Research Article - (2020)Volume 6, Issue 1
Bioremediation of Hydrocarbons from Kaduna Refining and Petrochemical Company Effluents Using Cladosporium
Magdaline Joseph Kwaji1*, Martha Onyinoyi Ahmadu2, Babalola Ayoade D3, Oghaego I Cyprian1 and Joy OgheneOchuko Ighodaye1Abstract
Bioremediation is the most effective management tool to manage the polluted environment and recover contamination. The process of bioremediation uses various agents such as bacteria, fungi, algae and highier plants as major tools in treating heavy metals present in the environment. Bioremediation both in situ and ex situ have also enjoyed strong scientific growth in parts due to the increase of natural attenuation, since most natural attenuation is due to biodegradation. Numerous works have been devoted in literature to biodegradation with the use of fungi. Therefore, in other to have clean and less expensive technologies to restore contaminated marine environment, bioremediation is one of the preferred approaches, because it is simple to maintain, eco-friendly, cost effective and may lead to complete or partial removal of pollutants. The aim of this research study is to bio remediate hydrocarbon from hydrocarbon contaminated water using Cladosporium spp. This study isolates and characterize Cladosporium spp. from hydrocarbon contaminated water samples and also use the isolate in bioremediating the hydrocarbon in the water. Bioremediation of the hydrocarbon contaminated water sample was achieved given the values of total hydrocarbon concentration before and after sterilization of samples. The Total hydrocarbon concentration decreased for both the sterilized and non-sterilized sample after bioremediation. Point A shows a decrease in concentration from 1.87-1.41 mg/l and 1.87-0.70 mg/l for sterilized and unsterilized samples respectively. It could also be depicted from this work that Cladosporium functions more effectively in the presence of other microorganisms since it has more effect in non-sterilized sample than the sterilized sample as clearly shown in the percentage removal (56.00%).
Keywords
Bioremediation; Mycelium; Hypercumulators; Hydrocarbons
Introduction
Bioremediation is defined as a process by which microorganisms are stimulate to rapidly degrade hazardous organic pollutants to environmentally safe levels in ground water. Microorganisms find food they eat in the oil or water where they live. However if a contaminant is present it can become an additional food source for the microrganisms. The contaminats serve two useful purpose for the microbes. First the contaminants provide a source of carbon needed for growth. Second, the microbes obtain energy by breaking chemical bonds and transferring electrons away from the contaminants. This is known as an oxidation reduction reaction. The contaminant that loses electron is oxidized while that which accepts is reduced. Mycoremediation is a form of bioremediation where fungi is used to degrade or sequester contaminants in the environment [1]. Fungal Mycelium Stimulates microbial and enzyme activity, reduces toxins, are hypercumulators and are capable of absorbing and concentrating hydrocarbons in their mushroom fruiting bodies .The decomposition of the fungi is usually performed by the mycelium .The mycelium secretes extracellular enzymes and acids that breaks down the organic matters [2]. Petroleum-based products are the major source of energy for industry and daily life. Leaks and accidental spills occur regularly during the exploration, production, refining, transport and storage of petroleum products. The amount of natural crude oil seepage was estimated to be 600,000 metric tons per year with a range of uncertainty of 200,000 metric tons per year. Release of hydrocarbons into the environment whether accidentally or due to human activity is a main cause of water and soil pollution. Soil contamination with hydrocarbons causes extensive damage of local system since accumulation of pollutants in animal and plant tissue may cause death or mutations. The technology commonly used for the soil remediation includes mechanical, burying, evaporation, dispersion, and washing. However, these technologies are deficient. The process of bioremediation using microorganisms to detoxify or remove pollutants owing to their diverse metabolic capabilities is an evolving method for the removal and degradation of many environmental pollutants including the product of petroleum industry [2]. In 2007, a similar method was used in San-Francisco. Oil had contaminated the shoreline after a cargo ship spilled 58,000 gallons of heavy oil. An experiment was designed that collected water samples from this shoreline and, the water was layered with oyster mushroom and straw: the mushrooms broke down the oil and after several weeks, the resulting soil was clean enough to be used for roadside landscaping. Bioremediation functions basically on biodegradation, which may refer to complete mineralization of organic contaminants (hydrocarbons) into carbon dioxide, fatty acid, water and inorganic compounds, and cell protein or transformation of complex organic contaminants to other simpler organic compounds by biological agents like microorganisms. Similar to the microbial mediated breakdown of organic matter, biodegradation mediated by indigenous microbial communities is the ultimate fate of majority of oil hydrocarbons. Fungi possess decomposing abilities to deal with the array of naturally occurring compounds that serve as potential carbon sources [3]. Hydrocarbon pollutants have similar or analogous molecular structures which enable the fungi to act on them as well. When an area is contaminated, the ability to deal with the contamination and turn it into an energy source is selected for within the fungal population and leads to a population that is better able to metabolize the contaminants [4]. Microorganisms with the capacity to degrade hydrocarbons are among the best microbial group in applied and environmental microbiology. Much progress has been made to determine the response of specific fungi taxa to oil contamination in aquatic environment impacted by oil spills [5].
The increased discharge of petroleum hydrocarbons in marine environment such as; diesel, crude oil and some distillate, is a major global concern, due to their toxic effects in fragile and sensitive environments of these area[6]. Petroleum substances are the main sources of pollutants stored in old waste pits. They cause degradation of biological life in the area of storage. Multicriteria effectiveness estimation of petroleum pollutant biodegradation in tested waste enabled determination of a role of fungi isolated from the waste during purification.
Oil waste results in significant and increasing biological degradation of an active surface of the earth. Nowadays, acceleration of petroleum hydrocarbon biodegradation through biotechnological processes with the use of effective fungal cultures (isolated from severely contaminated areas) has been among the most common research due to relatively low cost and high effectiveness. However, a huge amount of petroleum pollutants in weathered drill waste stored in pits causes difficulties in bioremediation works. Therefore, it had been claimed that the technological concept of waste purification, based on stage application of consecutive purification processes, will enable a gradual decrease in petroleum contaminant levels. This will lead to the use of successive methods for deeper purification of a polluted area.
Hydrocarbons are hydrophobic compounds and their persistence in the environment is chiefly due to their low water solubility. Hydrocarbons can be broadly classified into four types; saturated, unsaturated, cycloalkanes and aromatic hydrocarbons. Petroleum is a natural product, resulting from the anaerobic conversion of biomass under high temperature and pressure. Most of its components are subject to biodegradation, but at relatively slow rates. Crude oil is a highly toxic mixture of more than one thousand 1000 different hydrocarbons. An accidental spill causes severe contamination of marine ecosystems. Contamination due to spill of processed petroleum derivatives is an important problem in waters, generated from both natural and anthropogenic processes. These pollutants can be degraded by a great variety of soil and aquatic microorganism. Cladosporium spp. are known to be important hydrocarbon degraders. In most studies, degradation of crude oil by various species of fungi has been reported.
Justification
Cladosporium spp. enhances the bioremediation of large concentrations of hydrocarbon contaminants. Cladosporium spp. are densely populated in areas contaminated with hydrocarbons. Research had shown that Cladosporium spp. isolated from North pacific coast exhibited a high degrading ability after being cultured on media containing hydrocarbon. All components of crude oil are degradable by fungi, though at varying rates, [4] fungi mineralize them to nontoxic substances. The Cladosporium spp. are the best in terms of adaptability which is as a result of their flexibility in morphology and life cycle.
These advantages have placed Cladosporium as one of the best hydrocarbon degrading fungi. All components of crude oil are degradable by fungi, though at varying rates, and a variety of yeast and bacteria can also metabolize petroleum hydrocarbons. The World Health Organisation standard limit (2011) for total hydrocarbons concentration in water is 0.30 mg/l and this must not be exceeded for a safe water quality. In aquatic systems, hydrocarbons adsorb to suspended matter or sediments where they persist. Although most hydrocarbons are emitted to the atmosphere, sediments are the major environmental sink for these compounds. Based on these problems, it had been concluded that polycyclic aromatic hydrocarbons are entering the water in quantity or concentration or under condition that may have harmful effects on the environment thereby altering the water environment with regards to consumption. Pseudomonas are capable of utilizing hydrocarbons as energy and carbon sources producing bio surfactants [7] while Cyanobacteria on the other hand oxidizes alkanes to fatty acids and fungi mineralize them to nontoxic substances. Some chemical technologies were also developed and applied but due to their cost-sensitivity, technological complexity and increased risk exposure to contaminants for both workers at the site and nearby residents, these approaches were not widely accepted. Hence bioremediation seems to be only better option for the remediation of polluted sites [8].
In this work, fungi, will be used and tested for bioremediation of the hydrocarbon contaminated water.
Description of Study Area
Kaduna South Local Government area (LGA) is one of the 23 LGA’S of Kaduna state. It is surrounded by Kaduna North LGA to the north, Igabi LGA to the west and Chikun LGA to the south and east. It is located approximately between Latitude 90 54′ and 100 29′ N and between Longitude 60 59′ E and 80 09′ E.
It is one of the most industrialized cities in Nigeria. The entire land structure has an undulating plateau with many rivers including river Romi. There are basically two distinct marked seasons; rainy (April-October) and dry (November- March) with an average rainfall of 1000mm.On the average, this region enjoys a rainy season of about 5 months.
All Samples were collected at Kaduna Refining and Petrochemical Company, Kaduna. The Refinery occupies an area of 2.89 Square Kilometers, next to Ungwan Tanko and is located at KM 16 Kachia Road, Southern Kaduna, Kaduna State.
All the sampling points represented as A (point of discharge into the pond), B (point midway from the point of discharge) and C (the termination point) as shown in Figure 1.
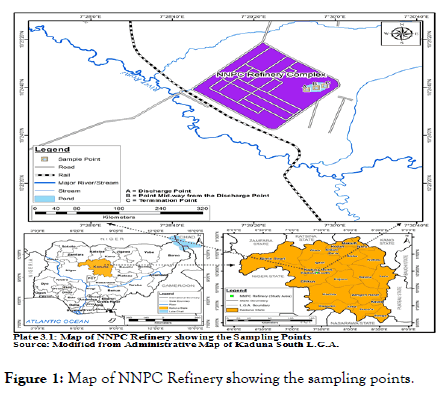
Figure 1. Map of NNPC Refinery showing the sampling points.
Methodology
Collection of samples
Hydrocarbon contaminated water samples were collected from the sampling area in clean sterile containers. The hydrocarbon contaminated water samples were collected by suspending the containers gently at a distance 15 cm from the surface of the pond.
Preparation of media used in the identification and characterization of Cladosporium from hydrocarbon contaminated water [9]
A portion (38 g) of Potato Dextrose Agar was suspended in 1 L of distilled water. This was boiled thoroughly and allowed to dissolve completely. A portion (10 ml) of it was then suspended on a sterile plate and was allowed to cool and solidify.
Total hydrocarbons concentration
To obtain the Total hydrocarbon concentration, the sample was properly mixed by shaking the bottle containing the sample, 50 ml of the samples was transferred into a beaker, the pH was adjusted to 2 or less to acidify the sample, the acidified sample was transferred into a 1000 ml graduated separatory funnel, 20 ml of tetrachloroethylene was added to the sample in the separating funnel, the separatory funnel was covered and shaken vigorously, the cap was removed at intervals to release pressure build up, the separatory funnel was kept standing to allow the content to settle and separate, all emulsions were allowed to break before drawing the extract, the bottom layer was transferred into a clean 50 ml volumetric flask. Another 20 ml of tetracloroethylene was added into the sample in the separatory funnel, the sample was fed into the quartz cell reagent to establish background spectra. The absorbance was recorded and the values obtained were entered into a graph to obtain the resultant concentration.
Isolation and characterization of Cladosporium spp. from hydrocarbon contaminated water samples (Khan et al., 2013)
A portion (1 ml) of hydrocarbon contaminated water samples was mixed and carefully filtered to remove unwanted debris using a sieve. This was transferred using a sterile pipette into a sterile test tube containing 9 ml of sterile distilled water. This gave a 10-1 dilution, and subsequently, three-fold (10-3) serial solutions were prepared from the 10-1 dilution. Diluted samples (1 ml) were poured on Potato Dextrose Agar plates and Sabouraud Dextrose Agar plates. Streptomycin (500 mgl-1) as an antibiotic to inhibit bacterial growth was added to media after sterilization [10]. Then, the plates were incubated at temperature 28 to 31°C for 72 h. To obtain pure cultures of the fungal isolates, fungal cultures were aseptically subcultured into fresh plates and incubated until the fungus began to sporulate and produce hyphae, followed by subsequent subculturing to obtain pure cultures consisting of only one type of fungal isolate. The pure isolates were identified appropriately using a microscope.
Colony growth was observed visually 3 days post incubation from the PDA and SDA plates using the method described by Larone [11].
Isolates subjected to further subculture on SDA and PDA plates were later observed after a period of incubation (3 days) under the microscope at 40 x magnifications. [12,13].
Bioremediation was carried out at room temperature using Singh Methodology [14].The water samples collected were initially sterilized by autoclaving at 121°C for 15 minutes, the isolate was introduced into the water sample using a sterile inoculation loop with internal diameter of 4 cm where each of the sampling bottles were innoculated with 3 loop full of the isolates, and these strains were allowed to grow respectively under white fluorescent light (to enhance spore formation) within the period of 10 days. Cladosporium isolates completely colonized the hydrocarbon contaminated water sample within 10 days of bioremediation.
Total hydrocarbon degradation in water sample was determined by analysing the concentrations of total hydrocarbons in the treated and untreated hydrocarbon contaminated water sample following the steps described earlier in 3.6.
Results and Discussion
Total hydrocarbon concentration
The highest concentration was recorded at point B (1.87 mg/l) with the lowest concentration at point C (0.40 mg/l) before bioremediation, but was highest at point A (1.41 mg/l) and lowest at point C (0.45 mg/l) with the introduction of the Cladosporium isolate (bioremediation) for both sterilized and unsterilized samples. The percentage removal was positive at point A (56.70%) with non-sterilized sample and negative at point B (-11.10%) with non-sterilized sample (Table 1).
| Absorbance | Before | Absorbance | After | Absorbance | %Removal(S) | (%Removal) (NS) |
|---|---|---|---|---|---|---|
| A 0.004 | 1.87 | 0.02 | 1.405 | 0.015 | 14 | 56 |
| B 0.035 | 1.64 | 0.025 | 1 | 0.03 | 45 | -11 |
| C 0.001 | 0.4 | 0.02 | 1.171 | 0.002 | 16 | -33 |
Table 1: Total Hydrocarbon Concentration in (mg/l) Before and After Bioremediation.
Key: mg/l- milligram per litre A, B, C refer to the sampling points; NS: Non Sterilized; S: Sterilized; S: Sample.
Isolation and characterization of Cladosporium spp. from hydrocarbon contaminated water samples 4.2.1 colony growth
Colonies ranging from grey to brown in color with distinct and erected elongations were isolated as shown in Figure 2.
Figure 2. Plate showing the growth of Cladosporium spp. on Potato Dextrose Agar.
Pure Black colonies with distinct and erected elongations after subculture on SDA plate were observed as shown in Figure 3.
Figure 3. Pure Isolate of Cladosporium spp. on Sabouraud`s Dextrose Agar.
Microscopic morphology
The observation of the isolate (Plate 4.2) under microscope revealed tangled masses of thin stalks (hyphae) with spongy appearance at (40x) objective as presented in Table 2 and Figure 4.
Figure 4. Growth of Cladosporium spp. on Potatoe Dextrose Agar at (40x) objective.
| Sample code | Growth on SDA | Hyphae | Inference |
|---|---|---|---|
| B | Black, cottony, powdery | Non septate, Vegetative, spongy | Positive |
Table 2: Growth of Hyphae on Sabraud Dextrose Agar (SDA).
Isolation and characterisation of Cladosporium isolate
Cladosporium spp. was isolated from the water sample. This is due to the fact that the organism is mostly associated with oil contaminated water. This is in agreement with the findings of Jesubunmi, (2014) where a low isolation rate of fungi from oil water source was reported. Kinglsey et al. (2015) in their study on the Isolation and Characterization of Microorganisms from Oil Polluted Soil in Kwata, Awka South, Nigeria also recorded the isolation of Cladosporium spp. among other organisms.
Microscopy revealed colonies with the same color as the mother culture. Vegetative hyphae and Spores were visible using (40x) objective. Spores were distinct from the vegetative hyphae, straight and were branched in the apical region with elongations. This is in agreement with the findings of Kingsley et al., (2015) where conidiophores with dark hyphae and sparsely branching long chains of conidia were observed.
Bioremediation of hydrocarbon contaminated water using Cladosporium isolate
Bioremediation of the hydrocarbon contaminated water sample was achieved given the values of physicochemical parameters, heavy metals and total hydrocarbon concentration obtained after bioremediation. Cladosporium isolates exhibited degrading potential for hydrocarbons. The concentrations reduced after treatment with Cladosporium spp. which shows that bioremediation was achieved and further demonstrates how the introduction of Cladosporium spp. (bioremediation) reduced the concentration of hydrocarbons and heavy metals in the sampling points.
Conclusion
Total hydrocarbon concentration after bioremediation
The Total hydrocarbon concentration decreased for both the sterilized and non-sterilized sample after bioremediation. Point A shows a decrease in concentration from 1.87-1.41 mg/l and 1.87-0.70 mg/l for sterilized and unsterilized samples respectively. Similarly, a decrease was observed in point B with sterilized samples from 1.64-1.17 mg/l for sterilized samples and 1.64-0.94 mg/l for non-sterilized samples. It can be inferred from these observations that Cladosporium spp. has potential to degrade hydrocarbons and it is in agreement with similar findings reported by Mohan and Strisvatra (2010). The decrease was higher with unsterilized samples than sterilized samples which depicts that the fungal isolate (Cladosporium spp.) could have a higher biodegrading potential in unsterilized samples than sterilized sample. It could also be depicted from this that Cladosporium functions more effectively in the presence of other microorganisms since it has more effect in non-sterilized sample than the sterilized sample as clearly shown in the percentage removal (56.00%). This suggests that treatment of samples could inhibit the biodegrading potential of Cladosporium spp. which is in agreement with the findings of Vincent et al (2014). The decrease in total hydrocarbon concentration in samples from point A was higher than the decrease in samples from point B. This could be as a result of treatment or chemical dispersant at this particular point which might have affected the hydrocarbons, and in turn the degrading ability of the Cladosporium spp. At point C, The total hydrocarbon concentration increased after bioremediation (0.40-0.45 mg/l for sterilized sample and 0.40-0.80 mg/l for non-sterilized samples). This could be as a result of other fungi which are able to synthesize the hydrocarbons. It could also be that as the Cladosporium spp. is breaking down the hydrocarbons, other microbial activities occurs such as the synthesis of hydrocarbons by other hydrocarbon synthesizing microbial consortium (Juhi Saxena, 2014).
References
- Margesin R, Hammale M, Tscherko D. Microbial activity and community composition during bioremediationof diesel oil contaminated soil: effects of hydrocarbons on incubation time, fetilizers and concentration. Microb Ecol. 2007;53 2:259-269.
- Medina-Bellver JI, Mann P, Deglado AA, Rodriguez-Sanchez E, Reyes JL, et al. Evidence for insitu crude oil biodegradation after the prestige oil spill. Environ Microbiol. 2005;7 6:773-779.
- Wolicka D, Suszek A, Borkowski A, Bielecka A. Application of aerobic microorganisms in bioremediation in situ of soil contaminated by petroleum products. Bioresour Technol. 2009; 100 13:3221-3227.
- Fernández-Luqueño F, Valenzuela-Encinas C, Marsch R Martínez-Suárez, C Vázquez-Núñez E, Dendooven L. Microbial communities to mitigate contamination of PAHs in soil-possibilities and challenges: a review. Environ Sci Pollut Res Int. 2011;1: 12-30.
- Berthe-Corti, L Nachtcamp, Timmis KNM. Bacterial communities in hydrocarbon contaminated marine costal environment. In handbook of hydrocarbon and lipid microbiology (springer Verlag; Berlin, Germany) 2010; pp: 2349-2359.
- Mohammed AZ, Hamidi AA, Mohammed HI. Effects of oil concentration and dispersion on crude oil. Biodegradation in contaminated sea water. Bull Environment Contaminated Toxicology.2010; 84: 438-442.
- Rahman KSM, Rahman TJ, Kourkoutas Y, Petsas I, Marchant R, et al. Enhanced bioremediation of n-alkane in petroleum slugde using bacterial consortium amended with rhamnolipid and micronutrients. Bioresour Technol. 2003;90 2:159-168.
- Rebeiro, JP, Lopes, Fachini, JA. Cleansing contaminated sea waters using marine cyanobacteria evaluation of trace metal removal from the medium. J Environment Analytical Chem.2008; 88: 701-710.
- Lee H, Jang Y, Choi YS, Kim MJ, Lee H. Biological procedures to select fungi white rot fungi for the degradation of Policyclic aromatic hydrocarbons.J basic microbial. 2014;53 2:195-199.
- Harrigan WF, McCance ME. Laboratory Methods of Food and Diary Microbiology. Academic Press, London.1990; p: 452.
- Larone DH. Medically important fungi. Atlas of Microbiology (4th ed). Amer Society Microbiol. 2002.
- Antonella A, Giovanna CV, Valeria FM. Isolation and identification of fungal communities in compost and vermicompost. Mycologia. 2005; 97 1:33-44.
- Roling WFN, Milner MG, Jones DM, Fratepietra F, Swannel RPJ, et al. Bacterial community dynamics and hydrocarbon degradation during a field scale evaluation of bioremediation in a mud flat beach contaminated with buried oil. Appl Environ Microbiol. 2004; 70 5:2603-2613.
- Singh G. Reoxidation of biogenic reduced uranium: A challenge toward bioremediation. Critical Reviews Environmental Sci Technol. 2014;44 4: 39.
Author Info
Magdaline Joseph Kwaji1*, Martha Onyinoyi Ahmadu2, Babalola Ayoade D3, Oghaego I Cyprian1 and Joy OgheneOchuko Ighodaye12Biochemistry Department, University of Ibadan, Ibadan, Nigeria
3Biodec Kano, National Biotechnology Development Agency, Bayelsa, Nigeria
Citation: Kwaji MJ, Ahmadu MO, Ayoade DB, Cyprian OI, Ighodaye JOO (2020) Bioremediation of Hydrocarbons from Kaduna Refining and Petrochemical Company Effluents Using Cladosporium. Appli Microbiol Open Access 6:167. Doi: 10.35248/2471-9315.20.6.167
Received: 16-Jan-2020 Accepted: 29-Jan-2020 Published: 05-Feb-2020 , DOI: 10.35248/2471-9315.20.6.167
Copyright: © 2020 Kwaji MJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.