Journal of Hematology & Thromboembolic Diseases
Open Access
ISSN: 2329-8790
ISSN: 2329-8790
Research Article - (2025)Volume 13, Issue 1
Objective: To demonstrate a clear link between predicted blood shear forces during valve closure and thrombogenicity that explains the thrombogenic difference between tissue and mechanical valves and provides a practical metric to develop and refine prosthetic valve designs for reduced thrombogenicity.
Methods: Pulsatile and quasi-steady flow systems were used for testing. The time-variation of Projected Open Valve Area (POVA) was measured using analog opto-electronics calibrated to projected reference orifice areas. Flow velocity determined over the cardiac cycle equates as instantaneous volumetric flow rate divided by POVA. For the closed valve interval, data from quasi-steady back pressure/flow tests was obtained.
Performance ranked by derived maximum negative and positive closing flow velocities, evidences potential clinical thrombogenicity via inferred velocity gradients (shear). Clinical, prototype and control valves were tested.
Results: Blood shear and clot potential from multiple test datasets guided empirical optimization and comparison of valve designs. A 3-D printed prototype valve design (BV3D) purposed for soft closure and reduced thrombogenic potential was assessed.
Conclusions: The relationship between leaflet geometry, flow velocity and predicted shear at valve closure illuminated an important source of prosthetic valve thrombogenicity. With an appreciation for this relationship and based on our experiment generated comparative data, we achieved optimization of valve prototypes with potential for reduced thrombogenicity.
Prosthetic valve; Laboratory simulation; Projected open valve area; Valve closure, Thrombogenicity; Valve flow velocity; Rebound
Our interest in prosthetic valve dynamics was initially stimulated during formation of the cardiac research development laboratory tasked with supporting the newly opened cardiac surgery unit at Royal Jubilee Hospital in Victoria, BC, Canada. Incorporated as Vivitro Systems Inc. (VSI), our primary focus was research, design and development of cardiac valve implant devices and on the laboratory test systems required. During this phase we studied valve motion in an early pulse duplicator using highspeed cinematography and photogrammetric analysis [1]. Subsequently, an innovative simpler method was devised to determine Projected Open Valve Area (POVA) similar to planimetric quantification of POVA from 16 mm cinematography and was published in new laboratory technique measures projected dynamic area of prosthetic heart valves [2]. In 2009, work transitioned into a separate independent research and development enterprise, ViVitro Laboratories Inc. (VLI) also based in Victoria, BC, Canada.
Consistent with a focus on evaluation of various prosthetic valve models, our pulse duplicator was modified to include a unique opto-electronic subsystem which we named Leonardo. The addition of this subsystem redirected our interest in heart valve dynamics to the closure phase and associated supra-physiologic backflow fluid velocities. Driven by ongoing curiosity and armed with new data from Leonardo, we reported our findings through a series of preprints and publications [3-5]. In a recent broader application of our investigative technology, results from in silico computational modeling and in vitro experimental studies confirmed or verified the characteristics of leaflet spatial oscillations in bioprosthetic valves (flutter) throughout the open period of the cardiac cycle Lee, et al. and prompted peer commentary [6,7].
Given the arc of success with prosthetic valves over decades including initiation and progressive expansion of transcatheter devices, long-term durability and thrombosis issues persist. Thrombosis is a physics-based phenomenon that nature evolved to stem bleeding after an injury. For both transcatheter and surgically implanted bioprosthetic valves, limited durability related to multiple factors has stimulated introduction of a variety of “rescue devices”. Intended to provide transcatheter based mitigation of complications in primary and valve-in-valve bioprosthetic valves implants, these devices are associated with their own unique complications. The longer term consequences of a transcatheter based multiple valve approach for patient morbidity; mortality and overall cost are yet to be determined. In the current paper, our focus returns to identification and assessment of sources of thrombogenicity in contemporary clinical and also experimental mechanical and bioprosthetic heart valves with particular attention to the central role of conspicuous transient fluid velocities during valve closure. Flow velocity manifests shear via flow velocity gradients that can trigger blood damage and clot formation in vascular disease processes and cardiovascular implant devices [8,9]. When adjacent fluid layers bypass with speed differentials, shear forces increase and blood damage results. We have sought to assess and compare the dynamic behavior of clinical and experimental valves which stimulated provocative conclusions regarding development of less thrombogenic devices.
Pulse duplicator experiments and computational modeling
Over time, progressive adaptations to the pulse duplicator and experiment outcomes have been reported [10]. This included the optical measurement of valve POVA kinematics and non-trivial in silico evaluations [11]. Test conditions, FSI parameters, and boundary conditions used in this study are reported in the Figure 1. Computational model results and experimental outcomes are in close agreement (Table 1) [12].
Figure 1: LeonardoLNS pulse duplicator system includes reduced models of compliance and resistance: CVIA1 120 mL; CVIA2 50 mL; Cper 615 mL; Croot 640 mL; Rper adjusted for normal mean aortic pressure of 100 mmHg; RVIA 200 c.g.s. units test fluid saline; Cardiac output ~5 L/min.
| Valve name | Description | Site |
|---|---|---|
| St. Jude Medical Regent™ (SJM) | Mechanical bi-leaflet | A, M |
| mock-TAVR (PVL pre-adjusted) | Simulated TAVR with preset trivial Paravalvular Leak (PVL) | A |
| On-X™ Life Technologies | Mechanical bi-leaflet | A, M |
| BV3DLNS | Printed prototype, bi-leaflet | A, M |
| Lapeyre-Triflo™-F6 (circa 2004) | Prototype, mechanical tri-leaflet | A, M |
| Edwards-Perimount™ | Bioprosthetic, pericardial tri-leaflet | A, M |
| Note: Valve seat size (TAD) ~25 mm. (A)ortic, (M)itral; Non-test valve Mitroflow** pericardial size 29 mm. **Sorin, Milan, Italy | ||
Table 1: Valves tested in aortic and mitral locations.
Test valves are listed in Table 1. Flow and pressure signals are filtered by analogue circuitry (bandwidth BW ~0-100 Hz). The POVA signal was unfiltered and had a measured rise time ~1.04 μs (BW ~0-337 KHz). Saline was used as an accepted test fluid per ISO 5840-3 with density ρ=1.0 g/cm3 and dynamic viscosity μ=1.0 cP (Figure 2).
Figure 2: (A): Photograph; (B): Rendering of prototype bi-leaflet mechanical valve BV3D.
Assessment of dynamic flow velocity
The Leonardo system enables assessment of dynamic flow velocity through test valves using measurements from an electromagnetic magnetic flow probe together with photodiode detection of POVA. Mathematically, flow velocity equates to the ratio of these two quantities as:
Flow velocity (cm/s)=volumetric flow rate (cm3/s)/POVA (cm2)
The assumption is POVA has a uniform flow profile but this may not hold true when valve occluder motion and flow are irregular. Experiments indicate that as POVA decreases, valve flow velocity increases and conversely, decreases when POVA increases. This behavior is governed by Bernoulli’s principle.
Pressure measurement
Left atrial, left ventricle aortic outflow tract, and aortic sites pressures measured via short catheters (~7.5 cm length) have internal diameter of ~1.8 mm connected to disposable pressure transducers (DeltranTM, model PT43-604)*** (***: Utah Medical Products Inc., Midvale, Utah 84047-1048, USA). Catheter ports measured wall pressures referenced to the mid-plane of the test valve. In Figure 1, aortic transvalve pressure is measured between the left ventricular outflow tract (LVP) and the aorta (AP). Mitral transvalve pressure is measured between the left atrium (LAP) and the left ventricular outflow tract (LVP).
Significant waveform characteristics
Several traits are clear in Figures 3-5. All signals were sampled synchronously. Regions relevant to the valve closure moment are near the dashed red line. Of importance are: Initial minimum valve POVA values and initial peak negative values attained in transvalve pressure, volume flow rate, and closure flow velocities. For bioprosthetic valves, we observed an upward-downward movement of both the valve frame and leaflets demonstrative of compliance reactivity. Hydrodynamic oscillations are also present post valve closure as seen in the unadjusted volume flow rate in some of the waveforms. Comparing the phasing of volume flow rate and POVA near valve closure, regional components having compliance influence hydrodynamic patterns.
Figure 3: Measured and derived AORTIC valve test data includes 10 consecutive cycles each experiment. A spatially averaged metric for mitral valve flow velocity (red curves) is equated for specified cycles to volume flow rate/valve projected open area. Mean flow velocity and 95% confidence limits are shown. Some peak flow velocities shown are outside the upper and lower bounds. An optical approach measures valve projected open area. Data acquisition sampling interval of white dots is 3.36 ms. Intravalvular Leakage rates are shown (IVL) and Paravalvular (PVL). Edited vs. unedited volume flow rates are shown dashed grey alongside edited solid black waveforms. Influence of reactive elastic and inertia elements are apparent in the unedited flows (dashed grey) which are edited out prior to estimation the red flow velocity waveforms. Partial re-opening of AORTIC valve projected open areas are shown highlighted in yellow and the shock water hammer zones are in blue. Control valve is Edwards Perimount.
Figure 4: Measured and derived MITRAL valve test data includes 10 consecutive cycles each experiment. A spatially averaged metric for mitral valve flow velocity (red curves) is equated for specified cycles to volume flow rate/valve projected open area. Mean flow velocity and 95% confidence limits are shown. Some peak flow velocities shown are outside the upper and lower bounds. An optical approach measures valve projected open area. Data acquisition sampling interval of white dots is 3.36 ms. Intravalvular Leakage rates are shown (IVL) and Paravalvular (PVL). Edited vs. unedited volume flow rates are shown dashed grey alongside edited solid black waveforms. Influence of reactive elastic and inertia elements are apparent in the unedited flows (dashed grey) which are edited out prior to estimation the red flow velocity waveforms. Partial re-opening of MITRAL projected open areas are shown highlighted in yellow and the shock water hammer zones are in blue. Control valve is Edwards Perimount.
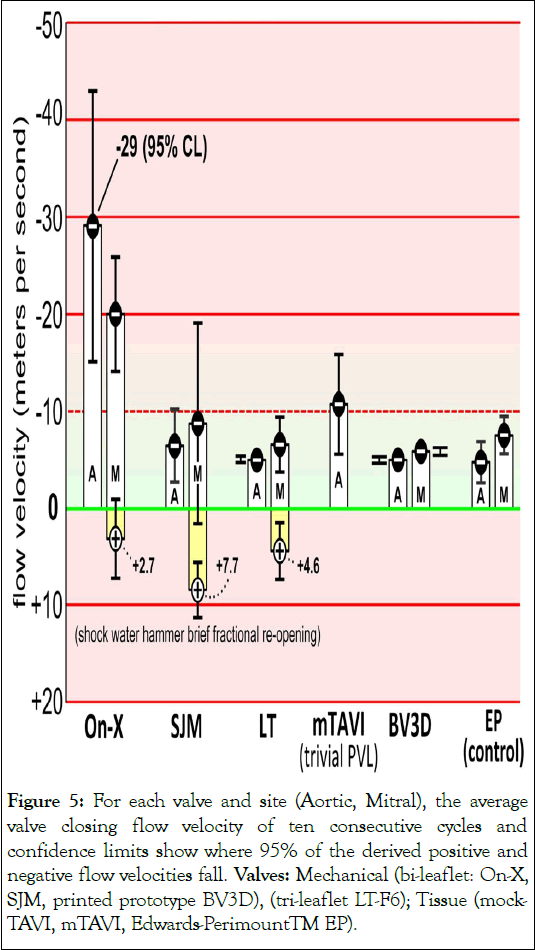
Figure 5: For each valve and site (Aortic, Mitral), the average valve closing flow velocity of ten consecutive cycles and confidence limits show where 95% of the derived positive and negative flow velocities fall. Valves: Mechanical (bi-leaflet: On-X, SJM, printed prototype BV3D), (tri-leaflet LT-F6); Tissue (mock- TAVI, mTAVI, Edwards-PerimountTM EP).
Signal synchronicity
As indicated in Figures 3 and 4, in the aortic and mitral valve sites, instantaneous minimum regurgitant volume flow rate and minimum POVA are synchronous with valve closure. The flow and pressure signals are processed through 100 Hz low-pass analogue filters whereas POVA is unfiltered to preserve maximum frequency response (~0-337 KHz).
Synchronized hydrodynamic oscillations emerge post valve closure due to the pooled reactive compliances of the test valve and holder. These damped oscillations are observed in the unadjusted volume flow rate signals (dashed grey) and are ascribed to the combined movement of test valve components (i.e., leaflets plus stent) within the elastic silicone rubber holder. Oscillations evident for all valves and sites have similar periodicity ~20 ms (50 Hz).
Statistical analysis
For each experiment, we acquired 10 consecutive cycles and report average velocity measurements and cycle-to-cycle variations of 10 negative peak flow velocities recorded using Confidence Limits (CL). Summary of the valve flow velocity test datasets utilize EXCEL**** (****: Microsoft, Redmond, WA, USA) data analysis tool labeled descriptive statistics with options Analysis ToolPak and Solver Add-in. In Figures 3-5, mean negative peak flow velocities and CL=95% indicated by CL bars adjacent to the flow velocity waveforms show the predicted uncertainty.
Experimental valve design
The BV3D rapid prototype valve design shown in Figures 2A and 2B show a working model that resulted from several designs trialed. Consideration of competing factors produced 56 laboratory constructed prototype valves with data from experiments producing empiric optimization through interaction of leaflet profile, pivot location and surface characteristics. These led to the rapid printed prototype model BV3D with advantageous forward and especially closing flow dynamics. In this work, Leonardo provided immediate feedback on the impact of even subtle geometric changes on valve dynamic behavior.
Aortic vs. mitral dynamics
When fluid in motion is abruptly halted or changes its direction (momentum change) water hammer is observed. This unwanted transient motion closely associates with valve closure timing, property of valve mountings and has been previously reported in vitro. Figures 3-5 illustrate snapshots of shock water-hammer dynamics with mean flow velocities reaching -29.0 m/s for the On-X mitral and -65.2 m/s for the On-X aortic valve and is absent for the Lapeyre-Triflo-F6, prototype BV3D, mock-TAVI, and Edwards-Perimount control valves. The mock-TAVI valve was untested in the mitral site.
For the On-X, SJM, and LT valves in the mitral site, a brief fractional reopening is evident in POVA post initial valve closure. In Figure 4, negative peak transvalve pressure spikes range over comparable levels in the mitral site (-75 to -90 mmHg). However, in the aortic position (Figure 3) pressure spikes encompass a wider range (-40 to -95 mmHg).
Signature shock water hammer dynamics for aortic and mitral valves are evident for POVA, transvalve pressures, volume flow rates, and derived valve flow velocity waveforms. We found that shock water hammer dynamics can be mitigated by valve designs optimized for soft valve closure which reduces retrograde valve flow velocity in the early closing phase as observed for the BV3D and EP valves.
Edited vs. unedited instantaneous volumetric flow rates
In Figures 3 and 4, measured and derived aortic and mitral valve test results include 10 consecutive cycles each experiment. Data acquisition sampling interval is ~3.36 ms (white data points). Evident are maximum negative flow velocities at valve closure ranging up to -65.2 m/s (On-X aortic). The oscillatory trait seen in the primary unadjusted (grey) flow rate waveforms was edited to produce the black volume flow rate waveform data prior to deriving the flow velocity data (red waveforms). The spatially averaged metric of mitral flow velocities (red curves) are obtained by dividing time-periodic volume flow rate by timeperiodic POVA. Average flow velocity and CL bars show where 95% of the derived negative peak valve flow velocities fall. An inherent advantage of Leonardo is that POVA is determined by a high resolution opto-electronic methodology with excellent spatial area resolution (details in supplementary information S7). Leonardo offers clear detection of initial and water hammer rebound events. Distinctive valve motion events such as: Initial POVA opening, POVA closure, shock water hammer rebound where POVA goes briefly positive just after valve initial closure are all discernible in the POVA waveforms. Volumetric flow rate, transvalve pressures, and derived flow velocity waveforms are acquired synchronously.
In Figure 4, the mock-TAVI valve produced aortic but not mitral site data. Leakage rates are indicated either Intra-Valvular (IVL) or Para-Valvular (PVL). Post valve closure, valve fractional reopenings, highlighted in yellow, are attributable to shock water hammer phenomena.
Readers will note that in prior publications, closing flow velocities in excess of those observed in this study were reported [13]. Previous records with excessive closing flow velocities have been rectified in this report by re-analysis of the historical experiment test datasets imported into revised EXCEL templates.
The relationship between POVA, flow velocity, and mass flow rate helps in understanding the fluid dynamics of valves. Factors such as fluid properties, valve design, and pressure differentials influence this relationship. For example, prototype valves with non-curved leaflet profiles and pivot locations near the orifice center show promise in optimizing valve designs for reduced thrombogenic potential. Partially closed valves evidenced by decreased POVA as well as an increase in flow velocity, aligns with the Continuity Principle. The continuity equation states that the mass flow rate of a fluid must remain constant along a continuous flow path and is expressed mathematically as Q=A1V1=A2V2 where Q is the fluid flow rate, A1 and A2 are the areas of the pipe or valve at two different locations, and V1 and V2 correspond to velocities of the fluid at those locations. Therefore, when the area available for flow is least (i.e., when the valve is approaching closure and almost closed), the velocity of the fluid is greatest. Transvalve pressure is amplified by increased flow velocity and, in accord with Bernoulli's principle, assists valve closure indicated by a reducing POVA.
Although high blood flow velocity alone may not cause blood damage directly, the presence of high flow velocity gradients which occur when flow velocity varies significantly over a short distance, can potentially trigger clotting. Such velocity gradients (shear) often arise in areas along the flow pathway where flow disturbances such as constrictions, or turbulence occur. Shear refers to the frictional force between adjacent fluid layers moving at different velocities. Steep velocity gradients contribute to increased shear potential. When shear force exceeds the physiological tolerance, cell fragmentation and activation of clotting processes can follow. While blood cells are capable of withstanding moderate shear levels due to their deformable membranes, certain implant devices like mechanical heart valves are deemed thrombogenic because shear forces expose cells beyond their physiologic limits. Consequently, individuals with such devices may require chronic anti-coagulation therapy to mitigate clotting risks.
Transvalve flow rate is determined primarily by valve open area and transvalve pressure. When valve open area is decreasing (partially closed), transvalve pressure and flow velocity increase. This relationship is governed by The Bernoulli’s principle states that as fluid velocity increases fluid pressure decreases. Therefore, when POVA is decreasing (valve not completely closed), fluid velocity increases, transvalve pressure increases and open area declines. Conversely, when the valve is fully open, and pooled open areas are maximal, fluid velocity declines in conjunction with transvalve pressure resulting in lower volumetric flow rate. High blood flow velocity may not be an independent trigger for blood damage, but generation of high flow velocity gradients can potentially lead to clotting activation. Velocity gradients come about when there is a significant change in flow velocity over a short distance and are often observed in areas having flow pathway disturbance, constriction or turbulence.
Shear is a frictional force exerted by adjacent fluid layers moving at different velocities. With steep velocity gradients, shear potential increases. When physiological tolerance for shear is exceeded, cell fragmentation and activation of clotting processes may result. While blood cells have deformable membranes that can tolerate moderate levels of shear, certain implant devices, such as mechanical heart valves, can be overtly thrombogenic when exposed to high shear force requiring chronic anticoagulation therapy for recipients.
Several notable points in Figures 3-5 are:
• The occurrence of positive flow velocities shortly after valve closure for the SJM, On-X and LT.
• The On-X, SJM, LT mechanical mitral valves show shock water-hammer patterns having momentary post closure fractional reopening.
• Shock water-hammer patterns are absent for the two bioprostheses (EP and mTAVI).
• The EP aortic and mitral control valves exhibit low closure flow velocity (<10 m/s).
• For the SJM valve, the differential between aortic and mitral maximum volumetric backflow rates consistently exceeded that observed in other tested valves ~ (-180 ml/s -150 ml/s).
• For current clinical mechanical mitral valves a disquieting abrupt closure and shock water hammer dynamics is observed and shown highlighted in yellow. In this region, a brief partial valve re-opening is triggered ~6 ms after valve closure with rebound duration of ~4-10 ms.
• Mitral valve closure generates oscillations in flow rate and transvalve pressure greater in amplitude but less dampened than valves in the aortic site.
• Mitral valve occluder rebound (highlighted yellow) associates with high negative regurgitant volume flow rate, transvalve pressure peaks and flow velocities which impacts on valve closing with certainty.
• Negative transvalve pressure spikes are near synchronous with valve closure.
• Transvalve pressure phase leads the flow.
• Important features underscored in the valve closure phase are: Near synchronous timing of valve closure ( valve minimum POVA), negative going transvalve pressures at closure, maximum retrograde closure volume flow rate, and maximum retrograde flow velocities.
• The velocity of unsteady pressures in water is ~1,500 m/s. Therefore, pressures in immediate proximity to the aortic test valve would, for example, have trivial phase (~0.07 ms) relative to the aortic wall pressure site which is ~10 cm distant from the mid-plane of the valve (Figure 6).
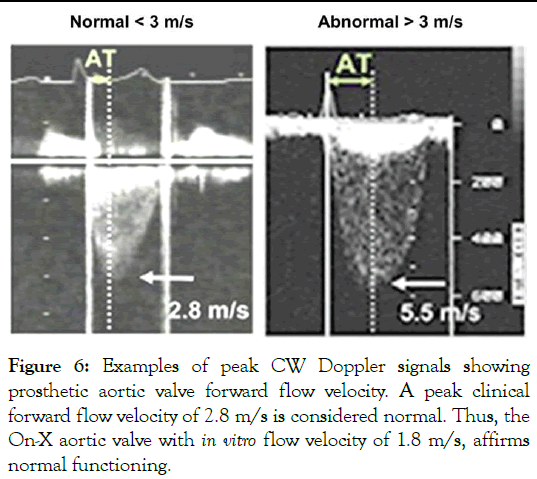
Figure 6: Examples of peak CW Doppler signals showing prosthetic aortic valve forward flow velocity. A peak clinical forward flow velocity of 2.8 m/s is considered normal. Thus, the On-X aortic valve with in vitro flow velocity of 1.8 m/s, affirms normal functioning.
Results in Figures 3-5 are based on predicted valve flow velocities. Notable near post valve closure are positive flow velocities for SJM, On-X and LT (Figure S2). An interesting mitral site pattern shows shock water-hammer phenomena associated with three of the four mechanical mitral valves tested (On-X, SJM, LT), where kinematic evidence shows momentary post closure fractional reopening and absence in the two bioprostheses (mTAVI and EP). The EP aortic and mitral control valves have low closure flow velocity (<10 m/s).
Important elements in Figures 3-5 are:
• The EP aortic and mitral control valve demonstrated the lowest predicted valve closing flow velocity which is consistent with their reported clinical experience of low thrombogenic potential.
• Driven by shock water hammer dynamics, backflow velocity is higher in the mitral than aortic site.
• Slow-motion and real time visualization of aortic and mitral valves qualitatively revealed both symmetrical and nonsymmetrical closing behavior of occluders. Quantitatively, flow velocity Confidence Levels (CL) may be a useful measure of occluder motion evenness over 10 consecutives cycles. Results show that the On-X and SJM have greatest CL values.
• The mTAVI valve sample with trivial Paravalvular Leak (PVL) had a peak average negative valve closing flow velocity of -10.7 m/s (± 5.25). However, it has been noted that valve samples with PVL=zero and counter intuitively, with >PVLs, have reduced valve closing flow velocities.
• Based on the control valve results, mean closure flow velocities <-10 m/s suggests potential for low/non-thrombogenic shear force.
• Valve closing dynamics are associated with shock water hammer are also associated with maximums in flow velocity, backflow rate and transvalve pressure.
• During valve closure, platelets subjected to jet flow velocity gradients are likely to accumulate more shear force damage relative to that from the forward flow phase [14].
Over the past 60 years, heart valve design and performance evolved to provide improved durability and hemodynamic function. While preferences for mechanical vs. bioprosthetic valves fluctuated widely, the advent of transcatheter delivered devices and their less invasive insertion methodology, the balance shifted progressively in favor of bioprostheses. Although initially restricted to use in the elderly or patients designated too fragile for conventional surgical implantation, use is now extended to younger and lower risk candidates.
Younger patient cohorts receiving bioprosthetic heart valves have longer life expectancies (>20 years) and thus a higher likelihood of re-intervention. The shift in favor of BHVs may prove to be premature as more (or lack of) long-term data is in hand. A recent surgical AVR study by in patients aged 50 to 69 years, found long-term survival was better in those who received mechanical compared to Perimount bioprosthetic valves and suggested a substantial survival advantage be recognized in patients with mechanical valves [15]. To respond to an incidence of technical failure in contemporary transcatheter valves and perhaps in anticipation of increasing frequency of degenerated valves in younger patients, a variety of transcatheter “rescue” devices are offered. Inserted within a degenerated or malfunctioning primary valve or a previously implanted rescue device, the predicted durability of rescue devices is speculative. In a prophetic article Russian Dolls; when valve-in-valve implantation is not enough, Tseng called attention to the possibility of rescue valve recipients who will represent persistent therapeutic challenges that may not be resolvable by transcatheter devices [16]. As a response to a possible increase in this initially small subset of patients, for those valve replacement candidates who favor the alternative of one valve durable for life but are hesitant because of concerns over anticoagulant related issues and the as-yet unfulfilled need by rheumatic patients in developing countries it is essential that research driven by purposeful curiosity continues toward achievement of a long awaited anticoagulant independent mechanical valve by research endeavors such as recently reported by [17].
At or near valve closure, flow dynamics can be considered analogous to transitory valve stenosis, whereby regurgitation is increasingly constrained until complete, motionless, closed-valve conditions arise. Thus, during brief crucial moments preceding and after valve closure, localized prothrombotic microenvironments may be relevant to generation of jet flow velocities with shear sufficient to induce blood element damage. These influences may impact multiple valve types with potential consequences that may include but not limited to:
• Reduced valve leaflet mobility, sub-clinical thrombosis.
• Potential for pannus formation.
• Cavitation and clinically detectable High Intensity Transcranial Signals (HITS).
• Transient Ischemic Attack (TIA), embolic acute/sub-acute stroke, other silent micro-infarction and adverse cerebrovascular events [18].
Shock water hammer phenomena and occluder rebound
Mitral valve closure dynamics shown in Figure 4 include a partial post closure transient opening (rebound) attributable to shock water-hammer, were found less prominent in the aortic site (Figure 3). Occluder rebound driven by water-hammer power (product of transvalve pressure and volumetric flow rate) for the SJM mitral valve is observed as a momentary post closure partial re-opening. This has been previously reported with high resolution magnified examples of POVA rebound data. Relative to the bioprosthetic control valve, mechanical mitral valves (On- X, SJM, LT) are driven by higher-level transvalve pressures and retrograde flows and reveal rebound behavior prior to final closure. Additional pro-thrombotic aspects may be related to sub-optimal valve forward flow energy losses (e.g. arterial sclerosis) and biomechanical and biochemical responses sufficient to exacerbate risk of pathologic thrombus formation and propagation.
Research outcomes reported:
• High amplitude valve closing flow velocities may result in supra-physiologic shear forces originating in small intra and para-valvular leak gaps and which become mechanistic initiators of the clotting cascade.
• Platelet activation is induced by high shear even with short exposure time [19].
• Closing dynamics disparity between mechanical (MHV) and bioprosthetic valves is chronically overlooked as a primary indicator of valve thrombogenicity.
• Current MHVs have overt valve closing flow velocity transients relating to occluder non-response to flow deceleration and residual valve POVA.
• For a given volumetric backflow rate near valve closure, the smaller the total residual leakage area, the greater the valve closing flow velocity.
• The highest valve closing flow velocity was for the On-X and the SJM valves compared to the tissue control valve (Edwards pericardial).
• The prototype BV3D valve had lowest predicted valve closing flow velocities in both aortic and mitral sites. Assessment of this dynamic behavior represents a practical means to qualitatively screen valves and controls for thrombogenic potential.
• Valve BV3D test data suggest that specific MHV leaflet geometries generate a closing force during forward flow deceleration and prior to flow reversal, a potentially beneficial “soft closure” response.
• Our novel laboratory methodology permits inference of shear damage to formed blood elements and potential for continuing thrombogenic response. This constitutes a mechanistic explanation for observed thrombogenic disparity in prosthetic valve types and designs, now broadened to include observations of TAVR related thromboembolic events.
• For MHVs, cyclic valve closing flow velocities appear to be related to a prothrombotic state requiring chronic anticoagulation.
• Bioprosthetic valves appear not to generate a pro-thrombotic closing phase.
• In both experimental and computational pulse duplicator studies, bioprosthetic valves in smaller diameters and/or with thicker leaflets generate higher flutter frequencies.
Strengths
A derived valve closing flow velocity provides a useful laboratory metric directly proportional to blood cell damage from shear force. For some mechanical mitral valves, a brief positive flow transient is noted. Such transients result from shock waterhammer dynamics and are indicative of high shear force, blood cell damage, biomechanical and biochemical responses that promote pathologic thrombus formation and propagation.
Limitations
Mechanical valves tested in the mitral position consistently manifested leaflet rebound observed as a transitory post closure partial re-opening driven by water-hammer power (product of transvalve pressure and volumetric flow rate). This has been previously reported with magnified examples of high resolution POVA rebound data.
In order to validate computational simulations, high fidelity amplitude and frequency responses are required to resolve small scale valve geometries and hydrodynamic features. This prerequisite may benefit from recently developed computational approaches and extensions needed to help fill lingering computational gaps [20].
This work exposes the central relationship between thrombogenicity and predicted high velocity flows and shear forces at valve closure. As practical application of our findings, specific valve geometric features were identified that led to prototype designs with potential for further development. The application of unique technology, rapid prototyping and ranking of flow velocity patterns near valve closure optimized experimental valve geometry for reduced thrombus potential compared with control valves.
We compared the hydrodynamics and kinematics of prosthetic heart valves at valve closure to control valves. The in vitro results suggest that the potential for blood clots caused by high velocity flows and shear forces can be reduced by focusing on specific valve geometric features, leading to the development of improved clinical devices. The study however raises the question of whether optimum mechanical valve performance requires similar, identical or lower valve closing flow velocities compared to contemporary clinical bioprostheses. The work opens an experimentally assisted pathway for developing new, durable and less thrombogenic devices and the prototype model BV3D serves as evidence that focused laboratory efforts can yield promising results. Additionally, the study indicates that valve flow velocity differentials and associated shear are significant activators of thrombogenicity for the On-X valve, emphasizing the importance of valve closure behavior in reducing thrombus potential and improving clinical devices, despite the challenges in introducing new prosthetic heart valves to the market. The Holy Grail goal of a mechanical valve independent of chronic anticoagulation still beckons.[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Scotten LN, Siegel R, Blundon DJ, Deutsch MA, Martin TRP, Dutton JW, et al. (2025) Canary in the Cardiac-Valve Coal Mine: Flow Velocity and Inferred Shear during Prosthetic Valve Closure–Predictors of Blood Damage and Clotting. J Hematol Thrombo Dis. 13:646.
Received: 16-Aug-2023, Manuscript No. JHTD-23-26131; Editor assigned: 18-Aug-2023, Pre QC No. JHTD-23-26131 (PQ); Reviewed: 01-Sep-2023, QC No. JHTD-23-26131; Revised: 11-Jan-2025, Manuscript No. JHTD-23-26131 (R); Published: 18-Jan-2025 , DOI: 10.35248/2329-8790.25.13.646
Copyright: © 2025 Scotten LN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.