]. Briefly, the packed RBC suspension (30 μl) was transferred into 0.6 ml of phosphate-NaCl buffer solution (pH 7.4) containing each of the carboxylic acids at 0, 0.1, 0.25, 0.5, 1, 2.5, 5, 10, 25, 50 or 100 mM in 1.5-ml micro test tubes (Nichiryo Co., Ltd., Tokyo, Japan). A suitable amount of NaCl was added to the buffer solution to adjust the osmolality for each substance tested. The RBC suspensions treated with the carboxylic acids were incubated by shaking (1 stroke/ sec) at 37ºC for 1 hr (Shaking Bath TBK 202 DA, Advantec Co., Ltd., Tokyo, Japan). Each RBC suspension was gently mixed using a mixer (Vortex Genie 2, Model-G560, Scientific Industry, Inc. NY., USA) following incubation, and 50 μl of each suspension was transferred into a 96-deep-well microplate (2 ml volume, Whatman Inc., Piscataway, NJ, USA) containing 1 ml of NaCl solution ranging from 0.1 to 0.8%. The deep-well microplate was immediately centrifuged at 1300 g (Plate Spin II, Kubota Inc., Tokyo, Japan) for 10 min at room temperature. The supernatants (200 μl) containing different concentrations of hemoglobin derived from the ruptured RBCs were transferred into another 96-well microplate (300 μl volume, Whatman Inc., Piscataway, NJ, USA) and determined colorimetrically at 540 nm (Microplate Reader Model 680, Bio-Rad Laboratories, Tokyo, Japan).
Statistical analysis
As complete hemolysis and no hemolysis of the RBC suspensions are induced in 0.1% and 0.8% NaCl solutions, respectively, the hemoglobin concentration in the 0.1% and 0.8% NaCl solutions were defined as 100 and 0%. The concentration of the NaCl solution inducing 50% hemolysis (EC50) of the treated RBCs was calculated from the hemolysis curve by using a straight-line equation between the points immediately adjacent to 50%. OF in the RBCs was defined as the EC50 value. All values are expressed as means ± S.D. (n=6). The significance of the differences between the control (0 mM) and subsequent concentrations (0.1-100mM) was calculated by Dunnett's test following one-way ANOVA. As apparent changes in OF were found by treatment with most of carboxylic acids at 10, 25, 50 and 100 mM, the differences from the control value at 0 mM were calculated and expressed as ΔEC50 (NaCl %). The partition coefficients of the carboxylic acids examined were mainly quoted from the chemical and physical properties of the PubChem [32] or the ChemSpinder [33] websites. The relationship between the partition coefficient of each carboxylic acid and the ΔEC50 of the RBCs was confirmed by regression analysis. Statistical analyses were performed using Excel Tokei for Windows 2012 (SSRI Co., Ltd., Tokyo, Japan). Statistical significance was fixed at a P value <0.05 or 0.01.
Effects of monocarboxylic and dicarboxylic acids possessing straight hydrocarbons
Acetic acid, which has one methyl group (C1; number of carbons in the hydrocarbon chain) bonded to one carboxylic group, did not affect OF at any concentrations from 0.1 to 100 mM in cattle RBCs (Figure 1A). On the other hand, malonic acid, which has one methyl group (C1) between two carboxylic groups, decreased OF in a dose-dependent manner, with a significant decrease in OF induced by the application of malonic acid at 100 mM (P<0.05). n-Caprylic acid (C7) did not change OF at 0.1 to 50 mM, but abruptly induced hemolysis at 100 mM (Figure 1B). Azelaic acid, which is a dicarboxylic acid corresponding to n-enanthic acid (C7), decreased OF dose-dependently, with significant decreases in OF observed at 50 and 100 mM (P<0.05). The degree of changes in OF at 10, 25, 50 and 100 mM for all tested monocarboxylic acids and dicarboxylic acids is shown in Tables 1 and 2, respectively. For the monocarboxylic acids tested in these experiments, formic acid (C0) to n-caprylic acid, except for 100 mM n-caprylic acid, did not change OF at 10 to 100 mM (Table 1). In contrast, all tested dicarboxylic acids (C0 to C7), except for glutaric acid (C3), decreased OF dose-dependently, with significant decreases in OF observed at 50 and/or 100 mM (P<0.05 or 0.01) (Figure 2).
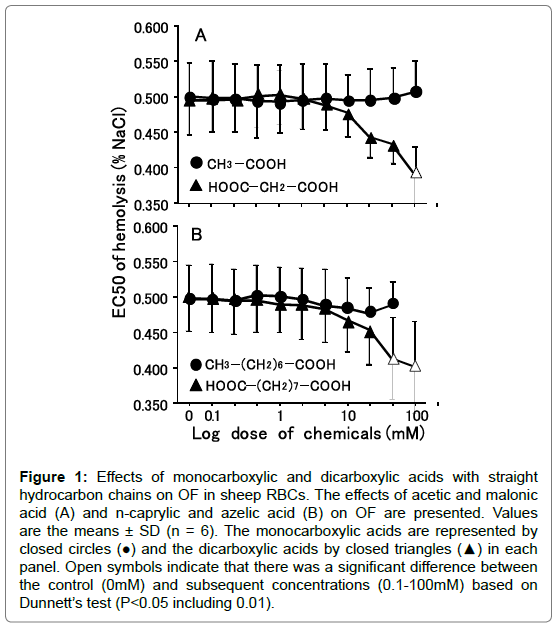
Figure 1. Effects of monocarboxylic and dicarboxylic acids with straight hydrocarbon chains on OF in sheep RBCs. The effects of acetic and malonic acid (A) and n-caprylic and azelic acid (B) on OF are presented. Values are the means ± SD (n = 6). The monocarboxylic acids are represented by closed circles (●) and the dicarboxylic acids by closed triangles (▲) in each panel. Open symbols indicate that there was a significant difference between the control (0mM) and subsequent concentrations (0.1-100mM) based on Dunnett’s test (P<0.05 including 0.01).
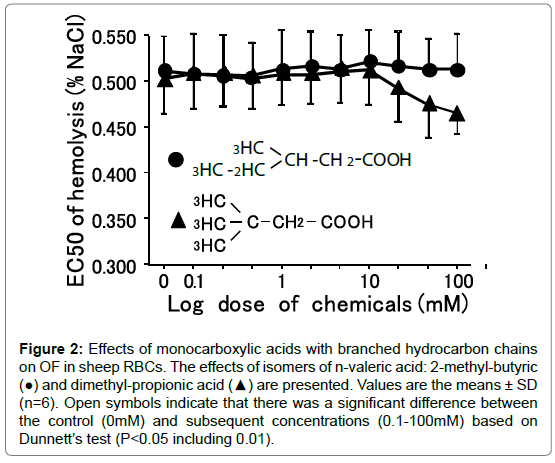
Figure 2. Effects of monocarboxylic acids with branched hydrocarbon chains on OF in sheep RBCs. The effects of isomers of n-valeric acid: 2-methyl-butyric (●) and dimethyl-propionic acid (▲) are presented. Values are the means ± SD (n=6). Open symbols indicate that there was a significant difference between the control (0mM) and subsequent concentrations (0.1-100mM) based on Dunnett’s test (P<0.05 including 0.01).
| No of hydrocarbon | Carboxylic acid | Partition coefficient | Dose (mM) | Change in OF ⊿EC50 (NaCl %) |
|---|---|---|---|---|
| 0 | Formic acid | -0.54 | 10 | -0.033 ± 0.042 |
| H-COOH | 25 | -0.039 ± 0.028 | ||
| 50 | -0.044 ± 0.035 | |||
| 100 | -0.026 ± 0.025 | |||
| 1 | Acetic acid | -0.17 | 10 | -0.005 ± 0.014 |
| CH3-COOH | 25 | -0.006 ± 0.008 | ||
| 50 | -0.002 ± 0.015 | |||
| 100 | 0.006 ± 0.014 | |||
| 2 | Propionic acid | 0.33 | 10 | 0.004 ± 0.012 |
| CH3-CH2-COOH | 25 | 0.001 ± 0.012 | ||
| 50 | -0.003 ± 0.021 | |||
| 100 | -0.008 ± 0.037 | |||
| 3 | n-Butyric acid | 0.79 | 10 | 0.000 ± 0.006 |
| CH3-(CH2)2-COOH | 25 | -0.010 ± 0.016 | ||
| 50 | -0.012 ± 0.019 | |||
| 100 | -0.005 ± 0.030 | |||
| 4 | n-Valeric acid | 1.39 | 10 | 0.002 ± 0.009 |
| CH3-(CH2)3-COOH | 25 | -0.003 ± 0.018 | ||
| 50 | -0.007 ± 0.021 | |||
| 100 | -0.004 ± 0.035 | |||
| 5 | n-Caproic acid | 1.92 | 10 | -0.011 ± 0.022 |
| CH3-(CH2)4-COOH | 25 | -0.019 ± 0.019 | ||
| 50 | -0.017 ± 0.018 | |||
| 100 | -0.020 ± 0.031 | |||
| 6 | n-Enanthic acid | 2.42 | 10 | 0.001 ± 0.015 |
| CH3-(CH2)5-COOH | 25 | -0.010 ± 0.021 | ||
| 50 | -0.029 ± 0.031 | |||
| 100 | -0.029 ± 0.023 | |||
| 7 | n-Caprylic acid | 3.05 | 10 | -0.012 ± 0.014 |
| CH3-(CH2)6-COOH | 25 | -0.020 ± 0.020 | ||
| 50 | -0.009 ± 0.044 | |||
| 100 | no data |
Table 1: Monocarboxylic acids possessing straight hydrocarbon chains, their chemical structure, partition coefficients and effects on OF in cattle RBCs in vitro. Values are means ± SD (n=6). The partition coefficients were obtained from the PubChem [32] or ChemSpider [33] website. Asterisks (* and **) indicate that there was a significant difference (P<0.05 and P<0.01) between the control (0 mM) and subsequent concentration (0.1-100 mM) based on the Dunnett’s test. As there were no significant changes for exposure to 0.1-5 mM of all tested monocarboxylic and dicarboxylic acids, the EC50 values at those doses are omitted and the data for 10, 25, 50 and 100 mM are presented.
| No of hydrocarbon | Carboxylic acid | Partition coefficient | Dose (mM) | Change in OF ⊿EC50 (NaCl %) | |
|---|---|---|---|---|---|
| 0 | Oxalic acid | -0.81 | 10 | -0.031 ± 0.022 | |
| HOOC – COOH | 25 | -0.068 ± 0.017 | |||
| 50 | -0.110 ± 0.018 | ** | |||
| 100 | -0.185 ± 0.020 | ** | |||
| 1 | Malonic acid | -0.81 | 10 | -0.017 ± 0.027 | |
| HOOC – CH2 –COOH | 25 | -0.052 ± 0.032 | |||
| 50 | -0.062 ± 0.031 | ||||
| 100 | -0.106 ± 0.029 | ** | |||
| 2 | Succinic acid | -0.59 | 10 | -0.013 ± 0.010 | |
| HOOC – (CH2)2 – COOH | 25 | -0.029 ± 0.017 | |||
| 50 | -0.043 ± 0.022 | ||||
| 100 | -0.085 ± 0.018 | ** | |||
| 3 | Glutaric acid | -0.29 | 10 | -0.007 ± 0.012 | |
| HOOC – (CH2)3 – COOH | 25 | -0.013 ± 0.012 | |||
| 50 | -0.035 ± 0.015 | ||||
| 100 | -0.039 ± 0.030 | ||||
| 4 | Adipic acid | 0.08 | 10 | -0.013 ± 0.017 | |
| HOOC – (CH2)4 – COOH | 25 | -0.025 ± 0.018 | |||
| 50 | -0.042 ± 0.014 | ||||
| 100 | -0.071 ± 0.027 | * | |||
| 5 | Pimelic acid | 0.61 | 10 | -0.021 ± 0.011 | |
| HOOC – (CH2)5 – COOH | 25 | -0.036 ± 0.014 | |||
| 50 | -0.063 ± 0.026 | ||||
| 100 | -0.102 ± 0.036 | ** | |||
| 6 | Suberic acid | 0.8 | 10 | -0.007 ± 0.013 | |
| HOOC – (CH2)6 – COOH | 25 | -0.031 ± 0.018 | |||
| 50 | -0.051 ± 0.021 | ||||
| 100 | -0.085 ± 0.025 | * | |||
| 7 | Azelaic acid | 1.57 | 10 | -0.030 ± 0.023 | |
| HOOC – (CH2)7 – COOH | 25 | -0.044 ± 0.024 | |||
| 50 | -0.085 ± 0.027 | * | |||
| 100 | -0.097 ± 0.041 | ** | |||
Table 2: Dicarboxylic acids possessing straight hydrocarbon chains, their chemical structure, partition coefficients and effects on OF in cattle RBCs in vitro. Values are means ± SD (n=6). The partition coefficients were obtained from the PubChem [32] or ChemSpider [33] website. Asterisks (* and **) indicate that there was a significant difference (P<0.05 and P<0.01) between the control (0 mM) and subsequent concentration (0.1-100 mM) based on the Dunnett’s test. As there were no significant changes for exposure to 0.1-5 mM of all tested monocarboxylic and dicarboxylic acids, the EC50 values at those doses are omitted and the data for 10, 25, 50 and 100 mM are presented.
Effects of monocarboxylic acids possessing branched hydrocarbons
3-Methyl-n-valeric acid (C6) did not change OF at any concentrations from 0.1-100 mM (Figure 3). In contrast, 3.3-dimetyln- butyric acid tended to decreased OF at 25 to 100 mM, but these changes were not statistically significant (N.S.). Although some compounds tended to decrease OF, those changes were N.S. (Table 3). Other monocarboxylic acids having branched hydrocarbons did not change OF at 10 to 100 mM.
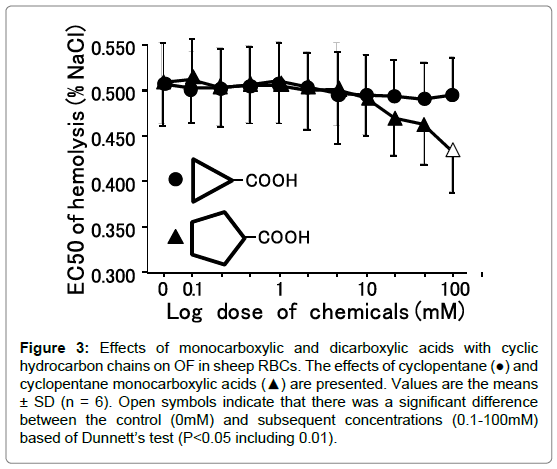
Figure 3. Effects of monocarboxylic and dicarboxylic acids with cyclic hydrocarbon chains on OF in sheep RBCs. The effects of cyclopentane (●) and cyclopentane monocarboxylic acids (▲) are presented. Values are the means ± SD (n = 6). Open symbols indicate that there was a significant difference between the control (0mM) and subsequent concentrations (0.1-100mM) based of Dunnett’s test (P<0.05 including 0.01).
| No of hydrocarbon | Carboxylic acid | Partition coefficient | Dose (mM) | Change in OF ⊿EC50 (NaCl %) |
|---|---|---|---|---|
| 3 | iso-Butyric acid | 0.94 | 10 | -0.001 ± 0.008 |
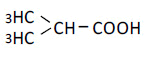 |
25 | -0.011 ± 0.017 | ||
| 50 | -0.013 ± 0.006 | |||
| 100 | -0.010 ± 0.013 | |||
| 4 | iso-Valeric acid | 1.16 | 10 | 0.004 ± 0.019 |
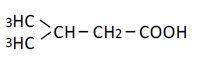 |
25 | 0.002 ± 0.014 | ||
| 50 | -0.011 ± 0.016 | |||
| 100 | -0.015 ± 0.014 | |||
| 4 | 2-Methyl-butyric acid | 1.18 | 10 | 0.007 ± 0.006 |
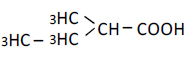 |
25 | 0.005 ± 0.009 | ||
| 50 | 0.006 ± 0.014 | |||
| 100 | 0.010 ± 0.013 | |||
| 4 | Dimetyl-propionic acid | 1.48 | 10 | -0.003 ± 0.017 |
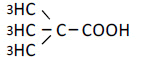 |
25 | -0.015 ± 0.014 | ||
| 50 | -0.036 ± 0.024 | |||
| 100 | -0.055 ± 0.023 | |||
| 5 | 2-Mrthy-n-valeric acid | 1.80 | 10 | 0.004 ± 0.010 |
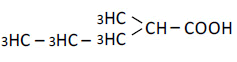 |
25 | -0.007 ± 0.012 | ||
| 50 | -0.030 ± 0.021 | |||
| 100 | -0.044 ± 0.031 | |||
| 5 | 3-Mrthy-n-valeric acid | 1.56 | 10 | 0.009 ± 0.010 |
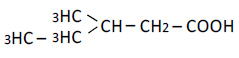 |
25 | 0.005 ± 0.015 | ||
| 50 | 0.002 ± 0.010 | |||
| 100 | 0.002 ± 0.018 | |||
| 5 | 4-Mrthy-n-valeric acid | 1.66 | 10 | 0.003 ± 0.005 |
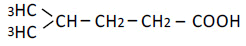 |
25 | -0.001 ± 0.005 | ||
| 50 | -0.015 ± 0.019 | |||
| 100 | -0.025 ± 0.010 | |||
| 5 | 2-ethyl-n-butyric acid | 1.66 | 10 | 0.002 ± 0.012 |
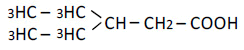 |
25 | 0.006 ± 0.007 | ||
| 50 | 0.002 ± 0.011 | |||
| 100 | 0.012 ± 0.019 | |||
| 5 | 3,3-Dimethyl-n-butyric acid | 1.47 | 10 | 0.010 ± 0.014 |
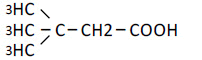 |
25 | -0.010 ± 0.011 | ||
| 50 | -0.026 ± 0.014 | |||
| 100 | -0.038 ± 0.016 |
Table 3: Monocarboxylic acids possessing branched hydrocarbon chains, their chemical structure, partition coefficients and effects on OF in cattle RBCs in vitro. Values are means ± SD (n=6). The partition coefficients were obtained from the PubChem [32] or ChemSpider [33] website. Asterisks (* and **) indicate that there was a significant difference (P < 0.05 and P < 0.01) between the control (0 mM) and subsequent concentration (0.1-100 mM) based on the Dunnett’s test. As there were no significant changes for exposure to 0.1-5 mM of all tested monocarboxylic and dicarboxylic acids, the EC50 values at those doses are omitted and the data for 10, 25, 50 and 100 mM are presented.
Effects of monocarboxylic acids possessing cyclic hydrocarbons
Cyclopropanecarboxylic acid (C3) did not change OF at any concentration applied in these experiments (Figure 4). In contrast, cyclopentanecarboxylic acid (C5) decreased OF in a dosedependent manner (P<0.05). For monocarboxylic acids having cyclic hydrocarbons, although cyclopentanecarboxylic (C5) and cyclohexanecarboxylic acids (C6) decreased OF at 100mM, the other carboxylic acids (C3, C4), including benzoic acid (C6), did not change OF in cattle RBCs (Table 4).
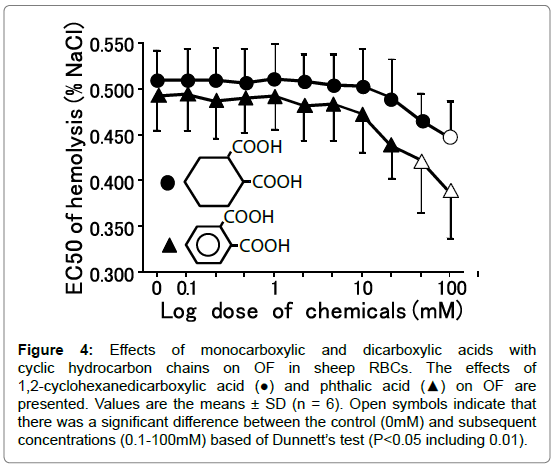
Figure 4. Effects of monocarboxylic and dicarboxylic acids with cyclic hydrocarbon chains on OF in sheep RBCs. The effects of 1,2-cyclohexanedicarboxylic acid (●) and phthalic acid (▲) on OF are presented. Values are the means ± SD (n = 6). Open symbols indicate that there was a significant difference between the control (0mM) and subsequent concentrations (0.1-100mM) based of Dunnett’s test (P<0.05 including 0.01).
| No of hydrocarbon | Carboxylic acid | Partition coefficient | Dose (mM) | Change in OF ⊿EC50 (NaCl %) | |
|---|---|---|---|---|---|
| 3 | Cyclopropane-carboxylic acid | 0.08 | 10 | -0.012 ± 0.006 | |
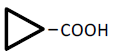 |
25 | -0.014 ± 0.007 | |||
| 50 | -0.016 ± 0.007 | ||||
| 100 | -0.012 ± 0.007 | ||||
| 4 | Cyclobutane-carboxylic acid | 0.65 | 10 | -0.004 ± 0.011 | |
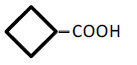 |
25 | -0.008 ± 0.005 | |||
| 50 | -0.008 ± 0.016 | ||||
| 100 | -0.004 ± 0.024 | ||||
| 5 | Cyclopentane-carboxylic acid | 1.21 | 10 | -0.016 ± 0.014 | |
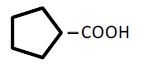 |
25 | -0.039 ± 0.015 | |||
| 50 | -0.046 ± 0.019 | ||||
| 100 | -0.076 ± 0.009 | * | |||
| 6 | Cyclohexane-monocarboxylic acid | 1.96 | 10 | -0.015 ± 0.016 | |
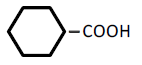 |
25 | -0.034 ± 0.029 | |||
| 50 | -0.051 ± 0.023 | ||||
| 100 | -0.067 ± 0.026 | * | |||
| 6 | Benzoic acid | 1.87 | 10 | -0.019 ± 0.015 | |
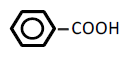 |
25 | -0.031 ± 0.027 | |||
| 50 | -0.039 ± 0.023 | ||||
| 100 | -0.040 ± 0.032 |
Table 4: Monocarboxylic acids possessing cyclic hydrocarbon chains, their chemical structure, partition coefficients and effects on OF in cattle RBCs in vitro. Values are means ± SD (n=6). The partition coefficients were obtained from the PubChem [32] or ChemSpider [33] website. Asterisks (* and **) indicate that there was a significant difference (P<0.05 and P<0.01) between the control (0 mM) and subsequent concentration (0.1-100 mM) based on the Dunnett’s test. As there were no significant changes for exposure to 0.1-5 mM of all tested monocarboxylic and dicarboxylic acids, the EC50 values at those doses are omitted and the data for 10, 25, 50 and 100 mM are presented.
Effects of dicarboxylic acids possessing cyclic hydrocarbons
1,2-Cyclohexanedicarboxylic acid and phthalic acid (C6) decreased OF dose-dependently, with significant decreases in OF observed at 100 mM and at 50 and 100 mM (P<0.05), respectively (Figure 5). The other dicarboxylic acids having cyclic hydrocarbons (C6), including a benzene ring, tended to decrease OF, but those changes were N.S. (Table 5).
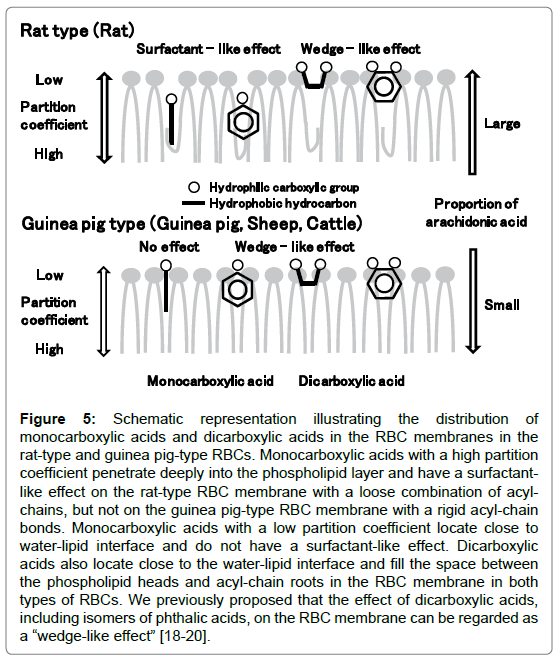
Figure 5. Schematic representation illustrating the distribution of monocarboxylic acids and dicarboxylic acids in the RBC membranes in the rat-type and guinea pig-type RBCs. Monocarboxylic acids with a high partition coefficient penetrate deeply into the phospholipid layer and have a surfactantlike effect on the rat-type RBC membrane with a loose combination of acylchains, but not on the guinea pig-type RBC membrane with a rigid acyl-chain bonds. Monocarboxylic acids with a low partition coefficient locate close to water-lipid interface and do not have a surfactant-like effect. Dicarboxylic acids also locate close to the water-lipid interface and fill the space between the phospholipid heads and acyl-chain roots in the RBC membrane in both types of RBCs. We previously proposed that the effect of dicarboxylic acids, including isomers of phthalic acids, on the RBC membrane can be regarded as a “wedge-like effect” [18-20].
| No of hydrocarbon | Carboxylic acid | Partition coefficient | Dose (mM) | Change in OF ⊿EC50 (NaCl %) | |
|---|---|---|---|---|---|
| 6 | 1,2-Cyclohexane-dicarboxylic acid | 0.64 | 10 | -0.006 ± 0.023 | |
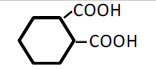 |
25 | -0.020 ± 0.019 | |||
| 50 | -0.043 ± 0.006 | ||||
| 100 | -0.064 ± 0.011 | * | |||
| 6 | 1,3-Cyclohexane-dicarboxylic acid | 0.46 | 10 | 0.005 ± 0.006 | |
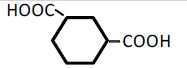 |
25 | -0.010 ± 0.017 | |||
| 50 | -0.031 ± 0.018 | ||||
| 100 | -0.048 ± 0.024 | ||||
| 6 | 1,4-Cyclohexane-dicarboxylic acid | 0.83 | 10 | 0.000 ± 0.008 | |
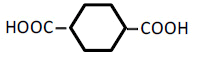 |
25 | -0.010 ± 0.016 | |||
| 50 | -0.028 ± 0.022 | ||||
| 100 | -0.044± 0.023 | ||||
| 6 | Phthalic acid | 0.73 | 10 | -0.020 ± 0.032 | |
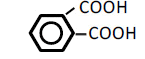 |
25 | -0.055 ± 0.025 | |||
| 50 | -0.070 ± 0.040 | * | |||
| 100 | -0.106 ± 0.039 | ** | |||
| 6 | Isophathalic acid | 1.66 | 10 | -0.017 ± 0.005 | |
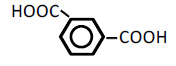 |
25 | -0.033 ± 0.015 | |||
| 50 | -0.040 ± 0.014 | ||||
| 100 | -0.053 ± 0.021 | ||||
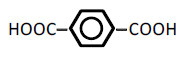
| 6 | Terephthalic acid | 2.00 | 10 | -0.008 ± 0.011 | |
 |
25 | -0.027 ± 0.011 | |||
| 50 | -0.038 ± 0.014 | ||||
| 100 | -0.060 ± 0.020 |
Table 5: Monocarboxylic acids possessing cyclic hydrocarbon chains, their chemical structure, partition coefficients and effects on OF in cattle RBCs in vitro. Values are means ± SD (n=6). The partition coefficients were obtained from the PubChem [32] or ChemSpider [33] website. Asterisks (* and **) indicate that there was a significant difference (P < 0.05 and P < 0.01) between the control (0 mM) and subsequent concentration (0.1-100 mM) based on the Dunnett’s test. As there were no significant changes for exposure to 0.1-5 mM of all tested monocarboxylic and dicarboxylic acids, the EC50 values at those doses are omitted and the data for 10, 25, 50 and 100 mM are presented.
Relationship between the partition coefficient of the compounds and their effects on OF
For both the monocarboxylic and dicarboxylic acids tested in this study, regression analysis did not demonstrate any clear relationship between the partition coefficient of the compounds and their effects on OF in cattle RBCs (Table 6).
| substances | n | mM | Intersept | Slope | r | P |
|---|---|---|---|---|---|---|
| Monocarboxylic acid 22 substances | 22 | 10 | -0.0073 | 0.0027 | 0.0027 | 0.3352 |
| 22 | 25 | -0.0118 | 0.0001 | 0.0039 | 0.9863 | |
| 22 | 50 | -0.0156 | -0.0021 | 0.106 | 0.6387 | |
| 21 | 100 | -0.0073 | -0.0116 | 0.356 | 0.1132 | |
| Dicarboxylic acid 15 substances | 14 | 10 | -0.0144 | 0.0011 | 0.1116 | 0.704 |
| 14 | 25 | -0.0345 | 0.0046 | 0.2348 | 0.4191 | |
| 14 | 50 | -0.0561 | 0.0062 | 0.2433 | 0.402 | |
| 14 | 100 | -0.0905 | 0.018 | 0.4266 | 0.1282 |
Table 6: Correlation between the partition coefficients of carboxylic acids and change in EC50 during hemolysis in cattle RBCs. Values were calculated by regression analysis (mean value of each carboxylic acid; n=6) between the partition coefficients and change in EC50 during hemolysis induced by each dose of the monocarboxylic and dicarboxylic acids, with benzoic acids, phthalic acid and its isomers included or not. Correlation efficient “r” and significance “P” are shown. P<0.05 is defined as statistically significant in the present study.
In this series of experiments, we clarified the effects of the application of monocarboxylic and dicarboxylic acids at 0.1 to 100 mM for one hour on OF in cattle RBCs. The results revealed that most monocarboxylic acids possessing straight hydrocarbon chains did not affect OF in cattle RBCs, except that the application of n-caprylic acid (C7) induced hemolysis at 100 mM. Although some monocarboxylic acids possessing branched hydrocarbon chain tended to decrease OF, these changes were N.S. For monocarboxylic acid with cyclic hydrocarbon chains, cyclopentanecarboxylic acid (C5) and cyclohexanecarboxylic acid (C6) decreased OF in a dose-dependent manner (P<0.05). Benzoic acid tended to decrease OF, but this change was N.S. Contrary to the monocarboxylic acids, most dicarboxylic acids possessing straight hydrocarbon chains, except for glutaric acid (C3), decreased OF dose-dependently (P<0.05). Glutaric acid tended to decrease OF, but this change was N.S. For dicarboxylic acids with cyclic hydrocarbon chains or benzene rings (C6), 1,2-cyclohexanecaroboxylic acid and phthalic acid decreased OF dose-dependently (P<0.05). 1,3- and 1,4-Cyclohexanecarboxylic acids, and isophthalic and terephthalic acids also tended to decrease OF, but these changes were N.S. Regression analysis failed to reveal any clear relationships between the OF responses to monocarboxylic acids or dicarboxylic acids, and their partition coefficients.
In our series of studies using the same experimental procedure, we have clarified the effects of monocarboxylic acids on OF in rat [17-19], guinea pig [19] and sheep RBCs [20]. OF in rat RBCs was increased by the application of most monocarboxylic acids possessing straight, branched and cyclic hydrocarbons, including benzoic acids [17-19]. There were positive and significant relationships between the partition coefficients of monocarboxylic acids with straight and cyclic hydrocarbon chains, and their effects on OF in rat RBCs [30,31]. On the other hand, in guinea pig and sheep RBCs, OF was not increased or was rather decreased by some monocarboxylic acids possessing branched or cyclic hydrocarbons [19,29]. No definite relationship between those two parameters was demonstrated in guinea pig [31] or sheep RBCs [20]. Thus, the response in OF in cattle RBCs to monocarboxylic acids and the relationship between the partition coefficient and OF response are close to those in guinea pig and sheep RBCs, but not in rat RBCs.
The octanol/water partition coefficient has been widely used as a useful parameter for estimating the distribution of amphiphilic chemicals in cells, tissues and the body in general [27-29]. On the other hand, it has been reported that for many chemicals, their actions on biological or artificial phospholipid membranes do not have a definite correlation to their octanol/water partition coefficient [34-36]. This can be explained not only by factors associated with the chemicals themselves, but also by the condition of membrane, such as the type and amount of phospholipids or cholesterol coming into contact with the chemicals [37-40]. In our previous experiment, the octanol/ water partition coefficient of monocarboxylic acids showed a positive and significant relation to the changes in OF in rat RBCs [18,19]. However, in guinea pig and sheep RBCs, the partition coefficients of monocarboxylic and dicarboxylic acids did not show any definite relationship to the changes in OF [19,20]. Monocarboxylic acids are amphiphilic compounds composed of a hydrophilic carboxylic group and a hydrophobic hydrocarbon chain with a number of carbons in various kinds of bonds. Many amphiphilic compounds are categorized as surfactants and monocarboxylic acids could also be recognized as surfactant-like substances in respect to their chemical structure and amphiphilic characteristics [41]. The mechanism for action of amphiphilic materials that act as surfactants on the biological membrane has been reviewed in both physiological and pharmacological terms [21-24]. According to these reviews, the first step is the permeation of the surfactants into the cell membrane, and the partition coefficient can be used as an index of their permeability. The second step is the production of mixed surfactant/lipid bilayers, known as mixed micelle formation, in the cell membrane. Each surfactant permeates the phospholipid layer and leads to the solubilization of the membrane until saturation of the surfactant to phospholipid ratio, referred to as the critical micellar concentration (CMC). The third step involves the destruction of the membrane and the bursting of the cell after the CMC is reached. The mechanism for the change in the RBC membrane and following hemolysis by surfactants has been well summarized and discussed in a previous review [41].
If we name the response in which OF is increased by monocarboxylic acids as “rat type” and the response in which OF is not changed or is rather decreased by monocarboxylic acids as “guinea pig type”, respectively. In respect to the OF response to monocarboxylic acids, only rat RBCs showed a rat-type response [17-19], while guinea pig [19] and sheep RBCs [20] showed guinea pig-type RBC reactions. The results of the current experiments showed that, for OF response to monocarboxylic acids, cattle RBCs show an obviously guinea pig-type response. We thought that, in rat-type RBCs, monocarboxylic acids could permeate the RBC membrane, form monocarboxylic acid/ phospholipid micelles, solubilize the RBC membrane and eventually induce hemolysis, as described above in the action model of surfactants [21-24]. We speculated that in guinea pig-type RBCs, since the process of permeation or micelle formation could not occur for some reason, solubilization of the RBC membrane leading to hemolysis would not induced by monocarboxylic acids.
We speculated that the differences in OF response could be explained by the differences in the phospholipid composition of the RBC membrane among animal species. Phospholipids are composed of a head group and an acyl-chain derived from fatty acids. It was reported that the composition of the head in phospholipids affects the fluidity of the RBC membrane [42]. In our recent report, we tried to explain the differences in OF response to monocarboxylic acids between rattype (rat) and guinea pig-type (guinea pig and sheep) RBCs [19,20]. However, it was difficult to explain the difference in OF response [19,20] based on the head group composition in phospholipids in the RBC membrane [43]. Differences in head group were recognized between rats, and guinea pigs and sheep. In the ruminant group (sheep, cattle and goats), the RBC membrane does not contain phosphatidylcholine, instead the proportion of sphingomyeline is larger in sheep (51.0%) than in rat (12.8%) and guinea pig RBCs (11.1%) [43]. The proportion of sphingomyeline is almost the same in rat and guinea pig RBCs. Further, the proportion of phosphatidylcholine dose not differ markedly between rat (47.5%) and guinea pig RBCs (41.1%) [43]. From the data in this report, it appears that the other head groups in phospholipids in the RBC membrane do not differ greatly among species. Thus, we could not explain the differences in OF response from the differences in the composition of the phospholipid head group in rat, guinea pig and sheep RBCs. In this report, the proportion of sphingomyeline in cattle is 46.2% which value is comparable with that in sheep (51.0%) [43].
In contrast, from the many kinds of fatty acids forming acyl-chain in phospholipids, we found that a specific fatty acid, arachidonic acid, comprised the most common fatty acid in the rat RBC membrane [44]. In addition, proportion of arachidonic acid in the RBC membrane was found to be noticeably larger in rats (30.0%) than in guinea pigs (18.0%) [44]. Another report showed that proportion of arachidonic acid in the RBC membrane is 22.7% in rats and only 1.5% in sheep [45]. The same report showed that the proportion of arachidonic acid among the fatty acids was 4.5% in the RBC membrane in cattle (oxen but not cows) [45]. The content of arachidonic acid in cattle RBCs is closer to that in guinea pig or sheep RBCs than to that in rat RBCs. It is assumed that arachidonic acid is one of the candidates for explaining the specific OF response in rat RBCs (rat type) compared to the responses in guinea pig, sheep and cattle RBCs (guinea pig type) [20].
Arachidonic acid, expressed as 20:4 (5,8,11,14), is a polyunsaturated carboxylic acid with a 20-carbon chain and four cis-double bonds. Due to the four unsaturated carbon bonds, arachidonic acid possesses a structure with a highly crooked acyl-chain in its moiety. This molecular structure is speculated to disturb the rigid binding of acyl-chains aligned linearly in the phospholipid layer of the RBC membrane. There is a report that long-chain cis-unsaturated fatty acids and their alcohol analogues, including arachidonyl alcohol, increased the membrane fluidity of cattle platelets [46]. The same report also demonstrated that the saturated fatty acids and trans-unsaturated fatty acid tested for comparison had either a much lower or no effect on membrane fluidity. In addition, the study also found that the position of the double bonds had less influence than did the number of double bonds [46]. It was reported that membrane fluidity is an important factor affecting Rb+(K+) efflux in RBCs isolated from seven mammalian species (cow, horse, pig, human, rabbit, cat and rat RBCs) [47]. The rate constant of Rb+(K+) efflux could be correlated with the order parameter obtained from electron spin resonance and with the number of double bonds in the fatty acids in membrane phospholipids. Among the seven species, excluding humans, rat RBCs demonstrated the highest rate constant of Rb+(K+) efflux and the number of double bonds in the fatty acids in the RBC membrane [47]. In addition, the study investigated the relationship between the rate constant of Rb+(K+) efflux and the percentage of arachidonic acid in the RBC membrane of mammalian species (cow, horse, pig, human, rabbit and rat RBCs) [48]. In this experiment, rat RBCs demonstrated the highest Rb+(K+) efflux and percentage of arachidonic acid among RBC membranes of mammalian species tested, excluding humans. The authors stated that there was no significant correlation between the rate constants and the relative contents of the four major phospholipids (phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine or sphingomyeline). In addition, a previous report showed that human RBCs will leak K+ if the native phosphatidylcholine is partly replaced by PC containing arachidonic acid [49]. Those reports suggest that a large amount of arachidonic acid may contribute to increasing membrane fluidity and the leakage of K+ from the RBC membrane. Further, they suggest that a large amount of arachidonic acid inhibits the formation of firm bond between acyl-chains and lead to greater space in the phospholipid layer in the cell membrane.
We have already proposed an explanation for different OF responses between rat-type and guinea pig-type RBCs in our recent report [19,20]. In cell membranes with a high proportion of arachidonic acid, such as rat RBCs, monocarboxylic acids with a certain length of hydrocarbon chain and high partition coefficients are speculated to invade the membrane matrix, which has loose acyl-chain bonds and a certain amount of free space, in the phospholipid layer. After permeation into the membrane, monocarboxylic acids, like as other surfactants, interact with the phospholipids, leading to micelle formation until CMC is reached. During this stage, membrane fluidity increases with increases in OF; however, after reaching CMC, the RBCs burst leading to hemolysis. On the other hand, in RBC membranes with a low proportion of arachidonic acid (guinea pigs, sheep and cattle), monocarboxylic acids possessing straight hydrocarbon chain cannot enter deeply into and disturb the membrane matrix as the acylchain bonds are strong and there is little free space in the phospholipid layer. In cases in which a monocarboxylic acid possessing a certain hydrocarbon chain structure (branched or cyclic) is applied to guinea pig, sheep or cattle RBCs, we assume that these hydrophobic elements cannot enter deeply into the acyl-chain layer but could enter to a sallow depth close to water/lipid interface, stabilizing the membrane and decreasing OF similar to the mechanism described dicarboxylic acids below.
In contrast to monocarboxylic acids, we demonstrated that most dicarboxylic acids possessing straight or cyclic hydrocarbon chain decrease OF in rat [17-19], guinea pig [19] and sheep RBCs [20]. In the present experiment, OF in cattle RBCs was also decreased or tended to be decreased by dicarboxylic acids, like as in rat, guinea pig and sheep RBCs. For the OF response to dicarboxylic acids, there is no clear difference in OF response; i.e., rat type and guinea pig type, among the animal species. These shared OF responses induced in the RBCs from different species could not be explained by the differences in phospholipid or fatty acids composition in the RBC membranes. We have previously proposed that the OF-lowering effect of dicarboxylic acids on the RBC membrane could be a “wedge-like effect,” and demonstrated the mechanism for the stabilizing effect on the cell membrane against low osmotic pressure [18-20].
In brief, the partition coefficients of the dicarboxylic acids are generally lower than those of the corresponding monocarboxylic acids, except for benzoic acid and terephthalic acid. Dicarboxylic acid is composed of a hydrophobic hydrocarbon chain and two hydrophilic carboxylic groups attached at the ends of this hydrocarbon chain. As two hydrophilic carboxylic groups could not enter deeply into the acyl-chain layer, these elements could be situated at the interface of phospholipid layer directed toward the water interface. A space enclosed by the roots of the two acyl-chains and the head group of the phospholipids could be formed in the lipid-water interface region of the RBC membrane. The physicochemical conditions of this region do not differ greatly between the RBCs of animal species as there are few unsaturated carbon bounds at the roots of acyl-chains bound to the head group of phospholipid compared to the deeper layer in which the acyl-chains are located. The hydrophobic hydrocarbon chain is speculated to enter the area, thereby changing its conformation to form a rigid U- or V-shaped structure [18-20]. This structure stabilizes the cell membrane and leads to an increase in membrane resistance to osmotic pressure, decreasing OF in the RBCs in various animal species.
Adding the concept of rat- and guinea pig-type RBC membranes and the results from this study using cattle RBCs to the previous model [20], we can propose a new model for the action of carboxylic acids and the wedge-like effect on RBC membranes (Figure 5). In RBC membranes with a higher proportion of arachidonic acid, such as in rat RBCs (rat type), monocarboxylic acids are speculated to permeate the phospholipid layer containing unfastened acyl-chains bonds and a certain amount of free space in the membrane matrix (rat type). Those substances permeate deeply into the RBC membrane, inducing formation of substance/phospholipid micelles and thereby increasing OF in rat RBCs. On the other hand, monocarboxylic acids cannot invade deeply into the membrane, resulting in a lack of any effect on the RBC membrane (guinea pig type). In cases in which monocarboxylic acids with a certain hydrocarbon structure are situated in the shallower area of the water/lipid interface, they make stable bonds with the phospholipids, thus stabilizing the cell membrane and decreasing OF in the RBCs of some animal species, except for rats. In addition, most dicarboxylic acids locate in the space close to water/lipid interface and increase resistance to osmotic pressure, resulting in decreased OF in the RBC membranes of various animal species.
At present, only rat RBCs are categorized as “rat type”, with the “guinea pig type” including guinea pig, sheep and cattle RBCs. This classification is based on the OF response to monocarboxylic acid; OF is increased by most monocarboxylic acids in the rat type and is not affected or rather decreased by these acids in the guinea pig type. This classification is well explained by the data based on the different proportion of arachidonic acid in the phospholipids in the RBC membrane. The same experiment using RBCs from other animal species will allow us to clarify our hypothesis, and confirm whether arachidonic acid is the key fatty acid in the RBC membrane in terms of inducing rat- or guinea pig-type OF response. Although we still need to find RBCs that show a rat-type OF response to monocarboxylic acids among other animals except for rats, the content of arachidonic acid in the RBC membrane will be an important clue in identifying these RBCs. On the other hand, OF is generally decreased by many dicarboxylic acids in the RBCs examined among different animal species to date (rats, guinea pigs, sheep and cattle). We need to elucidate whether our hypothesis of a wedge-like effect induced by dicarboxylic acids is a common phenomenon or not in the RBCs among animal species other than the above four. Thus, with regard to the OF response to monocarboxylic and dicarboxylic acids, it is necessary to clarify the cause of the differences in response among species and the mechanisms in detail. Advanced experiments using RBCs of other animal species will, therefore, be needed in future studies.
Citation: Mineo H, Moriyoshi M (2019) Carboxylic Acids with Certain Molecular Structures Decrease Osmotic Fragility against Osmotic Pressure in Cattle Erythrocytes in vitro: Appearance of a Wedge-like Effect Similar to RBCs in Other Animal Species. Biochem Pharmacol (Los Angel) 8:264. doi: 10.35248/2167-0501.19.8.264
Received: 27-Dec-2018 Accepted: 13-Mar-2019 Published: 20-Mar-2019 , DOI: 10.35248/2167-0501.19.8.264
Copyright: © 2019 Mineo H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.