Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Review Article - (2021)Volume 10, Issue 7
Caesalpinia bonduc L. is a pivotal medicinal plant that belongs to the family Caesalpiniaceae. The plant is widely distributed found throughout India especially in the coastal areas. It is extensively used in traditional medical systems such as Ayurveda, Siddha, Homoeopathy, and Unani. It is regarded as a valuable remedy for the treatment of numerous ailments in Indian traditional plant medicine. It is a unique medicinal plant used in conventional medicine because all plant parts have therapeutic properties. The plant has been reported to hold several pharmacological and medicinal properties such as anticancer, hepatoprotective, antioxidant, antimalarial, antimicrobial, antipyretic, antifertility, anti-inflammatory and antimalarial etc. C. bonduc seeds contain cassane diterpenoids like caesalpinins and caesalmins, as well as norcassane diterpenoids such norcaesalpinins. Cassane diterpenoids (e.g. taepeenins A-L) and norcassane diterpenoids (e.g. nortaepeenins A & B) have been isolated from the stems, roots, and seeds. Leaves include phenolic acids such as caffeic acid, chlorogenic acid, p-coumaric acid, ferulic acid, and gallic acid. This species has been found to contain numerous biologically and pharmacologically relevant bioactive secondary metabolites with unique structures and diverse mechanisms of action, which may entice pharmaceutical companies to produce novel therapeutic formulations based on herbal methods. With more research, it is possible to infer that C. bonduc could become the most acceptable source of medicine in the future for an array of illnesses. As a result, this review aims to provide a full overview of ethnopharmacology, phytochemistry, and pharmacology of C. bonduc.
Caesalpinia bonduc; Pharmacology; Phytochemistry; Seed
As a promising source of therapeutic aids, medicinal plants have played an increasingly important part in global health systems for both humans and animals, not only in disease conditions but also as a potential material for sustaining good health. First, however, it is necessary to understand which components of the medicinal herb are responsible for the therapeutic effects [1]. In recent years, there has been a greater emphasis on plant research worldwide, and a vast amount of scientific evidence has accumulated to demonstrate the enormous potential of medicinal plants employed in diverse traditional systems [2]. Today, there is a reinvigorated interest in traditional medicine and an increasing demand for more novel drugs from diverse plant sources. This revival of interest in plant-derived drugs is mainly due to the current widespread belief that “green medicine” is safe and more dependable than the costly synthetic drugs, many of which have reported severe adverse side effects. Herbal drugs or medicinal plants, their crude extracts and their isolated pure compounds have displayed a variety of interesting biological activities. Therefore, medicinal plants have been continuously used, especially as medicine in folklore systems or food supplements for managing countless disorders [3].
Plant pharmacological and therapeutic aspects are explored for a variety of reasons: (1) to learn more about native plant diversity's with potent medicinal potential; (2) to develop a rational basis for the therapeutic use of specific plant species; (3) to generate low-cost herbal medicines that have a pertinent biological activity to manage various disorders; (4) to explore novel drug prototypes; and (5) to understand more about our traditional medicines. Additionally, medicinal plants may be sources of bioactive compounds with unique structures and modes of action. These novel traits have inspired the pharmaceutical industry to emphasize research on the development of herbal medications [4].
For thousands of years, the Caesalpinia species has played a very major role in maintaining human health and improving the quality of human life by serving as useful components of medicine, seasonings, beverages, cosmetics, and dyes. Caesalpinia species have long been used in various traditional medicines of India as well as the rest of the world in managing varied symptoms and ailments. All part of this species (root, seed, bark, leaves, and flowers) has been utilised for various medicinal purposes, and the root, stem, leaves, bark, seeds, and nuts have all been used in some way. Moreover, these species are reported for several interesting biologically and pharmacologically important bioactive secondary metabolites that possess a novel structure with a diverse mechanism of action, attracting the pharmaceutical industries to develop novel drug formulation based on the herbal route.
Caesalpinia species
Caesalpiniaceae family mostly contains more than 152 genera and 2800 species predominantly dispersed in tropics and sub-tropics regions. Trees, shrubs, and woody climbers make up the majority of this family, but herbs are uncommon. Caesalpinia L. is one of the most significant genus of family Caesalpiniaceae [5]. Several species of this genus are essential in the fields of medicine, pharmaceuticals, horticulture, and economics. Moreover, several Caesalpiniaceae species have been employed in ethnomedicine in different parts of the world [6]. Earlier research found that this genus' species have a wide variety of pharmacological properties, including anticancer, anti-inflammatory, antipyretic, antimicrobial, antimalarial, antirheumatic, antiulcer, antidiabetic properties etc. The chemical investigation of this species revealed the presence of diverse chemical compounds such as triterpenoids, diterpenes, flavonoids, steroids and phenolic compounds. The diterpenes derivatives isolated from this genus are a group of extremely diverse and structurally different compounds and they exhibited a variety of pharmacological activities [7]. Following are some of the major species of Caesalpinia which are commonly used in traditional medicinal preparations. 1. C. bonduc (Linn.) Roxb., 2. C. benthamiana (Baill.) Herend. & Zarucchi. 3. C. cacalaco Humb. & Bonpl. 4. C. coriaria (Jacq.) Willd. 5. C. decapetala (Roth) Alston. 6. C. digyna Rottl etc. [8].
C. bonduc is an Indian herb reported in Ayurveda, the ancient medicine system of India. This species was reported to contain a diverse of biologically and pharmacologically properties along with various bioactive secondary metabolites with unique structures and diverse mechanisms of action. So this review highlights the phytochemistry and pharmacology of C. bonduc with special references to its seed.
Caesalpinia bonduc/Caesalpinia bonducella/ Caesalpinia crista
C. bonduc (L.) Fleming [Syn. C. bonducella (L.) Roxb, Syn. Caesalpinia crista Linn.] (Figure 1), which belongs to the family Fabaceae/Caesalpiniaceae, is a prickly shrub widely dispersed worldwide. It is expensively found in tropical and subtropical regions globally. It is richly found in India, Sri Lanka and Andaman and Nicobar Islands [9,10]. It is one the common scrub in the forest of the Eastern Ghats, India. As said above, the root, stem, leaves, bark, seeds and nuts are used for medicinal purposes [11,12]. This plant is commonly known as Ivy Gourd and is an important plant used in indigenous systems of medicine in managing various disorders. It is frequently used as antioxidant, antidiabetic, laxative, immune system modulator, and rheumatoid arthritis treatment. Two varieties of C. bonduc are found - (1) the white variety and (2) the black variety [13]. The name of the bonduc species is derived from the Arabic word “bonduce” which means little ball due to the globular shape of the seed [14,15]. The seeds of C. bonduc are coloured grey and resembles the eyeballs. Due to this, in Sanskrit it is known as Kuberakshi, which means eyes of Kubera, the Hindu God of Wealth [16,17].

Figure 1.C. bonduc (1) Plant; (2) Flower; (3) Fresh seed; (4) dry seed contain pod and seed; (5) Seed kernels.
The seed coat of C. bonduc is glossy, very hard, and greenish to ash grey and is traversed by circular and vertical faint markings of the cracks, forming uniform rectangular to squarish rectulations all over the surface. This particular plant was very much confused with Caesalpinia bonducella and was described under the same [18-24]. Besides this species like C. nuga [25,26] and C. jayoba are also sometimes mistakenly designated as synonyms for C. crista. Seeds were normally 1-2 oblong and is about approximately 1.3 cm long. A raised hilum with remains of the stalk lies in the centre of the dark spot, at the narrow edge of the seed. Adjacent to the hilum, lays a faint coloured circular to oval elevated micropyle. In the case of dry seed, kernel is usually often detached from the testa. Testa is about 1-1.25 mm in thickness and is composed of three separate layers. The outermost layer is thin and brittle, whereas the middle one is broad, fibrous and dark brown and the innermost layer is white and papery. The kernel surface is furrowed and ridged, hard, pale yellowish-white, circular to oval, flattened and about 1.23-1.75 cm in diameter. Ascar of the micropyle lies at one end of the kernel, from where arises a prominent ridge demarking the two cotyledons of the embryo. Plumule - radical axis is thick, cylindrical and straight. The taste of the seed is very bitter with a nauseating and unpleasant odour [27]. All parts of the plant have diversified medicinal properties, so it is a valuable medicinal plant utilized in traditional medicine.
Various names of C. bonduc
The diverse vernacular names [28-30] of C. bonduc are: Arab: Bunduk, Akitamaket; Bengali: Nata, Natakaranja; Tribal name: Kang Boi (Marma); Cannarese: Gajikekayi; Duke: Guchha; English: Fever Nut, Physic Nut, Molucca Bean, bonducella Nut, Nickar bean; French: bonducjaune, Guilandinabonduc; Hindi: Katkaranj, Katkaliji; Kannada : Gajjaga; Malayalam: Kalanji, Kazhanchi; Oriya: Kotakoleja, Glogila; Persian: Khayerhe-i-iblis; Sanskrit: Latakaranja, Putikaranja, Kuberakshi; Tamil: Kalarsikkodi, Kazha-shikkai, Gajega, Mulal, Kazharchi; and Telugu: Gache, Gatchkaya, Kalanju, Yalakhi [31].
Taxonomic classification
The taxonomical classification of C. bonduc is given below:
Kingdom : Plantae 28
Phylum : Magnoliophyta
Division : Magnoliopsida
Class : Angiospermae
Order : Fabales
Family : Fabaceae/Caesalpiniaceae
Genus : Caesalpinia
Species : bonduc
The following botanical characters have been described for C. bonduc [32].
Foliage : Evergreen
Roots : Deep roots, taproots
Type of stem : Hard and woody
Leaf type : Bipinnately compound, elliptical, ovate shaped
Leaf arrangement : Alternate
Leaf colour : Green
Leaf surface : Glossy
Seed type : Dicot
Odour : Characteristic
Taste : Bitter
Macroscopic characteristics
The C. bonduc has dark grey branches and yellow prickles that are very firm and straight.
Leaves: The leaves of the C. bonduc are large (30 to 60 cm long) and often very leafy and branching. The petioles on the dorsal side of the leaf are thorny. The leaf base has six to eight sets of pinnae with a few stipulary spines and has decreased pinnae with elongated mucronate point [33].
Flowers: The C. bonduc has dense blooms with axillary racemes that are narrow at the base and thick at the top. This plant's flowers range in length from 15 to 25 cm. Buds have short pedicles, flowers have nearly 5 mm pedicles, and fruits have around 8 mm pedicles [34].
Seeds: Because of the close squeezing of adjacent seeds, the seeds are hard-coated, greenish or grey, and slightly compressed on one side. The seeds are round, black, and have vertical fissures running through them. The testa, which is around 1 to 1.25 mm thick and consists of three layers, is separated from the dry seed kernels [35]. It depicts the hilum and micropyle as being close to one another. A dim zone usually surrounds hilum with a yellowish remnant to the funicle. Micropyle is near the edges of a dim district. It has a dim greenish to greyish seed coat that is fairly dim pale blue in colour [36].
Microscopic characteristics of seed
The seeds of C. bonduc showed numerous layers of vertical, columnar luminal cells when examined under a microscope. Columnar palisade cells are powdery, whereas parenchyma cells are black in colour, with strong bone-shaped walls and granules of starch loaded inside [37].
The pharmaceutically useful part of C. bonduc
Root, stem, leaves, bark, seeds and nuts are used for medicinal purposes.
Habit and habitat
C. bonduc can be found throughout Asia's tropical and subtropical climates. It is disseminated in India, Srilanka, Bangladesh, Burma, Myanmar, China and Vietnam. It's also found in the Andaman and Nicobar Islands, as well as in India's tropical regions. This plant is a viny perennial shrub that thrives in both the shade and the open. It is wild distributed throughout the plains, woodlands, hills, wastelands, and coastal locations, and up to 1,000 meters in the Himalayas. It can also be found on plain land as well as swampy land. The plant is a thorny shrub or woody vine that grows to a height of 10 meters. It is found primarily along the seacoast in India's hotter regions [38].
Growth & cultivation
At all stages of development, C. bonduc grows quickly. In 40 days after seeding, the seedlings will grow up to a height of approximately 26 cm. Plants that are older grow a metre or more per year. The coastline is the typical habitat for this species. C. bonduc prefers full sun and is shade-intolerant, but it can tolerate moderate shade. It grows in a wide range of soil pH, from mildly acidic to alkaline, and tolerates salt spray, saline soils, and occasional flooding with seawater. C. bonduc grows inland in disturbed regions and is usually found in beach vegetation, on coastal dunes, and at better-drained borders of mangrove forest. It thrives in grassy and herbaceous environments and scrambles onto the tops of low trees and bushes.
Seeds are used to propagate C. bonduc. These are planted at the beginning of the rainy season; they are first soaked overnight before being sown at 50 cm intervals to form a hedge. Irrigation is required as soon as the seeds are planted. The sandy loam soil provides the best conditions for growth. The plants sprout after 3-4 weeks and reach their maximum height in 2 to 2½ years. Therefore, irrigation is critical in the early stages, and plants should be pruned every two weeks.
Propagation
The plant grows in the wild and is spread by seeds. Acid scarification, light, and temperature treatments with concentrated sulphuric acid for 30-90 minutes can be used to break the seed's dormancy. These acid-treated seeds germinate 100% when exposed to blue light for 72 hours at 30°C.
Phytochemicals of C. bonduc
The preliminary phytochemical screening of the ethanolic and aqueous extracts of C. bonduc recorded a significant amount of flavonoids, tannins, proteins, alkaloids, carbohydrates reducing sugars, phytosterols, saponins, coumarins and triterpenoids [39,40]. However, Gurunath et al. reported that the seeds of the plant contained bonducin, proteins, saponin, starch, sucrose, enzymes, two phytosterols namely sitosterol and hepatsane, fatty acids such as palmitic acid, stearic acid, lognoceric, oleic, linolenic acid, as well as furanoditerpenes [41].
Many furano-cassane-diterpenes were isolated from the seeds of cassane diterpenes (another synonym: caesalpins) included (caesalpinin C-P), nor-cassane diterpenes (nor-caesalpine A-F) and neo-cassane diterpenes (neocaesalpins H and I), which characterized by α, and β-butenolide hemiacetal ring that is rare in nature, while they lack 5-hydorxy group which distinguishes them from cassane diterpenes (caesalpins). New Cassane-type diterpenes (taepeenin A-I) were also isolated from the plant [42]. Triterpenoids included lupeol acetate, ß-amyrin and α-amyrin were isolated from the cold macerated methanolic seed extract [43].
The phytochemical research on the methanolic extract of C. bonduc afforded two novel compounds, 2-hydroxytrideca-3,6-dienyl-pentanoate and octacosa-12,15-diene along with known compounds 3-O-methylellagic acid 3′O-α-rhamnopyranoside, β-sitosterol and sucrose [44].
Seed kernels of C. bonduc contained significant amount of protein which varies from 7.4 to 25.3%. They contained the following amino acids : aspartic acid 9.5%, lysine 7.9%, glycine 6.9%, leucine 6.3%, histidine 5.1%, isoleucine 5.1%, serine 3.8%, r-aminobutyric acid 3.7%, tyrosine 3.7%, citrulline 3.6%, glutamic acid 3.6%, threonine 3.6%, arginine 3.4%, proline 3.3%, L-alanine 2.5%, methionine 2.1%, phenyl alanine 1.4%, cystine 1.2%, valine 1.2% and tryptophan 0.8%. The non-protein amino acids usually found in the seed were r-ethylidene glutamic acid, r-methylene glutamic acid, r-ethyl glutamic acid and traces of r-OHr- methyl gltamic acid and B-OH r-methyl glutamic acid, accumulation of r-methyl glutamic acid being extremely large [45].
The seeds also contained 49% carbohydrates including pentoan (16.8%), starch (6.1%) 54 and water soluble mucilage (4.4%). 4-o-methyl myoinositol hydrate was isolated from C. bonduc grown in China [45,46]. The leaves mainly contain pinitol (4.1%), glucose and minerals like calcium (2%) and phosphorous (0.3%). bonducin, waxy material and an amorphous bitter principle (C2OH32O8, mp 119.12°C, yield 0.35%) have been isolated from the leaves. The waxy material yields mysteric acid and an alcohol [47,48].
Stem and root contained peltogynoids, pulcherrimin, 6-methox pulcherrimin, homoisoflavonoid, 8-methoxybonducellin, and the known compounds bonducellin, 2, 6- dimethoxybenzoquinone, 2', 4', 4-trihydroxychalcone and 2', 4-dihydroxy-4'-methoxy-chalcone [45]. C. bonduc methanolic extract (100 mg) yielded 50.23 ± 0.003 mg/mL gallic acid equivalent phenolic content and 106.83±0.0003 mg/ml quercetin equivalent flavonoid content [49].
However, Jana et al. found that the total phenols were (24.66 mg gallic acid equivalent/g dried extract) and flavonoids (136.65 mg quercetin equivalent/g dried extract) [49,50]. The chemical structure of major compounds isolated from the seed is shown in Figure 2.
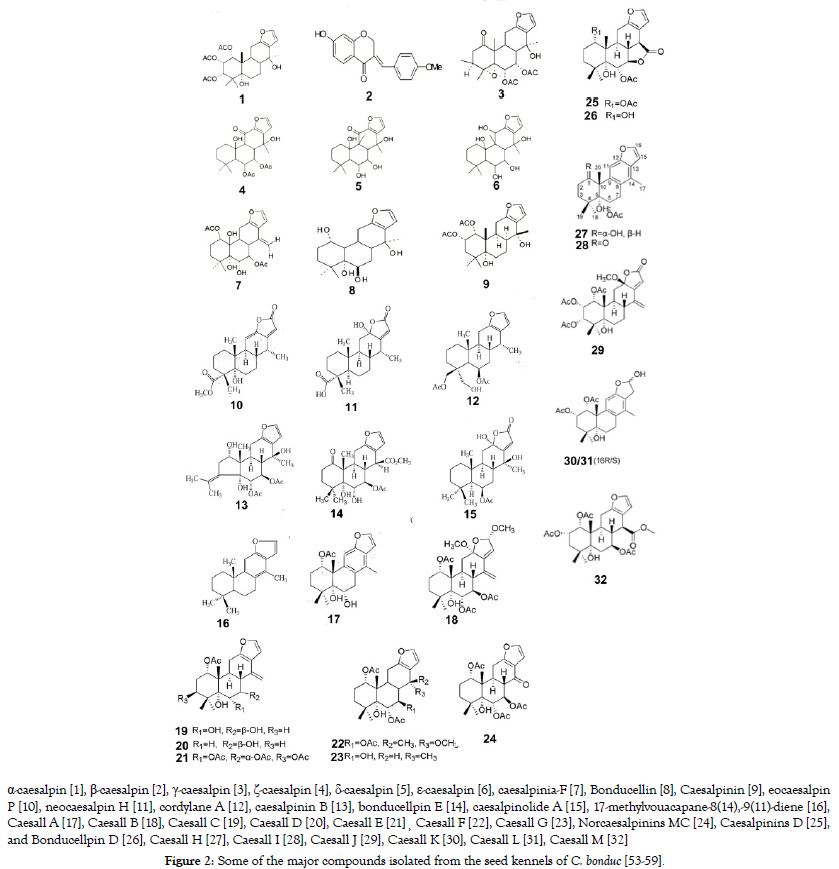
Figure 2. Some of the major compounds isolated from the seed kennels of C. bonduc [53-59].
Folk-medicinal uses of C. bonduc
C. bonduc juice is administered for two weeks after food in folk medicine to treat intermittent fever. Hydrocele is treated with ointment prepared from seed kernels. They are used to treat haemorrhages as an infusion. As an anthelmintic drug, karanjwa is combined with honey or castor oil. Internal administration of ground and roasted seeds is the most common method [51-57]. Simple, continuous, and intermittent fevers, asthma, and colic can all be managed with the help of seeds and root bark. Seeds are antiperiodic and febrifuge; they are also used for managing swelling, restraining haemorrhage, and infectious diseases [58,59]. Tender leaves are extremely effective in treating liver problems. Convulsions and nerve problems are highly benefited from the oil extracted from them. Doses administered: 1 to 2 g seed powder, 1 to 2 g root powder, 12 to 20 mL leaf infusion. The young leaves are used to treat infections and gargle for sore throats, elephantiasis, smallpox, liver diseases, and expel intestinal worms [58,59]. The seeds are astringent and have traditionally been used to cure infectious disorders, colic, hydrocele, skin ailments, and leprosy. Tumors have been treated with seed sprouts. Fever, intestinal worms, tumours, amenorrhoea, cough, and the removal of the placenta after childbirth are all treated with root bark [59-61]. An analysis of the literature on ethnobotanical data on C. bonduc revealed that the majority of workers reported using C. bonduc (Karanjwa) seed, followed by leaf and root (Figure 3), usually in crude powder form (53%), and followed by paste (33%). Nearly 7%, on the other hand, utilised Karanjwa as a decoction and infusion (Figure 3). Antipyretic was the most common ethnomedicinal usage mentioned by the workers, followed by hydrocele and diabetic treatment. Other disease conditions mentioned in the reports include pneumonia, piles, asthma, skin ailments, cough, and arthritis [62].
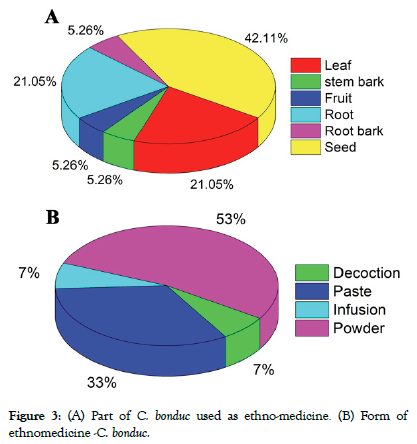
Figure 3.(A) Part of C. bonduc used as ethno-medicine. (B) Form of ethnomedicine -C. bonduc.
Pharmacological as well as medicinal properties of C. bonduc as evidenced by experiment
C. bonduc recorded several pharmacological properties (Figure 4). This section deals with the detailed pharmacological properties of C. bonduc.
Figure 4. The major pharmacological activity of Caesalpinia bonduc.
Antitumor activity
Gupta et al. has reported the antitumor activity of C. bonduc against Ehrlich Ascites Carcinoma in Swiss Albino Mice. The anticancer effect of a methanol extracts of C. bonduc leaves (MECB) against Ehrlich ascites carcinoma (EAC)-bearing Swiss albino mice were investigated in this work. The mice were given the methanolic extract at doses of 50, 100, and 200 mg/kg body weight/day for 14 days after the tumour was inoculated for 24 hours. The mice were then sacrificed and their haematological and biochemical profiles, including lipid peroxidation, glutathione content, superoxide dismutase, and catalase activities, were evaluated. Their findings showed that methanolic extract reduced tumour volume, packed cell volume, and viable cell count, as well as extending the lives of EAC-tumor-bearing mice. In methanol extract-treated mice, the haematological and serum biochemistry profiles were normal. Furthermore, when delivered daily (i.p.) for 14 days at the test dosages, the extract was found to have no obvious short-term toxicity in mice. Only at 300 mg/kg, the extract-treated animals demonstrate considerable toxic effects. These findings revealed that a methanolic extract of C. bonduc exhibited anticancer activity in EAC-bearing mice [63]. Deepika et al. investigated the anticancer potential of ethanolic extract and its various fractions of C. bonduc seeds against Ehrlich Ascites Carcinoma (EAC) cell lines and observed considerable anticancer activity in EAC cell lines [64].
Sandhia and Bindu investigated the cytotoxicity and anti-inflammatory activities of C. bonduc using the Trypan blue dye exclusion method in Daltons Ascites Lymphoma (DLA) cells and carrageenan-induced rat paw oedema at doses of 200, 100, 50, 20, and 10 g/mL. According to estimation studies using the Folin Cio-calteau method and the Aluminum chloride colorimetric method, the stem bark contains a lot of phenolic and flavonoid chemicals. Anti-inflammatory studies in-vitro and in-vivo reveal that extract has a stronger anti-inflammatory activity that increases in a dose-dependent way. The extract exhibits 100% cytotoxicity even at 100 µg/mL concentrations. The extract is completely cytotoxic even at doses of 100 g/mL. According to this study [65], the presence of significant levels of phenolics and flavonoids in the stem bark may be responsible for its anti-inflammatory and anticancer properties.
In-vivo anti-proliferative and pro-apoptotic properties of methanol extract of C. bonduc (MECB) on Ehrlich ascites tumour model were reported by Shivaprakash et al. in 2016. In their studies, methanol extract at 200 mg/kg concentration reduced the overall percentage of viable EAT cells (51.6%) and ascites volume (65%) in treated mice. Furthermore, the extract enhanced apoptosis, as evidenced by Giemsa and Acridine orange/Ethidium bromide (AO/EB) staining. More cells exhibited typical characteristics such as membrane blebbing, apoptotic body formation, and DNA fragmentation. Furthermore, the mice's survival time increased as a result of the treatment. Additionally, their FACS data suggested that the death of extract-treated EAT cells was caused by apoptosis rather than necrosis. Furthermore, the molecular mechanism investigation demonstrated that the extract decreased anti-apoptotic Bcl-2 expression while enhancing pro-apoptotic Bax expression. The immunoblot analysis confirmed the activation of PARP (Poly (ADP-ribose) polymerase), a substrate for executioner caspase-3, which causes DNA fragmentation throughout the apoptotic process. These findings support MECB's anti-proliferative and pro-apoptotic activities, which could lead to the development of therapeutic drugs to combat cancer in the future [66].
Pournaghia et al. investigated the cytotoxicity of a methanol extract of C. bonduc aerial parts (leaves and stems), seeds, and legumes against MCF-7 and PC-3 using the MTT test in 2021. The methanol extract exhibited higher inhibitory activity (IC50: < 500 µg/mL) in this case. As a result, they chose this extract for fractionation. Based on the substantial inhibitory activity (IC50: 170 ± 0.9 µg/mL), they selected the ethyl acetate (EtOAc) fraction for phytochemical analysis from the fractions. Finally, these researchers isolated three compounds from EtOAc fraction: (1) Quercetin-3-methyl ether, (2) Kaempferol, and (3) Kaempferol-3-O-L-rhamnopyranosyl12)—D-xylopyranoside. All isolated compounds were screened for cytotoxicity, and compound 1 showed better inhibitory activity than the other two. According to this study, C. bonduc could be investigated further as a natural source of biological chemicals having cytotoxicity activity [67].
Antioxidant activity
Antioxidants are compounds that can intercept or slow down cellular damage due to the generation of free radicals, which are unstable molecules produced by the body in response to environmental and other stressors. They are sometimes referred to as "free-radical scavengers." Antioxidant sources can be both natural and man-made. Kumar et al. discovered significant antioxidant activity in C. bonduc seed chloroform extract. In their work, they used DPPH free radical scavenging activity, total phenolic content estimation, and -carotene bleaching test to screen chloroform extract of C. bonduc seeds for antioxidant activity. According to the findings of their study, the IC50 value of chloroform extract was 170 ± 4.08 µg/mL. Total phenolic content was reported to be 21.96 ± 2.12 (for 1000 µg/mL), and total antioxidant activity was 24.96 ± 0.31, while standard BHA was 46.70 ± 0.43. Thus, the presence of antioxidant potential in a chloroform extract of C. bonduc seeds was revealed in this work [68].
Antioxidants with Reactive Oxygen Species (ROS) scavenging abilities may play an important role in preventing oxidative stress in our bodies. Shukla et al. in 2009 investigated the antioxidant activity and total phenolic content of C. bonduc seed ethanolic extract. Their study was aimed to test the in-vitro potential of ethanolic extract of C. bonduc seeds as a natural antioxidant. The DPPH activity result of the study clearly demonstrated that the extract (20, 40, 50, 100, and 200 µg/mL) recorded significant DPPH activity and that the activity increased in a dose-dependent manner, which was reported in the range of 38.93-74.77% as compared to standard ascorbic acid (64.26-82.58%). In the DPPH radical scavenging assay, the IC50 values of ethanolic extract of seed and ascorbic acid were reported to be 74.73 and 26.68 µg/mL. It has also been claimed that an ethanolic extract of the seed may scavenge superoxide using an EDTA/NBT system. Furthermore, the total phenolic content of the ethanolic extract of C. bonduc was found to be substantially higher than the reference standard gallic acid. The ethanolic extract inhibited the hydroxyl radical, nitric oxide, and superoxide anions with IC50 values of 109.85, 102.65 and 89.84 µg/mL. The IC50 values for standard ascorbic acid, on the other hand, were reported to be 70.79, 65.98, 36.68 µg/mL, respectively. These findings clearly show that C. bonduc seed has a high potential to use as a natural antioxidant agent [69].
Pandey et al. in 2019 investigated the antioxidant activity of C. bonduc extracts via DPPH radical scavenging and nitric oxide scavenging. They also measured the quercetin level of the C. bonduc extract, which was found to be 0.285 µg/mL. Furthermore, HPLC analysis of plant extracts revealed the presence of quercetin, the most prevalent dietary flavonol. According to the findings of this study, changes in bioactive component content could be a determinant for the medicinal capabilities of this plant, particularly for antioxidant activities with prospective applications in the food and pharmaceutical industries [70].
Sembiring et al. investigated the phytochemicals, total flavonoid and total phenolic levels, and antioxidant activity of an ethanol extract of C. bondoc’s root, stem, leaves, and seed kernel. Phytochemical analysis revealed that all the test samples contained flavonoid and saponin. Total flavonoid content was highest in the leaf and lowest in the root, whereas total phenols content was highest in the leaf and lowest in the seed kernel. The crude extracts demonstrated DPPH free radical scavenging activity, with the leaf extract having the highest value, followed by the root, stem, and seed kernel [71].
Adaptogenic activity
Adaptogens, or adaptogenic compounds, are used in herbal medicine to stabilise physiological processes and promote homeostasis in the body supposedly. In one study, C. bonduc seed extracts were screened for adaptogenic activity using cold stress model and swim endurance model. When the seed coat and kernel extracts were given orally at 300 mg/kg, they recorded strong antistress properties. The extracts also significantly increased the swim endurance time. The stress-induced rats in this investigation recorded considerable hypoglycemia, as well as a decrease in blood cortisol levels and an increase in total leukocyte count. The seed extracts were found to be effective in resolving these imbalances. Extracts were also found to have a substantial capability for regulating hyperlipidemia caused by the production of stress [72].
Antidiabetic activity
The term "antidiabetic" refers to a medicine that can help diabetes stabilise and control their blood glucose levels. These are frequently used in the treatment of diabetes. The oral anti-diabetic effects of various extracts of C. bonduc seed kernels were reported by Parameshwer et al. C. bonduc seed kernels have been reported to employ in the management of diabetes mellitus, mainly in the folklore medicine of Andaman and Nicobar, as well as the Caribbean Islands. In the experimental animals, the seed kernel powder was also reported to have hypoglycaemic action. Both the polar extracts (ethyl acetate and aqueous) and glibenclamide had a significant hypoglycaemic impact in diabetic-induced rats. Furthermore, it promotes in the restoration of diabetes-related alterations in lipid and liver glycogen levels. Non-polar extracts were also found to have an antidiabetic impact, according to the researchers. When it came to non-polar extracts, the ether extract had a moderate anti-diabetic activity, whereas the petroleum ether extract recorded no activity. Both the polar extracts were reported to contain triterpenoidal glycosides, so the antidiabetic activity is presumed to be due to these active principles. The aqueous extract was shown to have little free radical scavenging activity in in-vitro antioxidant studies; however, the ethyl acetate extract had substantial activity (49%) at the end of 1 hour. The antioxidant capability of ethyl acetate extract may help to combat diabetes-related oxidative stress, even though it's not necessary for hypoglycaemic activity [73].
The bark and root of C. bonduc have been shown to have antidiabetic properties by Patil et al. The antidiabetic effect of C. bonduc root extracts was investigated using a glucose tolerance test in normal and alloxan-induced diabetic rats. In a glucose tolerance test, both aqueous ethanol and chloroform extracts provided considerable protection and brought blood glucose levels back to normal. At a 250 mg/kg of body weight dosage, the highest reduction in blood glucose was observed after 3 hours in alloxan-induced diabetic rats. The aqueous chloroform and ethanol extract both provided 22.28 and 23% protection, respectively. After long-term treatment, the degree of protection was determined by monitoring blood glucose, triglycerides, cholesterol, and urea levels in alloxan-induced diabetic rats. The anti-diabetic activity of both aqueous chloroform and ethanol extracts was comparable to that of the conventional anti-diabetic medication glibenclamide [74].
In another study, C. bonduc seed extracts were tested for antidiabetic efficacy in alloxan-induced hyperglycemia. The oral administration of the extracts (300 mg/kg) had a significant anti-hyperglycemic effect as well as a considerable reduction in blood urea/nitrogen levels in this investigation. The effect of the extracts on diabetes-induced hyperlipidemia was studied in the same study, and the extracts significantly reduced high cholesterol and LDL levels. The extract's anti-hyperglycemic activity could be related to a blockage of glucose absorption. As a result, this extract has a lot of potential as an anti-diabetic and anti-hyperlipidemic medication [75].
In 2019, Widhiantara et al. discovered that C. bonduc seed had anti-diabetic properties in Wistar albino rats. Streptozotocin and nicotinamide were used to induce type 2 diabetes in male Wistar albino rats. The rats were split into three groups: distilled water control, glibenclamide positive control (10 mg/kg/d, oral), and C. bonduc seed extract test group (500 mg/kg/d, oral). After 14 days of treatment, blood glucose and plasma insulin levels were measured. The results showed that the postprandial blood glucose (PPBG) levels in both the positive control and test groups were considerably lower. In contrast, the PPBG levels in the control group were higher. In this investigation, glibenclamide was more effective than C. bonduc seed extract at lowering PPBG levels. In addition, the extract-treated group had a higher post-test fasting insulin level than the other groups. In conclusion, these findings imply that an ethanolic extract of C. bonduc seed has anti-diabetic efficacy against type 2 diabetes in mice [76].
Anti-inflammatory activity
The property of a medication or treatment that reduces inflammation or swelling is known as an anti-inflammatory. Inflammation is the body's natural response to damaging stimuli such as pathogens, irritations, and damaged cells. Several inflammatory diseases can be due to the excessive synthesis of inflammatory chemicals. As a result, preventing the over expression of inflammatory chemicals is critical. In ancient practise, medicinal plants were employed as an excellent source of anti-inflammatory substances. C. bonduc activity was evaluated for anti-inflammatory effects in rats using the formalin-induced arthritis and granuloma pouch techniques. The extract was found to be very effective in the granuloma pouch model at a dose of 250 mg/kg, and its activity was highly equivalent to that of the standard drug, phenylbutazone. At an oral dose of 1000 mg/kg, the seed extract showed more than 50% inhibitory action against carrageenan-induced hind paw oedema in rats when given 24 hours before carrageenan administration by IP. At a dose of 100 mg/kg, the antiinflammatory activity (66.67% inhibition) was comparable to that of phenylbutazone [77-79].
In 2015, Arunadevi et al. explored the anti-inflammatory properties of C. bonduc flower extract (CBFE). The CBFE was given orally (30, 100, and 300 mg/kg b.wt.) and its anti-inflammatory effect was investigated in carrageenan-induced inflammation, cotton pellet-induced chronic granulomatous inflammation, and autacoids-induced inflammation in their study. Studies in adjuvant-induced arthritis looked at the impact on the radiographic outcome. After all dosages (30, 100, and 300 mg/kg b.wt.), CBFE significantly reduced edoema volume in carrageenan-induced inflammation, with percentages of inhibition of 28.68, 31.00, and 22.48, respectively, compared to control at 5 hours after treatment. CBFE reduced the granuloma weight by 22.53% in a cotton pellet granuloma assay at 300 mg/kg treatment level. At 12, 1 and 3 hours after 5-hydroxytryptamine injection, CBFE (300 mg/kg) induced significant inhibition by 37.5, 44.44, and 35.29% edoema volume, respectively. When arthritic control animals were compared to animals treated with 300 mg/kg CBFE, the radiographic score of the animals treated with 300 mg/kg CBFE was significantly lower. All of these findings revealed that CBFE has significant anti-inflammatory effects [80].
Banupriya and co-workers investigated the phytochemical components, antioxidant, and anti-inflammatory activity of an ethanolic extract of C. bonduc leaf and seed kernel in 2018. Both the leaf and kernel extracts of C. bonduc effectively prevented albumin denaturation, proteinase action, and heat and hypotonic solution induced haemolysis of erythrocytes, indicating that they have anti-inflammatory potential. Overall, the findings showed that the ethanolic extracts of both the leaf and seed kernel of C. bonduc had substantial anti-inflammatory activity, primarily due to numerous secondary metabolites [81].
Suresh et al. recently looked at the anti-inflammatory properties of C. bonduc seeds. The anti-inflammatory effect of C. bonduc was investigated using an albumin denaturation assay. At 30 µL and 40 µL concentrations, the aqueous extract of C. bonduc seeds and the aqua-alcoholic extract of C. bonduc seeds are used. When compared to aqueous and aqua-alcoholic extracts of C. bonduc seeds, diclofenac had a greater percent inhibition value and anti-inflammatory action at higher concentrations, i.e., 50 µL [82].
In 2019, Jagdale et al. studied immediate anti-inflammatory activity in carrageenan-induced rat paw edoema, as well as chronic anti-inflammatory activity in a cotton pellet-induced granuloma paradigm. This study's GC-MS analysis revealed the presence of 32 phytochemicals. Among these 16 compounds, the presence of various biological activities has previously been observed. According to the findings, the extract dramatically reduced paw volume in acute inflammation and the weight of granuloma development in chronic inflammation in a dose-dependent manner. According to their observations, the C. bonduc extract has strong anti-inflammatory action, indicating that it can be used ethnopharmacologically to treat a variety of inflammatory disorders [83].
Anthelmintic activity
Jabbar et al. reported the anthelmintic property of C. bonduc for the first time using in-vitro and in-vivo investigations, and thus the study group further substantiated why C. bonduc was used in Pakistan's traditional medicine system [84]. C. bonduc leaves were tested for anthelmintic activity against Phertima posthuma and Ascardia galli. Different doses of the extracts were utilized in the trials, and the tested extracts showed strong anthelmintic action in the test organisms [85].
Gogoi and Yadav in 2016 studies the in-vitro and in-vivo anthelmintic effects of C. bonduc leaf extract against Syphacia obvelata (Nematoda) and Hymenolepis diminuta (Cestoda). They studied the extract's in-vitro anthelmintic activity on adult S. obvelata and H. diminuta worms in terms of physical motility and parasite mortality. They monitored the egg per gram of faeces count and worm count of animals after treatment with different doses of extract in an in vivo investigation in H. diminuta rats and S. obvelata mice. The extract had strong and dose-dependent anthelmintic effects on both parasites in this investigation. In the in vitro research, extract concentrations of 30 mg/mL killed H. diminuta in 2.5 ± 0.2 hours and S. obvelata in 3.57 ± 0.16 hours. The extract had a higher efficacy on S. obvelata in the in-vivo research, with an 800 mg/kg dose resulting in a 93% reduction in the worm load in mice, compared to an 85% drop in H. diminuta worm load in rats. These data imply that C. bonduc leaf extract has strong anthelmintic properties, indicating that it could be used as an anthelmintic in traditional medicine. This appears to be the first report of C. bonduc’s anthelmintic action against these parasites in-vivo [86].
Using Indian earthworms (Pheretima posthuma) and nematodes, Wadkar & Sayyad investigated the in-vitro anthelmintic effectiveness of ethanolic and aqueous extracts of C. bonduc root bark (Ascaris Gallis). The in-vitro anthelmintic potency of various extract concentrations (25, 50, 100 mg/mL) was determined by measuring the period of worm paralysis and death. The control drug was piperazine citrate (15 mg/mL). At a dosage of 100 mg/mL, both extracts showed considerable anthelmintic action. As a result of the findings, it can be concluded that C. bonduc has a bright future in treating helmintic infections [87].
Antifertility activity
The antifertility activity of ethanolic seed extract of C. bonduc in female Wistar albino rats was studied by Lilaram and Ahmed in 2013. In their experiment, pregnant rats were divided into two groups, each of which had eight animals. From gestation day 1 to 7, rats in the control group were given 1 mL/100 g b.wt of distilled water, while rats in the second group were given 300 mg/kg of the seed extract once daily. On gestation day 12, the animals were slaughtered twenty-four hours following the last treatment. In the seed extract-treated rats, a significant decrease in implantation index and a corresponding significant rise in resorption index, pre-implantation and post-implantation loss were observed. Rats given seed extract had significantly lower levels of progesterone. In treated rats, the corpora lutea had deteriorated. The embryos in the extract-treated group had craniofacial abnormalities. As a result, these findings revealed that C. bonduc seeds had antifertility effects, most likely due to their antiprogesterogenic hormonal features, which can modify the reproductive function of experimental rats [88].
Antifilarial activity
In experimental filarial infections, Gaur et al. discovered that C. bonduc had anti-filarial properties. Lymphatic filariasis is a severe microbiological illness that continues to wreak havoc on the health of tropical people. This research group carried out this investigation in order to assess the anti-filarial properties of C. bonduc seed kernel extract against rodent filarial parasites in an experimental model. Microfilaraemic cotton rats and Mastomys coucha carrying Litomosoides sigmodontis and Brugia malayi, respectively, were given crude extract or fractions of the C. bonduc seed kernel orally for 5 days in this investigation. The sterilizing effectiveness of microfilaricidal, macrofilaricidal, and female worms was also examined. From day 8 post-treatment, the crude extract showed a progressive decrease in microfilariae count in the L. sigmodontis-cotton rat model, achieving a reduction of more than 95% by the end of the study's observation period. In this investigation, it also demonstrated 96% macrofilaricidal and 100% female sterilizing action. Furthermore, when the fractions were evaluated for activity, the butanol fraction showed a 73.7% drop in microfilariae count and an 82.5% mortality rate in adult worms, as well as 100% female sterilization activity. The aqueous fraction also had a microfilaricidal activity of more than 90% and worm sterilization of 100%. Furthermore, two silica gel column fractions, namely the hexane soluble fraction and the hexane insoluble fraction, showed 64 and 95% macrofilaricidal activity. Both fractions showed a steady decrease in microfilaraemia as well as 100% worm sterilization activity. In the case of B. malayi-M. coucha model recorded gradual reduction in microfilaraemia and caused 80% sterilization of female parasites. Thus, microfilaricidal, macrofilaricidal, and female-sterilizing activity against L. sigmodontis and microfilaricidal and female-sterilizing efficacy against B. malayi were shown in animal models using C. bonduc seed kernel extract and fractions. This investigation demonstrated that this plant can serve as a foundation for future antifilarial medication development.
In 2017, Nondo et al. isolated Norcaesalpin D from C. bonduc dichloromethane root extract using column chromatography. Using the parasite lactate dehydrogenase assay, they investigated anti-plasmodial activity of crude extracts, fractions, and isolated compound against chloroquine-sensitive P. falciparum (3D7), chloroquine-resistant P. falciparum (Dd2, K1), and artemisinin-resistant P. falciparum (IPC 5202 Battambang, IPC 4912 Mondolkiri) strains. The researchers demonstrated that extracts of C. bonduc have antiplasmodial action against Dd2 parasites in their trials. The extract of C. bonduc roots was found to be particularly effective against parasites resistant to K1, Dd2, and artemisinin. Norcaesalpin D from C. bonduc root extract was active against 3D7, Dd2, and IPC 4912-Mondolkiri parasites, with IC50 values of 0.98, 1.85, 2.13 µg/mL, respectively. The antiplasmodial action of norcaesalpin D and C. bonduc extracts demonstrated in this work warrants additional investigation to identify antimalarial lead compounds for future medication development [89].
Antimicrobial activity
The antibacterial activity of seed extracts and the pure compound bondenolide isolated from C. bonduc has been reported by Simin et al. Bondenolide, the methanol extract, the ethyl acetate fraction, and the water soluble part of the methanol extract of C. bonduc were tested for antibacterial and antifungal activity, as well as a phytotoxicity test, in this work [90].
Arif et al. also reported on the antibacterial properties of C. bonduc seeds in-vitro and in-vivo assays [91]. The antibacterial activity of (2-hydroxy-2-methylpropyl)—(2-hydroxy-3-methylbut-2-en-1-yl) polymethylene from C. bonduc was similarly reported by Sagar et al. [92]. The compound, α-(2-hydroxy-2-methylpropyl)-ω-(2- hydroxy-3-methylbut-2-en-1-yl) polymethylene, isolated and purified from the ethyl acetate leaf extract of C. bonduc was checked for the antimicrobial activity against various clinical isolates like Proteus vulgaris, Pseudomonas aeruginosa, Klebsiella sp., Staphylococcus citrus, Staphylococcus aureus, Escherichia coli, Candida albicans and Rhodotorula sp. using the agar diffusion method. The tested compound showed a zone of inhibition at all concentrations tested, revealing concentration-dependent activity against all clinical bacterial and yeast strains tested. The activity is comparable to standard antibiotics such as streptomycin sulphate and gentamycin for bacteria. Fluconazole/griseofulvin for Candida albicans and Rhodotorula sp. C. albicans and Rhodotorula sp. had the best zones of inhibition. The zones of inhibition for Pseudomonas aeruginosa, P. vulgaris, and E. coli were greater than the standard antibiotics tested. In contrast, the zones of inhibition for Klebsiella sp. and S. aureus were equivalent to the standards [93]. The seed of C. bonduc comprising numerous active chemical constituents was also discovered to have antibacterial action in-vivo and in-vitro experiments [93].
In 2013, Billah et al. used the disc diffusion method to test the antimicrobial, antidiarrheal, and cytotoxic activities of the methanol extract and its ethyl acetate, chloroform and petroleum ether fractions of C. bonduc leaves against four Gram-positive and five Gram-negative bacteria at 300, 500, and 800 g/disc against four Gram-positive and five Gram-negative bacteria. As the standard medication, kanamycin (30 g/disc) was employed. In castor oil-induced diarrhoea model in rats, antidiarrheal effects of leaf extracts were assessed at 200 and 400 mg/kg and compared to loperamide. A brine shrimp lethality test was used to determine the fraction's cytotoxicity. At increasing concentrations, the methanol extract and the other three fractions showed stronger activity. At all three test concentrations, the chloroform fraction had the most activity. Except for the petroleum ether fraction, S. aureus and P. aeruginosa showed higher sensitivity to all extracts at all three concentrations. Out of nine bacteria tested, Bacillus megaterium and Klebsiella spp. showed lowest sensitivity to the extracts. With an 800 g/disc concentration of methanol extract, the maximum zone of inhibition was recorded against S. aureus. In the antidiarrhea test, all fractions showed dose-dependent and statistically significant effects. The ethyl acetate fraction inhibited defecation the most (51.11%), while loperamide inhibited it the least (57.75%). In a brine shrimp bioassay, the methanol extract and its three fractions showed moderate cytotoxicity when compared to the reference medication vincristine sulphate. Methanol crude extract, ethyl acetate, chloroform, petroleum ether fractions, and vincristine sulphate had LC50 values of 223.87, 281.84, 112.2, 199.53, 12.59 g/mL, respectively, in this investigation. From results, the ethyl acetate fraction was found to have the highest cytotoxicity, whereas the chloroform fraction had the lowest. The ethyl acetate fraction of C. bonduc leaves possesses antidiarrheal effects, according to this study. The methanol extract and the other three fractions of C. bonduc leaves have powerful antibacterial properties as well as moderate cytotoxicity, which could lead to the formulation of new drugs in the future [94].
The demand and use of herbal medicine is growing in tandem with the emergence of medication resistance in the bacterial population. Herbal treatments are safe and effective against bacteria and other pathogens, with no known negative effects. Reichal et al. investigate the antibacterial efficacy of C. bonduc leaf ethanolic extract against human oral infections using sterile discs in 2019. The minimal inhibitory concentration was determined using the agar well diffusion method. Their investigation demonstrated that the bacterial growth inhibition of C. bonduc leaf ethanolic extract is enhancing in a dose-dependently manner. In comparison to the common antibiotic ciprofloxacin, however, 100% inhibition was reported against Enterococcus faecalis. Finally, the extract was found to have antimicrobial properties. According to their findings, C. bonduc is a potential antibacterial agent against human oral pathogenic microorganisms, and hence the herbal medicine could be used as one of the antimicrobial agents [95].
Shukla et al. studied the antibacterial mechanism of action of C. bonduc seed oil on membrane permeability of Listeria monocytogenes NCIM 24563 and Escherichia coli ATCC 25922 by measuring the extracellular ATP concentration, release of 260-nm absorbing materials, leakage of potassium ions and measurement of relative electrical conductivity of the bacterial cells treated with MIC concentration of test materials. Release of extracellular ATP (1.42 and 1.33 µg/mL), loss of 260-nm absorbing materials (4.36 and 4.19 OD), leakage of potassium ions (950 and 1000 mmol/L), and enhance in relative electrical conductivity (12.6 and 10.5%) against L. monocytogenes and E. coli, respectively, confirmed its mode of action on membrane integrity. These findings reveal that C. bonduc oil's mode of action damages the membrane integrity, implying that it has considerable food and pharmaceutical potential [96].
Antiestrogenic activity
Kanchan et al. found that C. bonduc alcohol seed extract possesses antiestrogenic properties, attributed to the reduction of estrogen secretion [97].
Abortifacient activity
The seeds of C. bonduc have long been used in rural India to manage female fertility. The leaves are used as an emmenagogue and to help pregnant women deliver safely. The combination of C. bonduc seed powder and sesame oil causes abortion. It indicates that the herb is abortifacient [98].
Antispermatogenic
Kanerkar et al. observed in 2015 that C. bonduc seeds have antispermatogenic properties in male albino rats. Male albino rats had their sperm count reduced after taking an aqueous extract of C. bonduc seeds orally for 21 days. Furthermore, it considerably reduced sperm density and showed an average increase in antispermatogenic activity of 9.06%, 25.29%, and 39.79% with treatment at 50, 100, and 150 mg/kg, respectively. These findings imply that the seeds of C. bonduc might be utilized to create a safe and effective male contraceptive [99].
Sperm effect
Gradually increasing doses of alcoholic seed extract of C. bonduc caused morphological alterations in albino rats' sperm. Thus, the impact might be a direct outcome of disturbing protein effects and changes in the cauda epididymal milieu, most likely due to androgen deficiency caused by C. bonduc therapy [100].
Aphrodisiac activity (Improvement of male sexual performance)
In 2020 Sindete et al. reported the aphrodisiac activity of the ethanol extract from the root of C. bonduc in male Wistar rats. They divided the eighteen male rats into three groups: Group 1 received dimethyl sulfoxide (vehicle), Group 2 received 25 mg/kg body weight Viagra® (Sildenafil citrate), and Group 3 received 500 mg/kg body weight ethanol extract of C. bonduc root. With the exception of Viagra®, which was given 1 hour before each mating, the therapy was given once daily via gavages for 28 days. On the 1st, 14th, and 28th days, male rats were mated with artificially estrus female rats using benzoate estradiol and progesterone. Intromission Frequency (IF), Intromission Latency (IL), Mount Frequency (MF), and Mount Latency (ML) were examined as sexual behavior parameters. They also looked at the extract's impact on serum testosterone levels and testis histoarchitecture. When compared to the control group, C. bonduc root extract dramatically enhanced the IF and MF. They also observed that the rodents' ML and IL levels had dropped considerably. In addition, they reported significant increases in testosterone levels in the extract-treated group. This is substantiated by testicular cross-sections that demonstrated an increase in the diameter of the seminiferous tubes when compared to the control and Viagra® groups. This research revealed that C. bonduc root improved male rat sexual behavior and may have an aphrodisiac effect, justifying its usage in alternative medicine [101].
Anticataract activity
The ethanolic extract of C. bonduc seed kernels. (L) Fleming contains anticataract and antioxidant properties that may be effective in preventing or delaying cataract progression. The extract decreased opacity and tissue Malondehyde (MDA) levels while increasing the activities of catalase and superoxide dismutase (SOD). Water soluble protein levels and total protein levels both increased [102].
Antiulcer activity
The aqueous extract of C. bonduc was effective in treating ulcers and having antisecretory properties. Therefore, this plant has the potential to be used to treat stomach issues. In addition, the extract reduced stomach volume, total and free acidity, and elevated the pH of the gastric fluid. The aqueous extract of C. bonduc contained saponins, alkaloids, triterpenes, flavonoids, steroids, and tannins, and flavonoids were found to have antiulcer activity. The methanolic extract of C. bonduc leaves has considerable antiulcer activity [103].
Antimalarial activity
Pudhom et al. investigated the antimalarial activity of three new cassane furanoditerpenoids isolated from C. bonduc seed kernels, as well as recognized cassane diterpenes. The three novel cassane furanoditerpenoids showed promising antimalarial effectiveness against the Plasmodium falciparum K1 multidrug resistant strain [104]. Antimalarial activity of cassane and norcassane type diterpenes from C. bonduc was also reported by Kalauni et al., as well as the structure-activity link [105]. Linn et al. extracted cassane and norcassane type diterpenes from C. bonduc in Indonesia and shown antimalarial activity in Plasmodium falciparum [106].
Nondo et al. studied the in-vivo antimalarial activity of C. bonduc along with Erythrina schliebenii Harms, Holarrhena pubescens Buch-Ham, and Phyllanthus nummulariifolius Poir, which are all used to treat malaria in Tanzania, in 2016. The 4-day suppressive antimalarial experiment was used to assess in-vivo antimalarial activity. Mice were infected with Plasmodium berghei ANKA by injecting 2 × 107 erythrocytes into their tail veins. Extracts were administered orally once a day for four days starting on the day of infection. Positive and negative controls were chloroquine (10 mg/kg/day) and solvent (5 mL/kg/day), respectively. C. bonduc, E. schliebenii, H. pubescens, and P. nummulariifolius extracts all suppressed parasite growth in mice in a dose-dependent manner, with C. bonduc extract showing the greatest suppression. According to their research, all the plant extracts tested have the potential to create antimalarial compounds. In addition, the dichloromethane root extract of C. bonduc appears to be the most promising for obtaining active antimalarial chemicals. This study's in-vivo antimalarial activity validates traditional applications of C. bonduc roots in the treatment of malaria [107].
According to Bhattacharyya & Babu, tropical legume seeds contain a variety of proteinase inhibitors (PI), and this study focuses on the structural and functional characteristics of a strong serine PI isolated from the seeds of C. bonduc (CbTI-2). The N-terminal sequence of CbTI-2 indicated about 40% identity with a few serine PIs from the Caesalpinioideae subfamily after purification. CbTI-2 antitryptic activity levels varied dramatically when exposed to metal ions and ionic/non-ionic surfactants. When CbTI-2 was exposed to 1, 4-Dithiothreitol, Guanidinium HCl, H2O2, and Dimethyl sulfoxide, its inhibitory activity gradually decreased. The active site residue was suggested by chemical modification of amino acids. The native CbTI-2 Circular Dichroism spectrum revealed an unordered state. Following exposure to extreme conditions (heat, acidic/ alkaline environment, Guanidine hydrochloride, and DTT); the secondary structural composition of CbTI-2 was significantly altered, resulting in a significant loss of antiproteolytic action. CbTI-2 has a hydrodynamic radius of 2.2 nm, and the trypsin CbTI-2 complex has a 1:1 stoichiometry, according to DLS investigations. CbTI-2 was found to be a strong antiplasmodial agent in early experiments because it was very toxic to Plasmodium falciparum development, schizont rupture, and erythrocytic invasion [108].
Antibacterial, antifungal, antispasmodic activity
C. bonduc has been shown to have antibacterial, antifungal, and antispasmodic properties by Khan et al. [109]. Antibacterial activity in C. bonduc seeds has also been reported by Saeed and Sabir [110].
Antiproliferative activity
Cassane diterpenes were isolated from the C. bonduc by Yadav et al. The obtained compounds were then evaluated against MCF-7 (breast adenocarcinoma), DU145 (prostate carcinoma), C33A (cervical carcinoma), and Vero (African green monkey kidney fibroblast) cell lines for antiproliferative activities and recorded significant activity [111].
Antipsoriatic activity
Muruganantham et al. a traditional Siddha practitioner investigated the antipsoriatic action of C. bonduc in the Malabar region of Kerala, India, have employed the leaves of C. bonduc to treat psoriasis [112].
Anticonvulsant/Antiepileptic
In 2009, Ali et al. used pentylenetetrazole, maximum electro shock, strychnine, and picrotoxin-induced convulsions to investigate anticonvulsant activity. Except for the maximal electro shock model, which utilized phenytoin as a standard reference, all models used diazepam as a standard reference. C. bonduc seed kernels were ground and extracted with solvents such as petroleum ether, ethanol, methanol, and water using a soxhlet device. In all the tests, the extracts were given as a suspension in 2% gum acacia. Saponins, glycosides, starch, sucrose, proteins, sterols, and reported components including homoisoflavone (bonducillin) and a non-alkaloid bitter principle were found in a preliminary phytochemical analysis of C. bonduc petroleum ether extract. In their study, it was found to be non-toxic even at doses of 3000 mg/kg (LD50). Medium as well as high doses (600 and 800 mg/kg) of the extract demonstrated considerable anti-convulsant effect in pentylenetetrazole, maximum electro shock, strychnine-, and picrotoxin-induced convulsion models. The abundance of phytoconstituents in the drug, such as saponins, proteins, homoisoflavone (bonducillin), carbohydrates, and sterols, may have contributed to the petroleum ether C. bonduc's anticonvulsant action, as they have been previously reported for their anxiolytic and anticonvulsant actions [113].
Anxiolytic activity
Ali et al. studied the anxiolytic efficacy of C. bonduc seed extract in experimental animals and published their findings. In a stair-case model experiment, all three test doses of extract (400, 600, and 800 mg/kg) showed substantial and dose-dependent anxiolytic effects by increasing the number of steps ascended, but none of the three test dosages had a significant influence on rearing. Similarly, in the Elevated Plus Maze (EPM) model, medium and high doses showed activity, although low doses did not. The number of entries and time spent in open arms increased dramatically with the medium and high doses, while the number of entries and time spent in closed arms decreased significantly. Medium and high dosages of extract, i.e., 600 and 800 mg/kg, significantly increased the number, latency, and duration of head dipping but not the rearings in Hole-board model trials, in contrast, low doses failed to record any activity. However, in the LDT model, 800 mg/kg of extract significantly increased time spent, the number of crossings in the light compartment, decreased time spent in the dark compartment, and lowered the number of readings in both the light and dark compartments, indicating anxiolytic activity. Medium and high doses dramatically increased total movement, center locomotion, and grooming number in Open-Field Test (OFT) model studies, although immobility time was drastically reduced. All doses of the test extract had no discernible effect on rearing, feces, or urination. Similar to the above, both medium and high doses dramatically reduced the time latency to enter the mirror chamber and increased the number of entries and time spent in the room in the Mirror-chamber model of anxiety. Thus, the experimental model results clearly show that C. bonduc extract has anxiolytic properties [114].
Larvicidal activity
Saravanan et al. has reported the mosquito larvicidal properties of various leaf extracts as well as fixed oil from the seeds of C. bonduc. By adapting the WHO guidelines, this group conducted a preliminary laboratory experiment to determine the efficacy of petroleum ether, ethanolic, aqueous extracts of dried leaves, and fixed oil from the seeds of C. bonduc at various concentrations against the fourth instar larvae of Culex quinquefasciatus. In this experiment, 100% mortality was observed in 1% concentrations of petroleum ether and ethanolic extract of leaf, but 55% mortality was observed in 2.5% concentrations of aqueous extract and 92.6% mortality was observed in 2.5% concentrations of fixed oil. This group concluded that the active ingredient responsible for mortality should be separated. This may become a viable larvicidal agent in the near future that is cost-effective, non-polluting, and environmentally benign [115].
Toxicity and antifeedant activity
Natural pesticides have several advantages over synthetic pesticides, including being less expensive, biodegradable, and less hazardous to humans, animals, cattle, and wildlife. Furthermore, impoverished, marginalized rural communities in developing countries have easy access to natural biopesticides. The biotoxicity of crude extracts and fractions of C. bonduc against the cotton bollworm Helicoverpa armigera Hub was investigated. The chloroform extract of C. bonduc showed the most antifeedant, larvicidal, and pupicidal activity. As a result, the chloroform extract of C. bonduc was segregated using increasing polarity solvents. Six fractions were separated based on TLC characteristics. All fractions were tested on H. armigera. The 3rd fraction had the maximum antifeedant, larvicidal, and pupicidal properties, as well as the greatest treatment impact on larval-pupal duration. Overall, their research allowed others to evaluate 3rd fraction as a prospective source of botanical products helpful in the battle against agricultural pests, particularly the cotton bollworm, in an environmentally benign manner [116].
Immunomodulatory activity
C. bonduc extract has been shown to have antiinflammatory properties in numerous investigations. One study reported the immunomodulatory effects of an ethanolic extract of C. bonduc seeds among the research published. The immunomodulatory activity of C. bonduc ethanolic seed extract (200-500 mg/kg) was examined in this study. The experiment's findings revealed that the percentage of neutrophils adhering to nylon fibers increased significantly. The study also found that sheep red blood cells potentiated the delayed type hypersensitivity reaction by increasing antibody titre values in a dose-dependent manner. In-vivo investigations revealed neutrophil adhesion test, haemagglutinating antibody titre, delayed type hypersensitivity reaction, phagocytic activity, and cyclophosphamide-induced myelo-suppression [117,118].
The in-vivo immunomodulatory effects of the aqueous extract of C. bonduc seeds were described by Shukla et al. The impact of an aqueous extract of C. bonduc seeds on immune system cell mediated and humoral components in rats was investigated in this study. At a test dose of 400 mg/kg body weight, administration of C. bonduc seed extract resulted in an increase of 93.03 ± 4 mean hemagglutinating antibody titer and a change of 0.56 ± 0.058 mm in delayed type hypersensitivity compared to control. Finally, the findings of this investigation suggested that C. bonduc extract may be an effective immunostimulant. The immunomodulatory effects of the ethanolic extract of C. bonduc seeds were also found in another research by the same group. C. bonduc has possible immunomodulatory action and therapeutic potential for the prevention of different autoimmune disorders in humans, according to the findings of this study [119].
Hypoglycemic activity
As far as we know, there are currently no effective therapeutic alternatives for diabetes mellitus. Synthetic medicines, on the other hand, have a number of disadvantages. Many traditional herbs' antihyperglycemic properties are attributed to their capacity to treat diabetes mellitus. The antihyperglycemic and antioxidative effects of a hydromethanolic extract of C. bonduc seeds on streptozotocin-induced diabetes in male albino rats were investigated by Jana et al. For a total of 21 days, the hydromethanolic extract was given orally at a dosage of 250 mg/kg of body weight each day. The antihyperglycemic and antioxidative effects of a hydromethanolic extract of C. bonduc seeds on streptozotocin-induced diabetes in male albino rats were investigated by Jana et al. For a total of 21 days, the hydromethanolic extract was given orally at a dosage of 250 mg/kg of body weight each day. Other toxicity indicators such as serum glutamate oxaloacetate transaminase, glutamate pyruvate transaminase, and alkaline phosphates activities were examined, as well as glycogen levels in hepatic and skeletal muscles. When compared to the untreated diabetic group, therapy with the hydromethanolic seed extract of C. bonduc resulted in a considerable recovery in the activities of carbohydrate metabolic enzymes, as well as corrections in fasting blood glucose and glycogen levels. The activity of toxicity assessment enzyme parameters was also significantly improved by the extract. Antioxidant enzyme activity such as catalase and superoxide dismutase, as well as lipid peroxidation levels, improved considerably following treatment with the extract. The extract's effects were compared to those of the conventional anti-diabetic medication glibenclamide. This finding clearly portrayed that extract recorded possible antihyperglycemic and antioxidative action [120].
Innovative study on the hypoglycemic impact of C. bonduc in type 1 and 2 diabetes in Long Evans rat models was published by Chakrabarti et al. C. bonduc is extensively spread across India's coastal region and is utilized culturally by India's tribal people for blood sugar management, as previously described by the same researchers, and this is the motivation for doing hypoglycemia investigations in animals. This motivated them to conduct a detailed investigation in Long Evans rats using aqueous and ethanolic extracts of the seeds in both type 1 and type 2 diabetes mellitus. A substantial blood sugar lowering impact was reported in a type 2 diabetes animal in their investigation. A specific emphasis was placed on the mechanistic investigation of glucose and hepatic glycogen absorption in the intestine [121].
Moshi and Nagpa studied the impact of C. bonduc seeds on blood glucose levels in rabbits. The potential of a powdered seed kernel suspension in 0.5% carboxymethylcellulose (CMC) to decrease blood glucose in fasting and glucose-fed normal albino rabbits was evaluated in this study. There was no change in regions for the fasting blood glucose and oral glucose tolerance test curves following administration of 0.2, 0.4, and 0.8 g/kg body weight of the powder compared to controls that were given plain CMC. Whereas, 0.2 g/kg body weight of the powder, on the other hand, had no effect on fasting blood glucose or the clearance of a glucose load from the blood after 7 days. Further, 0.1 g/kg body weight chlorpropamide, on the other hand, dramatically reduced the area under the fasting blood glucose and OGTT curves when compared to a control group given CMC [122].
C. bonduc has an oral hypoglycemic effect, according to the findings of Biswas et al. In fasting, fed, glucose loaded, streptozotocin diabetic, and alloxan diabetic rat model studies, the blood sugar lowering effectiveness of the aqueous extract of C. bonduc seed shell was tested and reported. The extract was given orally to the rats at a dosage of 250 mg/kg of body weight. The results show that the extract significantly reduced blood sugar levels in glucose-loaded, streptozotocin-induced diabetic, and alloxan-induced diabetic animals. In the fasted and fed models, however, its effects were less noticeable. Thus, C. bonduc can be considered a good oral hypoglycemic agent in rats, according to the findings [123].
In streptozotocin-induced diabetic rats, Vijay and Joshi in 2020 investigated the antidiabetic effect of an aqueous extract of C. bonduc leaves. The extract was prepared by a cold maceration technique, and the animals were given a single dosage of streptozotocin to induce diabetes. A comparison was done between the effects of different concentrations of C. bonduc leaf extracts in doses of 100 and 200 mg/kg of body weight and glibenclamide, a well-known antidiabetic medicine (4 mg). In diabetic animals, the results reveal a gradual decrease in blood glucose levels. Antioxidants have been shown to protect the body from numerous diseases by preventing oxidative damage caused by free radicals. Enzymatic antioxidants such as catalase and superoxide dismutase have also been tested in the aqueous leaf extracts of C. bonduc and shown to be good sources of natural antioxidants used to treat disorders related with oxidative stress [124].
Wound healing
Chandra et al. conducted a study in 2017 to confirm the efficacy of C. bonduc and Cyclea peltata extracts on diabetic rats' experimentally generated excision wounds. For a period of 15 days, a methanolic and ethyl acetate extract of the test sample was administered in a PEG base and the wound healing effect was observed. Their findings revealed statistically significant wound contraction in the treated sample of up to 98%, compared to 90% in the diabetic control group. Their findings were connected to fasting blood glucose levels, demonstrating that hyperglycemia negatively affected wound healing. In animals treated with a high dose (100 mg/kg b.w) of methanolic extract of the aerial part of Cyclea peltata and the root of C. bonduc, histopathological investigations revealed mild granulation with significant epithelial enclosing and moderate hyperplasia. According to their findings, tested plant extracts enhance wound healing in diabetic mice, paving the path for further extensive investigation on the phytochemical ingredient for future medicinal uses. This investigation adds to the body of knowledge in the field of therapeutic medicine and may even serve as the foundation for the development of herbal-based gel formulations or ointments for treating diabetic wounds, avoiding the need for synthetic medications and their accompanying adverse effects [125].
Nootropic activity
This research group determined the effectiveness of dried seed kernels of C. bonduc extract as a learning and memory enhancer. The amnesic effect of scopolamine was reduced in mice using an aqueous extract of dried C. bonduc seed kernels. The aqueous extract of dried seed kernels of C. bonduc was compared to the standard drug piracetam in scopolamine-induced amnesia in mice using the redial arm maze and Morries water maze as exteroceptive behavioural paradigms. Finally, the statistical analysis was used to test the Morris water maze model for learning and memory and the radial arm maze model for learning and memory retention [126].
Cardioprotective property
Kumar et al. investigated the alcohol and aqueous seed extracts of C. bonduc in albino rats for their preventive properties against isoproterenol-induced myocardial infarction in 2013. The induced heart injury resulted in higher enzyme levels in the serum and increased lipid peroxide and lower glutathione levels in the heart homogenate. The increased enzyme levels in the serum and heart homogenate were dramatically reduced after pre-treatment with the extracts at a dose of 400 mg/kg orally for 30 days. Histopathological analysis revealed that the extract provided significant protection against cardiac necrosis [127].
Effects on muscle contraction
The effects of Caesalpinia crista extract on gallamine-induced relaxation in rat tibial muscular contractility were investigated using isometric-tension-anesthetized male rats aged 10 to 12 weeks. Intravenous administration of C. bonduc extract increased twitch contractions in a dose-dependent manner. The ED50 value was 2.75 × 10-4 g/kg bw. However, using C. bonduc extract or neostigmine abolished the effects of gallamine or puff adder venom on relaxation. According to the researchers, C. bonduc extract stimulates muscle contractile function by activating the cholinergic system [128].
In isolated pregnant rat myometrium preparations, C. bonduc leaf extract's calcium dependency and cholinergic action were investigated. In isolated strips, C. bonduc leaves extract increased contractile force in a concentration-dependent way. The results were similar to those seen with acetylcholine. In the presence of atropine, contractions elicited by C. bonduc leaf extract or acetylcholine were suppressed. Cholinergic receptors may be involved in the stimulating effects of C. bonduc leaf extract on contractile responses in isolated myometrium preparations. The leaf extract of C. bonduc elicited a tonic contraction (contracture) of the muscle in a calcium-free solution. Furthermore, the leaf extract of C. bonduc produced uterine smooth muscle contracture in a high-potassium calcium-free solution. In a calcium-free solution containing EDTA or EGTA, the leaf extract of C. bonduc was still able to induce contractions. These findings point to the existence of cholinergic receptors sensitive to C. bonduc leaf extract, which may influence calcium influx (phasic contraction) and calcium mobilization from cellular stores (tonic contraction), both of which are involved in the increase of contractile activity and development of uterine smooth muscle contracture [129].
C. bonduc extract inhibited spontaneous and high K+ (80 mM)-induced contractions of isolated rabbit jejunum preparations in a concentration-dependent manner, similar to verapamil [130].
Antiamyloidogenic and nootropic
Alzheimer's disease is thought to be caused by the generation and deposition of amyloid beta (A) peptides, which cause memory loss, cognitive dysfunction, and behavioural changes [131]. The ability to assess medicines for antiamyloidogenic characteristics using self-assembling monitoring of an in vitro. The thioflavin-T assay and transmission electron microscopy were used to investigate the effects of C. bonduc aqueous leaf extract on the development of oligomers and aggregates from monomers, as well as the generation of fibrils from oligomers [132]. The extract was found to be capable of inhibiting an aggregation from monomers and oligomers, as well as disaggregating pre-formed fibrils. The radial arm maze and the Morris water maze were used to test the aqueous seed extract of C. bonduc as a learning and memory enhancer (a nootropic medication) in rats with scopolamine-induced amnesia [133]. In the arm maze, mice given 50 and 150 mg/kg of the extract had memory retention of 33 and 45%, respectively, compared to 26% in the amnesic group. Learning performance was 65 and 58 seconds on average for three successful trials, compared to 113 seconds for the amnesic group. Memory retention was 39% and 52% in the arm maze, respectively, compared to 15% in the amnesic group, and learning performance was 33 and 23 seconds, respectively, compared to 38 seconds in the amnesic group.
Hepatoprotective activity
Hepatotoxicity is one of the unavoidable side effects of long-term medication use in a variety of conditions, persistent alcohol consumption, and certain infectious diseases. Despite the fact that there are few effective medications to treat such hepatotoxicity, hepatotoxicity-related mortality is rising. As a result, in our search for a more effective alternative, we discovered that C. bonduc, a shrub that grows in hotter parts of South Asia, has been successfully utilized in folk medicine to treat hepatotoxicity. Kumar et al. found that the methanol extract of C. bonduc exhibited hepatoprotective and antioxidant effects in Wistar albino rats. This study aimed to see if C. bonduc has hepatoprotective and antioxidant effects on rats with carbon tetrachloride-induced liver injury. Carbon tetrachloride (CCl4) (30% CCl4, 1 mL/kg b. wt. in liquid paraffin) was given to the rats in three doses (i.p.) at 72-hour intervals in this investigation. The CCl4-treated rats were next given methanol extract at dosages of 50, 100, and 200 mg/kg, and silymarin 25 mg/kg. In hepatotoxicity-induced rats, the effects of methanol extract and silymarin on Serum Glutamyl Pyruvate Transaminase (SGPT), serum glutamyl oxalacetic acid transaminase (SGOT), Serum Alkaline Phosphatase (SALP), bilirubin, uric acid, and total protein were assessed. Further, they measured effects of the extract on Lipid Peroxidation (LPO), enzymatic antioxidant (Superoxide Dismutase (SOD) and Catalase (CAT)), and non-enzymatic antioxidant [glutathione (GSH), vitamin C and vitamin E] were assessed. The methanol extract, as well as silymarin, had a substantial hepatoprotective impact by lowering serum enzyme activity, bilirubin, uric acid, and lipid peroxidation, as well as increasing SOD, CAT, GSH, vitamin C, vitamin E, and protein levels in a dose-dependent manner. It can be concluded from these findings that methanol extract has substantial hepatoprotective and antioxidant effects [134].
Sumalatha et al. tested the hepatoprotective properties of aqueous extract of C. bonduc in a rat model induced by carbon tetrachloride (CCl4) in 2014. In CCl4-induced hepatotoxic rat models, increased levels of blood ALT, AST, and ALT enzymes were observed. When these animals are given C. bonduc either before or after the induction of hepatotoxicity, ALP, AST, and ALT levels in their blood are significantly lower than in the untreated hepatotoxic group. Hepatotoxic rats also have necrotic alterations and vacuolation in their hepatocytes, as well as altered hepatic architecture and congested hepatic sinusoids. When these mice were given C. bonduc, however, such histological alterations were reduced. Thus, C. bonduc functions as a preventive and curative hepatoprotector, according to the findings of this study [135].
Kumar et al. explored the hepatoprotective properties of C. bonduc leaves, stem bark extract, and phytoconstituents in CCl4-induced rat models in 2015. CCl4 was administered to induce hepatotoxicity in Wistar albino rats of either sex, weighing around 180-200 g. For a period of 15 days, animals were given various leaves, stem bark extracts (300 mg/kg b.wt.) and the isolated phytoconstituent (70 mg/kg b.wt.) orally once a day. Except for the vehicle control group (15% (v/v) DMSO, 1 mL/kg body weight, p.o.), all groups received a 50% CCl4 intraperitoneal dosage every 72 hours. Animals were sacrificed via cervical decapitation at the end of the experiment. Biochemical markers were determined using serum and liver samples. The animals treated with stem bark chloroform extract showed a significant amelioration effect, with elevated levels of MDA and lowered levels of SOD, CAT, GPx, and GST. The isolated phytoconstituent SC2 (Methyl (4E)-5-{2-[(1E)-buta-1,3- dien-1-yl]-4,6-dihydroxyphenyl}pent-4-enoate) was more significant by reducing the serum markers level similar to standard drug silymarin. In the liver sections of rats treated with stem bark chloroform extract and its isolated compound SC2 against the toxicant CCl4, normal hepatic architecture, absence of necrosis, and few fatty lobules were observed. Hepatoprotective properties of C. bonduc extract and isolated phytoconstituent [136].
Antipyretic and analgesic activity
C. bonduc seed kernel extract has been shown to have antipyretic and analgesic properties by Archana et al. Adult albino rats or mice of either sex were given ethanol extract of C. bonduc seed kernel for antipyretic and antinociceptive effects at concentrations of 30, 100, and 300 mg/kg orally. In experimental rats, the ethanolic extract has significant antipyretic efficacy against Brewer's yeast-induced pyrexia. In hot plate and tail flick procedures, the extract demonstrated significant central analgesic efficacy. Furthermore, both the acetic acid-induced writhing test in mice and the Randall-Selitto assay in rats showed a significant peripheral analgesic effect. It also inhibited mice from licking their hind paws after being injected with formalin. After analyzing all of their findings, it can be concluded that the ethanolic extract of C. bonduc seed kernel has significant antipyretic and antinociceptive properties, and thus could be used to treat pain and pyretic illnesses in the future.
In 2015, Shruti et al. investigated the antipyretic, antiinflammatory, and analgesic effects of an ethanolic extract of C. bonduc whole seeds in albino rats. Three doses of the whole seed ethanolic extract produced as a suspension in 2 ml of 2% gum acacia were used: 100, 200, and 400 mg/kg. Carrageenan-induced paw edema and brewer's yeast-induced pyrexia models were used to assess acute inflammatory and antipyretic effects in experimental animals. When compared to control animals, experimental animals had a substantial reduction in paw volumes and pyrexia. In terms of paw edema, percent writhes inhibition, and rectal temperature, the ethanol see extract (400 mg/kg) showed anti-inflammatory, antipyretic, and analgesic properties in vivo, with reductions of (0.24 ± 0.03), (31.38%), and (36.2 ± 0.1), respectively. At all concentrations tested, the whole ethanolic seed extract demonstrated significant anti-inflammatory, antipyretic, and analgesic effects. Their research findings convincingly showed C. bonduc's ethno-medicinal potential in treating pain and inflammation-related illnesses, verifying its efficacy as a natural analgesic, antipyretic, and antiinflammatory agent [137].
Animal toxicity studies of C. bonduc
The acute toxicity investigations of C. bonduc extract in albino mice were reported by Muddapur et al. To evaluate the toxicity experiments in mice, various doses of extract ranging from 5 to 2000 mg/kg bodyweight were given orally to the test groups of mice, while distilled water was provided to the control group. In the treated group, histological investigations of the liver, heart, and kidney revealed minimal or minor changes. Tubular congestion, tubular desquamation, glomerular congestion, and interstitial inflammatory cells of the test animal, on the other hand, showed a modest change in liver cellular architecture. The extract's LD50 was found to be greater than 2000 mg/kg, and no alterations in behavioural parameters in mice were detected [138].
Preeja et al. investigated the acute and subacute toxicity of C. bonduc methanolic extract in albino mice. The acute toxicity experiments were carried out in accordance with OECD standards 420, with a limit test dose of 2000 mg/kg. After 2 hours, 4 hours, and 8 hours of therapy, the animals were evaluated for respiration rate, heart rate, and behavioural indications such as lethargy, reduced locomotor activity, and licking every seven days. Three groups of six mice were given distilled water (control), 200, and 400 mg/kg of extract orally every 24 hours for 28 days in sub-acute toxicity experiments. The results show that after 28 days of therapy, there was no significant difference in body and organ weights between the control and treated groups. Furthermore, the extract had no harmful effects according to the hematological analysis and clinical blood chemistry. Gross abnormalities and histological alterations were not recorded in pathological examinations. Furthermore, no deaths were reported throughout the study [139].
The tribal inhabitants of the Kolli Hills in Tamil Nadu, India, utilize C. bonduc leaves in combination with other herbs to treat tumors, liver issues, inflammation, and other ailments. These plants were said to have anticancer properties in ancient Ayurvedic medicine. The haematology and hepatorenal function of C. bonduc were studied by Kumar et al. Because there are no scientific data on the toxicological characteristics of this plant, our group's research focuses on sub-chronic toxicity tests of C. bonduc methanol extract in Swiss albino mice. Swiss albino mice were given the methanolic extract intraperitoneally twice a week for thirteen weeks. At dosages of 100 and 200 mg/kg body weight, no significant changes in haematological, biochemical, or histological markers were seen in the treated groups after therapy. The administration of 400 mg/kg body weight of methanolic extract increased serum enzyme levels and changed haematological parameters. These findings revealed that mice were unaffected by methanolic extract at levels of 100 and 200 mg/kg body weight. Only at a dose of 400 mg/kg body weight was an adverse effect reported [140].
In Wistar female albino rats, Lilaram & Ahamed investigated the effect of ethanolic seed extract of C. bonduc on the histoarchitecture of various essential organs and clinical chemistry. The participants were placed into four groups for the study. Group I was given distilled water as a vehicle-treated control group. Groups II, III, and IV were given a 100, 200, or 300 mg/kg b.wt dose of seed extract orally for 10 days and then euthanized 24 hours following the final treatment. The vital tissue histoarchitecture in the treated groups looked to be normal. In all of the seed extract treated groups, hematological examination revealed a significant rise in RBC count, various types of WBCs, platelet count, haemoglobin levels, and packed cell volume levels. In all the treated groups, serum biochemistry revealed significantly lower cholesterol, triglycerides, and creatinine levels, whereas HDL levels were significantly higher. Our findings imply that the ethanolic seed extract of C. bonduc contains chemical compounds with cytoprotective potential, and that it should be investigated further as a natural medicine source [141].
Nutritional values
C. bonduc has been found to possess the following nutrients: crude fibre 12.79-14.07%, protein 18.65-20.32%, fat 6.54-7.23%, carbohydrate 16.91-18.56%, food energy (Kcal/100 g) 376.27-402.12, and food energy (Kcal/100 g) 376.27-402.12. Calcium ranges from 0.150% to 0.184%, Phosphorus from 0.17% to 0.22%, Sodium from 0.07 to 0.08%, Iron from 0.22% to 0.5%, Vitamin C from 0.016 to 0.043 (IU/g), and Vitamin A from 416.75 to 700.14 (IU/g).
Modern health care facilities with expensive drugs are still out of reach for the rural segment in a developing country. As a result, it's important to evaluate into herbal medicine possibilities. As a result, ethnomedical studies have received a lot of interest because they reveal a lot of recognized and unknown medical virtues, mainly of plant origin, that need to be studied using current scientific methods such phytochemical analysis, pharmacological screening, and clinical investigations. Numerous studies involving Caesalpinia species have confirmed their efficacy as a natural source of new chemical entities and therapeutical applications, revealed their structural diversity, demonstrated useful properties for the development of traditional medicines, and produced scientific guidance for the use of herbal medicines. However, of the approximately 500 species of this genus found around the world, only around 30 have been examined in terms of phytochemicals and pharmacological properties. C. bonduc was one of the most prominent of them. Antiulcer, anticancer, anti-diabetic, anti-inflammatory, anti-rheumatic, antimicrobial, antibacterial, and cytotoxic properties were all demonstrated by C. bonduc. As stated in this review, C. bonduc has a number of essential pharmacological properties. As a result, the data accessible in the literature to far, when combined with the diversity of metabolites, clearly show that chemical/pharmacological research of C. bonduc could lead to novel drug prototypes. Furthermore, more pre-clinical and clinical investigations and the implementation of stronger quality control systems are required to understand this plant's untapped potential fully.
The authors would like to thank Managing Director and Director Pankajakasthuri Herbals India Pvt. Ltd., Poovachal, Kattakada, Trivandrum, India for providing the necessary infrastructure, facilities and financial support. The authors thank Dr. Suresh Kumar C MD (Ayurveda), Professor, Department of Shalyatantra, Pankajakasthuri Ayurveda Medical College & P.G. Centre, Killy, Kattakada, Thiruvananthapuram, Kerala, India and Prof. (Dr.) K.G. Revikumar, Former Chief & Head, Clinical Pharmacy, Govt. Medical College Hospital, Trivandrum, Kerala, India for their valuable technical advice.
The authors have not declared any conflict of interests.
Citation: Sasidharan S, Srinivasa Kumar KP, Kanti Das S, Hareendran Nair J (2021) Caesalpinia bonduc: A Ubiquitous yet Remarkable Tropical Plant Owing Various Promising Pharmacological and Medicinal Properties with Special References to the Seed. Med Aromat Plants (Los Angeles) 10: 394.
Received: 26-Jun-2021 Accepted: 09-Jul-2021 Published: 16-Jul-2021 , DOI: 10.35248/2167-0412.21.10.394
Copyright: © 2021 Sasidharan S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.