Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2017) Volume 7, Issue 1
In chemical reactions, bifurcation points are determined by triangulation of a system. With the components interacting, a bifurcation sequence is formed, and the system evolves following one of the branches with formation of a stable compound. Transformations of the components at the point of bifurcation are determined according to "The Gibbs function normalized to the total number of electrons". All conversions are described by the chemical potential, affinity, thermodynamic force. Production of entropy, the flow of entropy and the reaction rate constant allowing the system to choose a specific path of evolutionary conversions have been calculated. The correlation dependence between the chemical potential, affinity, conversion temperature, production and flow of entropy, reaction rate constant is stated.
<Keywords: Non-equilibrium thermodynamics; Bifurcation; Chemical potential
In the systems far from equilibrium the procedure of a chemical reaction between the components does not always correspond to the macroscopic equations [1]. The elementary act of collision of the components and the nature of the reaction products being formed depend on the energy of the colliding molecules, on their correct spatial orientation. Collision of the initial components and formation of the reaction products are proportional to the motive force of the process which is normally perceived as affinity. According to de Donder [2], affinity is determined by the relationship of chemical potentials of substances undergoing a chemical reaction. If several reactions proceed in the system, affinity and transformations of the components must be determined separately for each reaction. In the chemical reaction at the bifurcation point, the system loses stability, and passes to a nonequilibrium state, which makes it possible to assume a new quality of evolution of the dynamic system. There takes place a change in the nature of motion and structure of the system. When the system moves to the bifurcation point, an important role is played by the chemical potential and affinity of the system. At the bifurcation point, the system begins to produce entropy, and the reaction procedure is characterized by a certain definite rate constant. It is these factors that the evolutionary path of the system after it passes the point of bifurcation. In the course of interaction of chemical reagents in a real system it is possible to determine all the paths of the system evolution. All evolutionary processes between two points of bifurcation obey regularities [3]. The instability, bifurcation, symmetry breaking are being actively studied on the basis of the general theory of stability of nonlinear differential equations [3-6]. For this, the basic relations characterizing the loss of stability, the ambiguity of solutions at the point of bifurcation, symmetry breaking, etc. are used. However, as noted by Prigogine, "nonlinear non-equilibrium thermodynamics is, first of all, thermodynamics of chemical reactions" [1]. Therefore, it is proposed to study non-equilibrium thermodynamics on the basis of chemical reactions. To determine the ways of the system evolution in chemical reactions, we use two concepts:
- “The Gibbs function normalized to the total number of electrons” [7,8].
- Triangulation of a multicomponent system [9].
The physical meaning of the new concept “The Gibbs function normalized to the total number of electrons”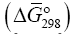 [7,8] is determined by the chemical bond as the collective effect of electronnuclear interaction. It is calculated by dividing the value of the Gibbs energy of formation of the compound by the total number of electrons in this compound, that is, it determines the density of the energy of the compound formation per electron. This function characterizes accumulation of the Gibbs energy density on molecular bonds. At the formation of bonds, “The Gibbs function normalized to the total number of electrons” adequately responds to all changes in the structure of the compound, allows to judge the reactivity of the compound its stability [8], to determine the value of the chemical potential and affinity, to calculate the production and flow of entropy, thermodynamic force and flow, thermodynamic "probability" of the interaction, the conversion degree of components in the course of a chemical reaction, to describe the processes of "self-organization" [10], to carry out triangulation of multicomponent systems and to determine mechanisms of reactions. “The Gibbs function normalized to the total number of electrons” is interpreted by us as the chemical potential of a substance, that is
[7,8] is determined by the chemical bond as the collective effect of electronnuclear interaction. It is calculated by dividing the value of the Gibbs energy of formation of the compound by the total number of electrons in this compound, that is, it determines the density of the energy of the compound formation per electron. This function characterizes accumulation of the Gibbs energy density on molecular bonds. At the formation of bonds, “The Gibbs function normalized to the total number of electrons” adequately responds to all changes in the structure of the compound, allows to judge the reactivity of the compound its stability [8], to determine the value of the chemical potential and affinity, to calculate the production and flow of entropy, thermodynamic force and flow, thermodynamic "probability" of the interaction, the conversion degree of components in the course of a chemical reaction, to describe the processes of "self-organization" [10], to carry out triangulation of multicomponent systems and to determine mechanisms of reactions. “The Gibbs function normalized to the total number of electrons” is interpreted by us as the chemical potential of a substance, that is 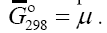 In accordance with the full quantum Boltzmann statistics for localized systems (for solid systems), particles lose their indistinguishability. By Liouville's theorem and the ergodic hypothesis which use canonic conjugated variables of coordinates and momentum, “The Gibbs function normalized to the total number of electrons” can represent a set of infinitesimal transformations. “The Gibbs function normalized to the total number of electrons”, being a quantity which characterizes the state of matter in equilibrium conditions, is regarded as the average of the functions of the coordinates and momenta of the system and allows to evaluate both the energy state and the rate of movement of separate parts of the system. Therefore, on the basis of the chemical potential (“The Gibbs function normalized to the total number of electrons”) the transformation of a substance in chemical reactions can be considered. The chemical potential μi is a partial molar quantity and one can speak about the chemical potential of a component at every point of the system in the same way as we speak about its concentration or mole fraction [11]. The processes of evolution in chemical reactions can be described on the basis of conversion of the phase composition of the system compounds as the causal relationship between the phases. This problem can be solved using the teaching of Kurnakov by triangulation of systems [9,12]. The plot structure-property is an exact geometric model of the complex function that displays the dependence between the temperature, volume, concentration and other physical and chemical factors that determine the state of an open system, i.e., in the diagram structure-property, chemical transformations of a substance and geometric transformations of space are inseparably linked. Using the diagram, it is possible to determine the boundaries of existence of different phases in the system, and identify not sharply pronounced processes and note weak interparticle interactions. On the basis of the continuity principle and correspondence principle Kurnakov proposed a method of triangulation of multicomponent systems and the possibility of determining the reaction mechanism [9]. Singular triangulation gives a geometric representation of the dissolution, combination, substitution, exchange reactions in a multicomponent system, i.e., it allows to determine the direction of the reactions, the nature and character of the separate phases in any part of the system, to make the conclusion on the nature of the system under study. When studying natural and artificial systems, triangulation allows to choose that part of the system which corresponds to the specified conditions by the optimal composition, temperature and chemical interaction [9,13]. The main feature of triangulation of systems is determination of the components that form only eutectic compositions. Triangulation of a system makes it possible to determine phase transitions of the system components in exchange, dehydration and polycondensation, hydration, conversion, complexation reactions, to predict all bifurcation paths of components as well as to predict the existence of new compounds.
In accordance with the full quantum Boltzmann statistics for localized systems (for solid systems), particles lose their indistinguishability. By Liouville's theorem and the ergodic hypothesis which use canonic conjugated variables of coordinates and momentum, “The Gibbs function normalized to the total number of electrons” can represent a set of infinitesimal transformations. “The Gibbs function normalized to the total number of electrons”, being a quantity which characterizes the state of matter in equilibrium conditions, is regarded as the average of the functions of the coordinates and momenta of the system and allows to evaluate both the energy state and the rate of movement of separate parts of the system. Therefore, on the basis of the chemical potential (“The Gibbs function normalized to the total number of electrons”) the transformation of a substance in chemical reactions can be considered. The chemical potential μi is a partial molar quantity and one can speak about the chemical potential of a component at every point of the system in the same way as we speak about its concentration or mole fraction [11]. The processes of evolution in chemical reactions can be described on the basis of conversion of the phase composition of the system compounds as the causal relationship between the phases. This problem can be solved using the teaching of Kurnakov by triangulation of systems [9,12]. The plot structure-property is an exact geometric model of the complex function that displays the dependence between the temperature, volume, concentration and other physical and chemical factors that determine the state of an open system, i.e., in the diagram structure-property, chemical transformations of a substance and geometric transformations of space are inseparably linked. Using the diagram, it is possible to determine the boundaries of existence of different phases in the system, and identify not sharply pronounced processes and note weak interparticle interactions. On the basis of the continuity principle and correspondence principle Kurnakov proposed a method of triangulation of multicomponent systems and the possibility of determining the reaction mechanism [9]. Singular triangulation gives a geometric representation of the dissolution, combination, substitution, exchange reactions in a multicomponent system, i.e., it allows to determine the direction of the reactions, the nature and character of the separate phases in any part of the system, to make the conclusion on the nature of the system under study. When studying natural and artificial systems, triangulation allows to choose that part of the system which corresponds to the specified conditions by the optimal composition, temperature and chemical interaction [9,13]. The main feature of triangulation of systems is determination of the components that form only eutectic compositions. Triangulation of a system makes it possible to determine phase transitions of the system components in exchange, dehydration and polycondensation, hydration, conversion, complexation reactions, to predict all bifurcation paths of components as well as to predict the existence of new compounds.
As an example, let us consider the system CaO-SiO2-H2O. In this system, formation of 86 compounds was stated (Table 1), triangulation of the system was carried out on the basis of Kurnakov’s teaching [9] (Figure 1) (projection at standard conditions) using “The Gibbs function normalized to the total number of electrons”. The principle of triangulation of systems is given in [7]. Thermodynamic indices of compounds are calculated based on the method of ion increments and increments of Aldabergenov [7]. Calculations show a good consistency with the literature data and the relative error does not exceed 0.87%. For many compounds thermodynamic indices are calculated for the first time. The triangulation of three-component system is performed by partitioning of the large basic triangle into several small “subtriangles”, which are named by Kurnakov as a simplex [9]. AS Trunin calls them a single phase unit (SPU) [14,15]. SPU is characterized by formation of only a eutectic composition in them. Binary eutectic in CaO-SiO2 system are given in [16] and in the system CaO-SiO2-H2O in [17,18]. Compounds of one SPU do not interact with each other, and those referring to different SPUs interact. On this basis, it is possible to consider any interactions between the components of the system.
Figure 1: Triangulation of the system СaO-SiO2-H2O (#48 and #53 compounds are in the picture at one point. Such combination are available for other compounds: #66 and #67; #59 and #73; #49 and #75; #65 and #81; #35 and #71, #83; #41 and #84; #8 and ##9, 10, 11, 12, 13; #21 and #22; #24 and #25; #26 and ##27, 28, 29, 30. Therefore, the figure shows only one of these compounds combined).
| Compounds | -∆fG0298, kJ/mol | % av. | 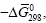 kJ/mol.el |
|
|---|---|---|---|---|
| by increments | [14] | |||
| 2 | 3 | 4 | 5 | |
| СaO | 603.5 | 21.55 | ||
| SiO2 | 892.6 | 29.75 | ||
| H2O | 237.2 | 23.72 | ||
| H2Si14O29 | 12437.23 | 28.92 | ||
| H2Si8O17 | 7274.17 | 29.10 | ||
| H2Si2O5 | 2111.11 | 30.16 | ||
| H10Si6O17 | 7113.49 | 30.93 | ||
| H2SiO3 | 1250.60 | 31.26 | ||
| H4Si2O6 | 2501.19 | 31.26 | ||
| H6Si3O9 | 3751.79 | 31.26 | ||
| H8Si4O12 | 5002.38 | 31.26 | ||
| H10Si5O15 | 6252.98 | 31.26 | ||
| H12Si6O18 | 7503.58 | 31.26 | ||
| H10Si4O13 | 5392.47 | 31.72 | ||
| H8Si3O10 | 4141.87 | 31.86 | ||
| H6Si2O7 | 2891.28 | 32.13 | ||
| H4SiO4 | 1640.68 | 32.81 | ||
| H8SiO6 | 2099.28 | 29.99 | ||
| Ca(OH)2 | 897.5 | 23.62 | ||
| Ca3SiO5 | 2788.84 | 2785.1 | 0.13 | 24.46 |
| Ca2SiO4 | 2185.34 | 2191.3 | 0.27 | 25.41 |
| Ca6Si3O12 | 6460.24 | 25.04 | ||
| Ca8Si5O18 | 9548.14 | 25.53 | ||
| Ca3Si2O7 | 3724.29 | 3756.7 | 0.87 | 25.86 |
| Ca6Si4O14 | 7362.80 | 25.57 | ||
| CaSiO3 | 1538,95 | 1543.9 | 0.32 | 26.53 |
| Ca2Si2O6 | 3077.90 | 26.53 | ||
| Ca3Si3O9 | 4616.85 | 26.53 | ||
| Ca6Si6O18 | 9233.70 | 26.53 | ||
| Ca7Si7O21 | 10772.65 | 26.53 | ||
| Ca5Si6O17 | 8587.31 | 26.84 | ||
| Ca3Si4O11 | 5509.41 | 27.01 | ||
| CaSi2O5 | 2431.51 | 27.63 | ||
| CaSi3O7 | 3324.07 | 28.17 | ||
| CaSiO3.0,16H2O | 1574.15 | 26.41 | ||
| CaSiO3.H2O | 1758.95 | 25.87 | ||
| CaSiO3.2H2O | 1978.95 | 25.37 | ||
| CaSiO3.2,5H2O | 2088.95 | 25.17 | ||
| CaSiO3.3H2O | 2198.95 | 24.99 | ||
| Ca2SiO4.H2O | 2405.34 | 25.06 | ||
| Ca2SiO4.1,17H2O | 2442.74 | 25.00 | ||
| Ca2SiO4.4H2O | 3065.34 | 24.33 | ||
| CaSi2O5.H2O | 2651.51 | 27.06 | ||
| 1 | 2 | 3 | 4 | 5 |
| CaSi2O5.2H2O | 2871.51 | 26.59 | ||
| CaSi2O5.3H2O | 3091.51 | 26.20 | ||
| CaSi3O6(ОН)2 | 3553.37 | 27.76 | ||
| Ca2Si2O6.H2O | 3297.90 | 26.17 | ||
| Ca3Si2O6(ОН)2.2H2O | 4393.59 | 25.25 | ||
| Ca2Si3O8.2H2O | 4367.57 | 26.31 | ||
| Ca2Si3O8.2,5H2O | 4477.57 | 26.18 | ||
| Ca2Si5O12.1,5H2O | 6085.58 | 27.54 | ||
| Ca3Si2O7.H2O | 3944.29 | 25.61 | ||
| Ca3Si2O7. 3H2O | 4384.29 | 25.20 | ||
| Ca3Si3O9.H2O | 4836.85 | 26.29 | ||
| Ca3Si6O15.7H2O | 8834.53 | 26.45 | ||
| Ca3Si6O13(ОН)4 | 7753.13 | 27.30 | ||
| 3[Ca3Si6O13(ОН)4].4H2О | 24139.39 | 27.06 | ||
| Ca4Si3O10.H2O | 5473.24 | 25.82 | ||
| Ca4Si3O10.1,5H2O | 5583.24 | 25.73 | ||
| Ca4Si4O12.H2O | 6375.80 | 26.35 | ||
| Ca4Si6O13(ОН)6.4H2O | 9508.82 | 26.27 | ||
| Ca5Si2O9.H2O | 5108.40 | 24.33 | ||
| Ca5Si5O15.H2O | 7914.75 | 26.38 | ||
| Ca5Si6O17.3H2O | 9247.31 | 26.42 | ||
| Ca5Si6O17.5,5H2O | 9797.31 | 26.13 | ||
| Ca5(Si6O18H2).8H2O | 10576.61 | 25.80 | ||
| Ca5Si6O17.9H2O | 10567.31 | 25.77 | ||
| Ca5Si6O17.10H2O | 10787.31 | 25.68 | ||
| Ca6Si2O7(ОН)6 | 6222.69 | 24.12 | ||
| Ca6Si3O12.H2O | 6680.24 | 24.93 | ||
| Ca6Si6O18.H2O | 9453.70 | 26.41 | ||
| Ca7Si16O39.H2O | 18768.35 | 27.36 | ||
| Ca8Si6O17(ОН)6 | 11085.71 | 25.54 | ||
| Ca8Si10O28.H2O | 14316.72 | 26.81 | ||
| Ca8Si12O30(ОН)4.6H2O | 17960.44 | 27.05 | ||
| Ca9Si6O21.H2O | 11221.31 | 25.39 | ||
| Ca9Si6O21.7H2O | 12541.31 | 24.98 | ||
| Ca9(Si3O9H)(Si2O7H)(OH)8. 6H2O | 12651.03 | 24.71 | ||
| Ca9(Si6O18H2)(OH)8. 6H2O | 13467.81 | 24.85 | ||
| 2[Ca10Si12O31(ОН)6].3H2O | 36385.04 | 26.56 | ||
| Ca10Si12O31(ОН)6.8H2O | 19622.52 | 26.16 | ||
| Ca10Si12O31(ОН)6.18H2O | 21822.52 | 25.67 | ||
| Ca6Si6O17(ОН)2. | 9463.00 | 26.43 | ||
| Ca12(Si6O17)2(ОН)4.12Ca(OH)2 | 29696.00 | 25.34 | ||
| Ca14Si24O58(ОН)8.2H2O | 31828.10 | 27.16 | ||
| Ca14Si24O61(ОН)2.6H2O | 32020.20 | 27.09 | ||
Table 1: The values of the Gibbs energy of formation (-ΔfGo 298) and "The Gibbs function normalized to the total number of electrons" ( ) 298 ΔGο compounds of CaO-SiO2-H2O system in a solid state.
In the system CaO-SiO2-H2O, the most active and reactive is Ca(OH)2. Therefore, we considered the interaction of Ca(OH)2 (compound # 19) with Ca3Si2O6(OH)2 .2H2O (compound #48) (Table 1) in the exchange reactions. To do this, between the poles of these compounds we draw a straight line 19-48 that intersects the stable secants: 42-69; 18-69; 39-69; 69-78; 69-79; 69-17; 69-82 (Figure 1). The points of intersection of lines are points of bifurcation. At this point, the system becomes unstable and there appear new solutions of evolution due to the branching (bifurcation). Line of 19-48 [Ca(OH)2-Ca3Si2O6(OH)2 .2H2O] shows that in the presence of a small displacement of the initial components, the system is capable of spasmodic transitions between stable states. “The Gibbs function normalized to the total number of electrons”, "average electron heat capacity", " average electron entropy" for the participants of reactions are shown in Table 2.
| Compounds | 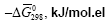 |
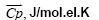 |
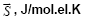 |
|---|---|---|---|
| Са(ОН)2 | 23.62 | 2.302 | 2.194 |
| Ca6Si2O7(OH)6 | 24.12 | 1.744 | 1.801 |
| Ca2SiO4.4H2O | 24.33 | 2.219 | 2.464 |
| Ca3Si2O6(OH)2.2H2O | 25.25 | 1.878 | 2.034 |
| H8SiO6 | 29.99 | 3.189 | 5.785 |
| CaSiO3.3H2O | 24.99 | 2.268 | 2.510 |
| Ca9(Si3O9H)(Si2O7H). (OH)8. 6H2O |
24.71 | 1.969 | 2.124 |
| H4SiO4 | 32.81 | 2.956 | 6.343 |
| Ca10Si12O31(ОН)6.18H2O | 25.67 | 2.048 | 2.231 |
| Ca9Si6O18H2(OH)8.6H2O | 24.85 | 1.942 | 2.087 |
Table 2: Values of “The Gibbs function normalized to the total number of electrons” 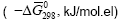 "average electron heat capacity"
"average electron heat capacity"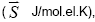 "average electron entropy" ( S , J/mol.el.K) for the components of reactions in a solid state.
"average electron entropy" ( S , J/mol.el.K) for the components of reactions in a solid state.
Results and Discussion
Triangulation of three-component system, for example, CaO- SiO2-H2O, reflects all the possible transformation processes of the system components in both non-equilibrium and equilibrium states. The bifurcation points are in the system at the intersection of lines of interacting components with a stable secant. On this basis, it is possible to describe chemical reactions of interaction of Ca(OH)2 with Ca3Si2O6(OH)2.2H2O at bifurcation points:
19+48=42+69
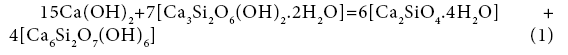
A=-1.34 kJ/mol.el.
19+48=69+18
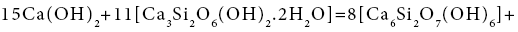
 (2)
(2)
A=4.32 kJ/mol.el.
19+48 = 69+39
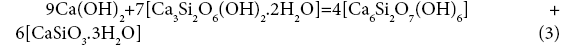
A=-0.68 kJ/mol.el.
19+48=69+78
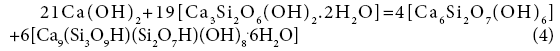
A=-0.96 kJ/mol.el.
19+48=69+79
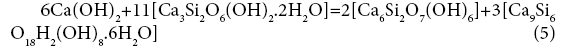
A=-0.82 kJ/mol.el.
19+48=69+82
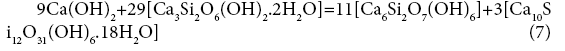
A=0 kJ/mol.el.
The affinity is determined for the elementary collision acts. If the affinity is positive, the reaction rate is positive too, i.e., the reaction proceeds from left to right. A negative value of the reaction affinity indicates impossibility of spontaneous occurrence of this reaction. As seen from the examples, reactions (1), (3-5) do not proceed spontaneously, while reactions (2) and (6) - do. The affinity of reaction (7) is equal to zero, i.e. the reaction components are in the equilibrium state. It is the affinity that is the driving force of chemical reactions. According to the transition state theory [19], a chemical reaction is associated with formation of an intermediate complex between the initial components. Further transition of initial substances to the final product depends on the energy of the colliding molecules, on their correct orientation in space, on the structural changes. There takes place the breakage of chemical bonds in the initial compounds and formation of new chemical bonds to yield reaction products, leading to an spasmodic change of properties. At the bifurcation point, the system becomes unstable, having the possibility of transition to both initial compounds and the reaction products. The system can pass to one of several discrete stable states with a higher degree of organization, ordering, which was called dissipative by Prigogine, i.e., organized due to dissipation of energy. In our example, the compounds Ca(OH)2 and Ca3Si2O6(OH)2.2H2O, colliding with each other, can form products according to reactions (1) - (7), this forming, on the one hand, the compound Ca6Si2O7(OH)6 (#69) and, on the other, compounds Ca2SiO4.4H2O (#42), H8SiO6 (#18), CaSiO3.3H2O (#39), Ca9(Si3O9H) (Si2O7H)(OH)8.6H2O (#78), Ca9(Si6O18H2)(OH)8.6H2O (#79), H4SiO4 (#17), Ca10Si12O31(OH)6 .18H2O (#82) (Figure 1). There appears a symmetric bifurcation sequence, conversion of the initial components will depend on the process temperature, entropy production, structural changes and conversion rate constant. In this case it is necessary to consider the change of chemical potential at individual conversions of components #19 [Ca(OH)2] and #48 [Ca3Si2O6(OH)2.2H2O] into all others: ## 69, 42, 18, 39, 78, 79, 17, 82 according to the bifurcation sequence. It should be noted that the irreversible conversions may be realized during transition to only stable states with the decrease of chemical potential. During phase transition, the chemical potential is the factor of intensity, that is transition of the component can occur spontaneously only from the phase with high potential to the phase with less potential. According to Lewis, the chemical potential should be considered as a measure of the tendency of the substance to scatter from the space occupied by it, i.e., a measure of the substance reactivity. According to Reaction (1), compounds Ca(OH)2 and Ca3Si2O6(OH)2.2H2O transform into Ca6Si2O7(OH)6 and Ca2SiO4.4H2O, therefore, it is necessary to consider individual conversion of Ca(OH)2 to Ca2SiO4.4H2O and Ca6Si2O7(OH)6, as well as conversions of Ca3Si2O6(OH)2.2H2O to Ca6Si2O7(OH)6 and Ca2SiO4.4H2O.
The chemical potential of the components changes as follows:
It should be noted that conversions of Ca3Si2O6(OH)2.2H2O to Ca2SiO4.4H2O and Ca6Si2O7(OH)6, are characterized by the increase in the chemical potential (0.92 kJ/mol.el) and (1.13 kJ/mol.el), respectively; i.e., Ca3Si2O6(OH)2.2H2O is a more stable compound than Ca2SiO4.4H2O and Ca6Si2O7(OH)6. According to Reaction (1) it can be concluded that conversions of Ca(OH)2 to Ca2SiO4.4H2O (#19-#42) and Ca6Si2O7(OH)6 (#19-#69) are possible and of the two initial compounds, Ca(OH)2 is more reactive than Ca3Si2O6(OH)2.2H2O.
Average electron energy density is only a function of temperature. Therefore, one can determine the temperature of the process at which the reaction procedure based on the chemical potential is possible. The temperature of the process of the component conversion to a stable compound accompanied by the decrease in the chemical potential can be determined as the difference of “The Gibbs function normalized to the total number of electrons” of the components divided by the "average electron heat capacity" of the compound being formed (Table 2). The process temperature of Ca(OH)2 conversion to Ca2SiO4.4H2O is determined:
[-23.62-(-24.33)].1000/2.219=320K
All irreversible processes are described with the help of thermodynamic forces and thermodynamic flows. Thermodynamic flows are caused by thermodynamic forces. The thermodynamic force (F) is determined [1] as follows:
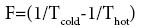
Тcold-for solid-state reactions the standard temperature of 298 K is taken.
Тhot-transformation temperature.
F=(1/298 - 1/320)=0.231.10-3 1/K
then production of entropy for conversion of Ca(OH)2 to Ca2SiO4.4H2O:
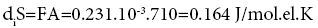
The total change of entropy for conversion of Ca(OH)2 to Ca2SiO4.4H2O is determined as the difference between "average electron entropies" of the compounds (Table 2), i.e., dS for conversion is equal to:
dS 2.464–2.194=0.27 J/mol.el.K
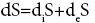
After deS=dS - diS=0.27 - 0.164=0.106 J/mol.el.K.
According to the transition state theory [20], the transformation rate (K is the rate constant), is determined by the formula
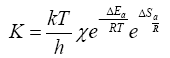
Where, k is Boltzmann’s constant, T is temperature, h is Planck's constant, χ is transmission ratio (assumed to be equal to unity, since we consider only irreversible processes), ΔEa is activation energy, R is gas constant, ΔSa is entropy of activation.
Activation energy and activation entropy are determined as the difference between the “The Gibbs function normalized to the total number of electrons” and "average electron entropy" of the reagents:
ΔEa=710 J/mol.el
ΔSa=0.27 J/mol.el.K
All calculations of component transformations are given in Table 3. Analysis of the table data shows that in all chemical transformations there takes place reduction in the chemical potential. The transformation temperature ranges from 205 to 3109 K. All conversions retained diS > 0, that corresponds to the requirements of the second law of thermodynamics (Tables 3 and 4).
| Conversion | A, J/mol.el | Т, К | F, 1/K | diS, J/mol.el.K | dS, J/mol.el.K | deS, J/mol.el.K | K, 1/s |
|---|---|---|---|---|---|---|---|
| 19-42 Ca(OH)2 - Ca2SiO4.4H2O | 710 | 320 | 0.231.10-3 | 0.164 | 0.27 | 0.106 | 5.267.1012 |
| 19-69 Ca(OH)2- Ca6Si2O7(OH)6 | 500 | 287 | 0.128.10-3 | 0.064 | -0.393 | -0.457 | 4.619.1012 |
| 19-18 Ca(OH)2- H8SiO6 | 6370 | 1997 | 2.855.10-3 | 18.186 | 3.591 | -14.595 | 4.356.1013 |
| 48-18 Ca3Si2O6(OH)2.2H2O- H8SiO6 | 4740 | 1486 | 2.682.10-3 | 12.712 | 3.751 | -8.961 | 3.305.1013 |
| 19-39 Ca(OH)2 - CaSiO3.3H2O | 1370 | 604 | 1.7.10-3 | 2.329 | 0.316 | -2.013 | 9.931.1012 |
| 19-78 Ca(OH)2- Ca9Si6O18H2(OH)8.6H2O | 1090 | 554 | 1.551.10-3 | 1.691 | -0.07 | -1.761 | 9.017.1012 |
| 19-79 Ca(OH)2- Ca9Si6O18H2(OH)8.6H2O | 1230 | 633 | 1.776.10-3 | 2.184 | -0.107 | -2.291 | 1.028.1013 |
| 19-17 Ca(OH)2 - H4SiO4 | 9190 | 3109 | 3.034.10-3 | 27.882 | 4.149 | -23.733 | 7.456.1013 |
| 48-17 Ca3Si2O6(OH)2.2H2O - H4SiO4 | 7560 | 2557 | 2.965.10-3 | 22.347 | 4.309 | -18.038 | 6.251.1013 |
| 19-82 Ca(OH)2 - Ca10Si12O31(OH)6.18H2O | 2050 | 1001 | 2.357.10-3 | 4.832 | 0.037 | -4.795 | 1.635.1013 |
| 48-82 Ca3Si2O6(OH)2.2H2O - Ca10Si12O31(OH)6.18H2O | 420 | 205 | 1.522×10-3 | 0.639 | 0.197 | -0.442 | 3.414×1012 |
Table 3: Conversion parameters of components.
| Reactions | A, J/mol.el | T, K | F, 1/K | diS, J/mol.el.K | dS, J/mol.el.K | deS, J/mol.el.K | K, 1/s |
|---|---|---|---|---|---|---|---|
| 1 | 1210 | 305 | 0.077×10-3 | 0.093 | -0.123 | -0.216 | 3.881×1012 |
| 2 | 11610 | 1429 | 2.656×10-3 | 30.836 | 6.949 | -23.887 | 2.578×1013 |
| 3 | 1870 | 466 | 1.21×10-3 | 2.262 | -0.077 | -2.339 | 4.515×1012 |
| 4 | 1590 | 428 | 1.02×10-3 | 1.622 | -0.463 | -2.085 | 5.39×1012 |
| 5 | 1730 | 469 | 1.224×10-3 | 2.118 | -0.5 | -2.618 | 5.89×1012 |
| 6 | 17250 | 2253 | 2.912×10-3 | 50.232 | 8.065 | -42.167 | 4.92×1013 |
| 7 | 2970 | 509 | 1.391×10-3 | 4.131 | -0.159 | -4.29 | 5.153×1012 |
Table 4: Conversion parameters of chemical reactions.
There is a straightforward dependence between the affinity of the elementary stages of the reagents and the entropy production (Figure 2a) and the process temperature (Figure 2b) for individual transformations. The more entropy the system produces, the higher the temperature of transformations and entropy flow released into the environment (Figure 2c). A negative value of the entropy flow indicates the "leakage" of entropy from the system into the environment. The components out flowing from the system, carry more entropy than the components in taken (Table 3). The increase in affinity leads to the increase in the thermodynamic force (Figure 2d). Thermodynamic forces and thermodynamic flows are not in linear relation, the ratio of A/RT in all transformations (Table 3) is less than unity. In this area, the increase in affinity results in a non-linear increase in thermodynamic force, i.e., the system moves to the nonlinear regime. The maximum production of entropy requires maximum flow of entropy (Figures 2e). The reaction rate constant also has a linear dependence on the entropy production (Figure 2f), and as well as on the affinity. There is a nonlinear dependence of the entropy production (Figure 2g) and the rate constant (Figure 2h) on the thermodynamic force. Table 3 shows that conversions with formation of H8SiO6 and H4SiO4 are characterized by the high value of entropy production per electron (from 12.712 to 27.882 J/mol.el.K) and high temperature (from 1486 to 3109 K, Table 3). These transformations are characterized by the increase in the rate constant. H8SiO6 and H4SiO4 may be formed both on the basis of conversion from Ca(OH)2 and from Ca3Si2O6(OH)2.2H2O. For conversions of 19-82 [Ca(OH)2-Ca10Si12O31(OH)6.18H2O] and 48-82 [Ca3Si2O6(OH)2.2H2O-Ca10Si12O31(OH)6.18H2O] with formation of the reaction product Ca10Si12O31(OH)6.18H2O, production of entropy makes up 4.832 and 0.639 J/mol.el.K at the process temperatures of 205 and 1001 K, and at the rate constant of 3.414*1012 and 1.635*1013 1/s. Thus, the most interesting reactions are (2), (6) and (7) resulting in formation of stable compounds at transformations of both initial reagents. In accordance with the procedure, the transformation parameters are determined for chemical reactions (1) - (7) (Table 4). Only conversions of the components with reduced chemical potential were taken into account. For example, for reaction (1), the affinity is determined as the sum of two conversions: Ca(OH)2 to Ca2SiO4 .4H2O and Ca(OH)2 to Ca6Si2O7(OH)6 (А=710+500=1210 J/mol.el). Accordingly, the conversion temperature is determined as 1210/(2.219+1.744)=305 K and so on. Analysis of transformation parameters for chemical reactions shows that they retain linear dependences of production and flow of entropy, process temperature on affinity (Figure 3) identically as for both individual transformations (Figure 2). In chemical reactions of the system, there occurs the flow of energy and substance leading it to a non-equilibrium state in the nonlinear regime. In the reactions, the ratio of A/RT is less than unity (0.21-0.38) (but not A/RT«1) (Table 4), and one can observe the nonlinear dependence of the thermodynamic force on affinity (Figure 3d), of entropy production and the rate constant on the thermodynamic force (Figures 3g and 3h). In chemical reactions (2) and (6), there is a sharp increase of affinity, process temperature, entropy production and entropy flow, thermodynamic force and rate constant (Table 4). These chemical reactions are characterized by the interrelation of component transformations. Conversion of the reaction components according to (7) differs from the parameters of reactions (2) and (6) in low temperature (509 against 1429 and 2253 K) and low production of entropy (4.131 against 30.836 and 50.232 J/mol.el.K). In accordance with the second law of thermodynamics, the sum of entropy changes of the system and the environment cannot decrease. According to reaction (7), the sum of the system entropy changes makes up -0.159 J/mol.el.K, affinity for this reaction is zero; for reactions (2) and (6), the entropy change is positive (+6.949 and +8.065 J/mol.el.K, respectively), the affinity of the reaction is also positive (+4.32 and +7.14 kJ/mol. el, respectively). According to the parameters of transformations at the bifurcation point, the evolution of interaction of Ca(OH)2 and Ca3Si2O6(OH)2.2H2O can follow reactions (2) and (6) with formation of H8SiO6 and H4SiO4.
Figure 2: Correlation dependence of entropy production (a), conversion temperatures (b), the entropy flow (c), thermodynamic force (d) on the chemical affinity and the entropy flow (e), the rate constant (g) on the entropy production; the entropy production (k) and the rate constant (l) on the thermodynamic force for individual conversions.
Figure 3: Correlation dependence of the entropy production (a) transformation temperature (b), the flow of entropy (c), thermodynamic force (d) on affinity and entropy flow (e), the rate constant (g) on the entropy production, the entropy production (k) and the rate constant (l) on the thermodynamic force for chemical reactions.
“The Gibbs function normalized to the total number of electrons” and triangulation of the system allow to determine the points of bifurcation during interaction of components. There appears the bifurcation sequence and further transition of the system to evolutionary path with formation of a stable compound depends on entropy production, entropy flow and reaction rate constant. All conversions of the components at the point of bifurcation are described in terms of the chemical potential, affinity, thermodynamic force. The correlation dependence between the chemical potential, affinity, conversion temperature, entropy flow, entropy production and rate constant is stated.