Journal of Depression and Anxiety
Open Access
ISSN: 2167-1044
ISSN: 2167-1044
Research Article - (2025)Volume 14, Issue 1
Background: The brain and the immune system are not fully formed at birth, but rather continue to mature in response to the postnatal environment. The two-way interaction between the brain and the immune system makes it possible for childhood psychosocial stressors Early Life Stress (ELS) and Parental Schizophrenia (PSZ) to affect immune system development, which in turn can impact brain development and its long-term functioning.
Objective: The present study aimed to explore the complex multifaceted interactions between early-life stress, Schizophrenia (SZ), and neuro-inflammation, and their collective effect on social interaction, locomotor function and spatial memory.
Methods: Male and female Sprague-Dawley rat pups were randomly assigned to eight groups: Control, ELS, schizophrenia, and ELS+schizophrenia. ELS was induced by Gestational Stress (GS) and Maternal Separation (MS) during the first two weeks of life, while SZ was induced by subcutaneous administration of ketamine. To assess behavioral changes, social interaction tests and sucrose preference tests were conducted. In addition, Neuro-inflammation was determined through DNA methylation, complete blood cell count and furthermore, we used ELISA kits to measure the expression levels of Interleukin-6 (IL-6), Matrix Metalloproteinase-9 (MMP-9), and Interferon-gamma (IFN-g).
Results and conclusion: Our results revealed that both differential ELS and PSZ had a significant impairment in social interaction, social preference and spatial memory on the experimental groups compared to the control group (p<0.05). In addition, there was a decreased intersection of GFAP astrocyte processes in the hippocampus and Prefrontal Cortex (PFC) (p<0.01) and alterations in DNA methylation patterns. Furthermore, differential reductions were observed in the Neutrophil-to-Lymphocyte Ratio (NLR) (p<0.05), Interleukin-6 (IL-6), Matrix Metalloproteinase-9 (MMP-9), and Interferon-gamma (IFN-g) were altered (p<0.05), indicating an inflammatory response. Gaining insights into the neuro-inflammatory pathways that contribute to motor and cognitive impairments could offer new therapeutic avenues for addressing the multifaceted challenges associated with this disorder.
Neuro-inflammation; Early-life stress; Schizophrenia cognitive functioning; Motor functioning; Astrocytes; DNA methylation; CBC count; Cytokines
ACE: Adverse Childhood Experiences; MDD: Major Depressive Disorder or Depression; ELS: Early Life Stress; SZ: Schizophrenia; MS: Maternal Separation; DL: Developmental Impact; CNS: Central Nervous System; HPA: Hypothalamic-Pituitary-Adrenal; PFC: Prefrontal Cortex; PTSD: Post-Traumatic Stress Disorder
The notion that stress could have detrimental effects on the health of children has been present for quite some time. Around fifty years ago, established a clear dose-response correlation between family-related stress and children outcome [1]. However, even after numerous decades, our understanding on the connections between ELS or PSZ and immunity remains incomplete. The mechanisms by which ELS or PSZ disrupts the developing immune system, and how these mechanisms might influence brain and behavioral development during childhood, still elude us. This stands in sharp contrast to the abundance of accumulated research findings in these realms, particularly concerning animals, and to a lesser degree, human studies. Interestingly, several studies have identified both ELS and PSZ as significant risk factors for the development of cognitive and motor impairments later in life [2].
One possible mediator candidate is the immune system. The activation of neuro-inflammatory and immune processes has been proposed as a pivotal factor influencing the progression of diseases within various pathologies that impact the Central Nervous System (CNS) [3]. A lasting imprint of ELS on inflammation and immune system reactivity is evident. Clinical research has revealed a distinct "immune phenotype" that characterizes the blood of individuals exposed to Childhood Maltreatment (CM), encompassing sustained low-level inflammation, expedited immune aging, and conceivably compromised cellular immunity [4,5]. These alterations in the immune system have been intertwined with the vulnerability of ELS individuals to psychiatric and neurological disorders, considering the indispensable role of immune processes and neuro-immune communication in CNS development and homeostasis. Another possible mechanism is that ELS initiates a permanent rewiring of innate immune cells and stress systems, including the sympathetic nervous system and the hyperactivation of the Hypothalamus-Pituitary-Adrenal (HPA) axis, regulators of immune responses [6,7]. Taken together this rewiring could lead to heightened inflammatory responses upon successive challenges. Such rewiring could potentially trigger amplified or dysfunctional neuro-inflammatory reactions to protein aggregates and tissue damage, potentially sparking the commencement or escalation of CNS pathologies in ELSaffected individuals. Furthermore, ELS or PSZ could potentially serve as a catalyst for neurological disorders by reshaping immune pathways that influence the maturation of the brain, especially in microglia. In individuals subjected to maltreatment, irregular neuronal function and synaptic plasticity might prompt premature aging and susceptibility to insults within circuits influenced by neurodegenerative ailments and injuries [8,9].
Astrocytes are integral components of the CNS, and also glial cells that play pivotal roles in modulating neuro-inflammatory responses and maintaining CNS homeostasis. These star-shaped glial cells possess a remarkable ability to detect shifts in their microenvironment and respond by releasing a diverse array of signaling molecules, including cytokines like interleukins and tumor necrosis factor-alpha, as well as neurotransmitters like glutamate and ATP [10,11]. This molecular repertoire empowers astrocytes to significantly influence microglia, the resident immune cells of the brain, thereby intricately shaping the balance between neuroprotection and neurodegeneration. Moreover, astrocytes play a direct role in synaptic function and plasticity. By controlled release of glio-transmitters such as glutamate, they actively engage in synaptic transmission and modulate neural communication. Additionally, they contribute to vital processes like synapse formation, synaptic pruning, and the maintenance of optimal neurotransmitter levels, thereby fundamentally shaping neural circuitry. In the realm of neuro-inflammation, astrocytes manifest dual roles. On one hand, they possess mechanisms to counteract inflammation, releasing anti-inflammatory factors and promoting tissue repair. On the other, they have the potential to escalate inflammatory responses under specific conditions, further underscoring their intricate involvement in CNS health and pathology. Astrocytes are also active participants in neurodevelopmental processes, guiding neuronal migration, supporting synaptogenesis, and regulating neurogenesis. The dysregulation of astrocytic functions due ELS or PSZ periods has been implicated in various neurodevelopmental disorders [12,13].
Multiple animal models, such as MS, GS, and limited nesting and bedding time, have been widely used to replicate ELS and childhood traumatic experiences [14,15].
These studies underscore the adverse effects of MS or GS on social preference, spatial memory, and the architecture of the Hippocampus and PFC, ultimately impacting physical health. Yet, the mechanisms by which ELS induces astrocytopathy and related behaviors, including pro-inflammatory mediators like MMP-9, IL-6, and TNF-α, remain not well explored [16,17]. Moreover, the interplay between ELS and PSZ has yet to be modeled in animal research, providing a novel avenue for exploration, of the multifaceted EL experiences in humans.
To address these questions, we performed impact of ELS (MS, GS) and PSZ on neuro-inflammation and its implications for cognitive and motor impairments in later in life. In this study we investigate the impact of ELS or PSZ on locomotor, social preference and spatial memory, anxiety-like behaviors as well as astrocytes intersection process and epigenetic modifications associated with neuro-inflammation on the Hippocampus and PFC in rats. Moreover, we evaluate the Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Lymphocyte-to-Monocyte Ratio (LMR), as indicators of systemic inflammation.
Experimental animals and treatment
A total of twenty-four (24) SD rats, comprising of sixteen (16) nulliparous females in the fertile phase and eight (8) stud male, were procured from the Biomedical Resource Unit (BRU) breeding centre at the University of KwaZulu-Nata (UKZN). The animals were bred and raised within the controlled environment of the Animal House, located at the UKZN's BRU. All animals were subjected to standard laboratory conditions, including being housed in pairs within standard cages (29 cm by 22 cm by 14 cm). The temperature was maintained at (20–23°C), and a 12 hour dark photoperiod was implemented between 600hrs and 1800hrs. Food and water were provided=ad libitum. The present study adhered to ethical guidelines and obtained prior approval before commencing the research. The study protocol was assigned the reference number AREC/00003119/2021.
Animal housing and surgery
Sixteen nulliparous females in the fertile phase and eight provenstud male rats were mated to obtain 64 pups for the experiments. After the animals were collected from the BRU, the females were paired in cages for one week to acclimatize and minimize stress, and to synchronize their oestrous cycles. The males were individually housed for one week prior to the mating to build up sperm count and maximize their fertility. Vaginal smears were taken to assess their oestrous cycle, and when they were at proestrus, two females were transferred into a stud male cage. On the subsequent morning, rats examined for the presence or absence of a vaginal plug [18]. The presence of a plug was marked as gestation day one. After mating, males were removed from the cages and euthanized to avoid stalk contamination.
Animal groupings and stress factors
The study consisted of eight distinct groups, each comprising of eight pups. On the day of delivery 4 pups were randomly selected from each dam and labeled accordingly for proper follow up. The distribution of these groups and the corresponding number of pups are presented in Table 1. Dams and any additional pups were euthanized post weaning.
| Groups | Number of pups (n) | Treatment conditions | Stressors |
| Control | 8 | No stressor | No stressor |
| Prenatal maternal stress+ketamine injected pups (group 1) | 8 | Early life stress+parental psychopathology | Re-strainer maternal stress and ketamine injection on the pups PND-7,9,11 and 15. |
| Prenatal maternal stress (group 2) | 8 | Parental psychopathology only | Re-strainer maternal stress |
| Maternally separated pups (group 4) | 8 | Early life stress only | Maternal separation for 3 hours from PND-1 to PND-14 |
| Maternal separated+ketamine injected pups (group 5) | 8 | Early life stress only | Maternal separation for 3 hours from PND-1 to PND-14 and Ketamine injection on the pups PND-7,9,11 and 15. |
| Ketamine injected parent (group 6) | 8 | Parental psychopathology only | Ketamine will be injected to the parents on PND-2,4 to 6 |
| Ketamine parent+ketamine pups (group 7) | 8 | Early Life stress+parental psychopathology | Ketamine will be injected to the parents on PND-2,4 to 6 and ketamine pups. |
| Ketamine (positive control) (group 8) | 8 | Early life stress only | Ketamine injection on the pups PND-7,9,11 and 15. |
Table 1: Experimental design and behavioral tests in early life stress study. The table, outlines different treatment groups, the specific stressors the animals were exposed to, and the behavioral tests performed.
Maternal confinement stress during gestation: To mimic human prenatal stress, pregnant rats were subjected to daily GS induction from days 15 to 18. The experimental procedure entailed confining pregnant rats within transparent cylindrical restrainers (8-10 cm in diameter and 20-24 cm in length) for 45 minutes [19]. This confinement took place in a well-illuminated environment maintained at a temperature range of 21-22°C. The dimensions of the restrainers were modified in accordance with the body mass of the laboratory rats throughout the gestational phase of the experimental investigation, which was anticipated to span duration of approximately 21 to 23 days.
Dams and pups pychopathology: This study examined the association between parental psychopathology and the likelihood of passing down mental health issues. Offspring susceptibility to disorders arises from a mix of genetic, environmental, and psychosocial factors [20]. We used an experimental model, inducing SZ in dams from post-natal day 1 to 7. The dams and their offspring were grouped per table 1 conditions. We utilized ketamine, an N-methyl-D-aspartate (NMDA) receptor antagonist, which mimics cognitive, positive, and negative symptoms of SZ. Dams received (30 mg/kg, i.p) daily basis for five consecutive days while the control group received saline (0.5 ml/kg, i.p) and pups were injected with ketamine (16 mg/kg, subcutaneously) three times a week (on Mondays, Wednesdays, and Fridays) from postnatal day 1 to 14. The selection of dosage, route of administration, and injection schedule were determined through a comprehensive analysis of prior scientific investigations that have reported the induction of psychotic-like alterations subsequent to a 5-day regimen of ketamine treatment. On postnatal day 21 the pups were weaned while the dams were euthanized. The pups were left undisturbed until postnatal day 60, at which point they were subjected to behavioral testing.
Maternal separation: The impact of mother-child separation: To study MS effects, we separated dams from their offspring for 3 hours daily (9 a.m. to 12 p.m.) from postnatal day 1 to 14. The dams were relocated from their original cages. The pups were subsequently removed from the experimental rooms and relocated to a distinct room (to establish a barrier to communication between the offspring and their respective mothers) (Figure 1).
Figure 1: Illustration of the maternal separation paradigm in early life stress study.
Following a period of 3 hours (9 a.m. to 12 p.m.), the pups were reintroduced to the designated animal room, and the dams returned to their respective home cages. Figure 1 presents a visual representation showcasing the MS paradigm utilized in the investigation of ELS.
Behavioral assessment: Understanding actions and reactions
Social interaction behaviors: Social interactions play a crucial role in the biology of various species, contributing to the structure and stability of social networks and relationships within societies. In this study, the effects of stress on social interaction was assessed using Crawley's sociability and preference for social novelty test. On postnatal day 60 (PND 60), all animal cages were transferred to the behavioral room 30 minutes prior to the commencement of the experiment. The experimental setup consisted of three chambers created using Plexiglas walls: one in the middle, and one on each side. The subject rat was placed in the center of the middle chamber for a 5 minute adaptation period. Following adaptation, the walls separating the chambers were removed, allowing the subject rat to freely explore all three chambers. During the experiment, the following parameters were monitored and recorded: The duration and number of direct (active) contacts between the subject mouse and the containment cup housing either a stranger mouse or remaining empty, recorded separately for each chamber. Active contact was counted when the subject mouse made direct contact with the containment cup or stretched its body within 3-5 cm of the cup. Other behaviors of the subject mouse in each compartment, such as walking, self-grooming, freezing (no body movements for more than 5 seconds), as well as any unusual behaviors like jumping or repetitive actions, were also recorded in terms of duration and frequency. Additionally, the duration and number of entries into each compartment were documented.
After each trial, the chambers were cleaned using 70% ethanol (between rats) and Clidox 1:5:1 (between cages) to prevent olfactory cues and ensure proper disinfection, respectively. A rat was considered to be in a chamber when its head and all four paws had entered. Each session of the test lasted for 10 minutes.
Sacrifice and neurochemical analysis
Animal sacrifice and tissue collection: Prior to sacrifice, animals were anesthetized using a combination of ketamine and xylazine or Halothane, inducing a state of deep unconsciousness to minimize any potential distress or pain. Once the desired level of anesthesia was confirmed, transcardial perfusion was performed or decapitation procedure. Sacrifice was done by trained personnel experienced in the procedures, maintaining a focus on precision and compassion. Detailed records of each sacrificing event, including animal identification, date, time, and personnel involved, were documented for regulatory compliance and transparency. The date of sharpening of the guillotine was not more 3 than weeks from the decapitation date.
Transcardial perfusion fixation: Twenty-four hours after the behavioral assessment, a cohort of SD rats was injected with ketamine (80 mg/kg, i.p) and xylazine (10 mg/kg, i.p) using a 27- gauge needle and a 1cc syringe. After the injection, the rats were further anesthetized using sodium pentobarbital according to their body weights.
The rats were perfused using a solution of Phosphate-Buffered Saline (PBS) and 4% Paraformaldehyde (PFA). To optimize perfusion, a minor laceration was created at the caudal aspect of the left ventricle following the exposure of the heart. A perfusion needle (15 gauge) with either a blunt or olive tip, specifically designed for this purpose, was carefully inserted through a surgical incision. The needle was inserted into the ascending aorta. If required, supplementary anesthesia was administered to maintain the rats at an optimal level of surgical anesthesia.
Decapitation, post fixation, tissue collection and storage: At postnatal day 60, the SD rats were anesthetized using Halothane in accordance with their body weight. The head of each rat was decapitated using a sharp guillotine. Blood samples were collected from the jugular vein and stored in vacutainers for further analysis. To expose the skull, a midline incision was made along the skin from the neck to the nose, and the neck muscles were trimmed to reveal the base of the skull. An identical incision was made on the opposite side, extending to the posterior edge of the skull surface.
Subsequently, the brain was removed from the skull and then placed in a vial containing fixative solution, ensuring that the volume of the fixative was at least 10 times greater than the brain's size. The vial was intermittently swirled while kept in the fixative solution for 24 hours at a temperature of 4°C. The brain was rinsed with phosphate-buffered saline through three media exchanges, with swirling performed after each exchange. The harvested hippocampal astrocytes were stored in phosphatebuffered saline with sodium azide and kept at a temperature of 4°C for further use.
Complete blood cell count
Biomarkers analysis: The trunk blood was harvested, the Neutrophil Lymphocytes Ratio (NLR) and the Monocyte- Lymphocyte Ratio (MLR) was measured. Neutrophil- Lymphocyte Ratio (NLR): NLR is often used as an indicator of systemic inflammation and stress. We calculated the NLR by dividing the absolute neutrophil count by the absolute lymphocyte count. Monocyte-Lymphocyte Ratio (MLR): MLR, like NLR, is also a marker of inflammation and immune system activity. It is calculated by dividing the absolute monocyte count by the absolute lymphocyte count.
ELISA kits: ELISA kits were employed to quantify the expression levels of IL-1b, IL-6, Matrix metalloproteinase (MMP-9), and Brain-Derived Neurotrophic Factor (BDNF) in rat samples.
Astrocyte cell cultures
Tissue was then incubated for 48 hours at 4°C. During this incubation, a mouse monoclonal antibody (MAB360, Millipore) specifically designed to target GFAP was utilized. The antibody was diluted to a ratio of 1:10,000 in a working buffer solution composed of PB containing 2% NGS (normal goat serum) and 0.3% TX (Triton X). Following the incubation period, the sections were subjected to a series of three 10-minute washes in the working buffer and then transferred to a working buffer maintained at a temperature of 25°C. The working buffer contained a 1:500 dilution of goat anti-mouse IgG conjugated to Alexa Fluor 633 (Inqaba Biotechnical SA). After a 3-hour incubation period, the sections were subjected to a subsequent 15-minute incubation step at 25°C in a working buffer solution that was prepared by diluting DAPI (Sigma) at a ratio of 1:2000. The sections were washed and then carefully mounted in Prolong Gold antifade reagent. Astrocytes and principal neurons were identified using a confocal microscope, which relies on the distinct characteristics of their soma in terms of size and shape.
Immunofluorescence labeling was used to detect the presence of the astroglial marker S100b. To achieve this, free-floating sections were subjected to an overnight incubation with a rabbit anti-S100b antibody (Switzerland), at a dilution of 1:400. Tissue was then incubated for 1 hour with 20 mg/ml carbocyanine (Cy) 2-conjugated donkey anti-rabbit IgG (Dianova). To inhibit the unoccupied binding sites of Cy2-anti-rabbit IgG, the sections were incubated for 3-hour with 50% rabbit antiserum. The sections were subsequently subjected to an overnight incubation with 10 mg/ml rabbit anti-GFAP antibodies. The sections were subjected to a 1-hour incubation period with Cy3-anti-digoxin (20 mg/ml; Dianova) to detect the presence of GFAP immunereactivity.
Morphological attributes of hippocampal neurons were examined under a stereological system manufactured by ZEISS, at × 2.5 magnification. The experimental design incorporated a predetermined area sampling fraction of 2% to ensure representative data collection. In order to accurately quantify the number of GFAP+cells (astrocytes) within a given tissue sample, a "guard zone" with a width of 3 μm was implemented at the uppermost surface of the sections. Subsequently, the number of GFAP+ cells was counted within a depth of 15 μm below the guard zone, which corresponds to the height of the dissector. The estimation of the total number of astrocytes (N) was conducted using the formula: n=ΣQ−× 1/ssf × 1/asf × 1/hsf] ΣQ is denoted as the quantitative representation of GFAP+cells observed in the specimens. The section sampling fraction (ssf), area sampling fraction (asf), and height sampling fraction (hsf) are used to account for the respective fractions of the total sample that were analyzed in terms of sections, areas, and heights.
Data analysis and statistical measures
All statistical analysis was performed in paleontological statistics software package (PAST-4) and the significance level was set at p<0.05. Results were expressed as mean ± SEM. To address the issue of multiple comparisons and to control the family wise error rate, the adjusted significance level was set at p<0.0018. Comparisons within the groups on behavioral test and astrocytes were done by one-way ANOVA. Power analysis was conducted to test for the effect size in results. Furthermore, after the ANOVA result if the results indicated a significant overall effect we performed a Post hoc Tukey's test to further analyze and compare the means of different groups. Image-J was used to quantify Hippocampal Astrocytes Density (HAD) and the process intersections. The data on the effects on cognition, memory, and motor were recorded and plotted in bar graphs, box plots and radar plots.
Social interaction behaviour
In our study examining social approaches among various groups (control, group 1 (prenatal maternal stress+ketamine injected pups), group 2 (GS), group 4 (MS), group 5 (maternal separated +Ketamine injected pups), group 6 (PSZ), group 7 (ketamine parent+ketamine pups), and group 8 (schizophrenia pups), the ANOVA revealed significant differences in social interaction patterns (F=18.21, df=7, 56, p<0.001, ω²=0.6531 Figure 2A). Subsequent post hoc tests revealed that both the stressed and schizophrenia groups exhibited significantly different social approach patterns when compared to the control group (nonstressed). The post hoc analysis of group comparisons yielded significant findings (p<0.001 Figure 2B) in the study of social approach latency among various groups. Notably, control differed significantly from group 4 (p<0.001), group 5 (p=0.0005), group 6 (p<.001), and group 7 (p<0.001). Group 1 and group 2 also exhibited significant differences compared to group 4 (p=0.0167 and p<0.001, respectively), while group 5 differed significantly from group 6 (p=0.0024). However, no significant differences were observed between group 5 and group 8 (p=0.4041), as well as group 6 and group 7 (p=0.0035).
Finally, on the context social interaction dynamics, the post hoc analysis revealed noteworthy findings. Firstly, when comparing the control group to all other groups (p<.001 Figure 2C), signifying significant distinctions in social interaction patterns between the control group and the remaining groups. Furthermore, within the inter-group comparisons, we observed statistically significant variations, group 1 and group 2 displayed a highly significant difference in social interaction (p<.001), as did group 2 when compared to group 4 (p<.001). Group 7 and group 8 also exhibited a highly significant discrepancy in social interaction (p=.00488).
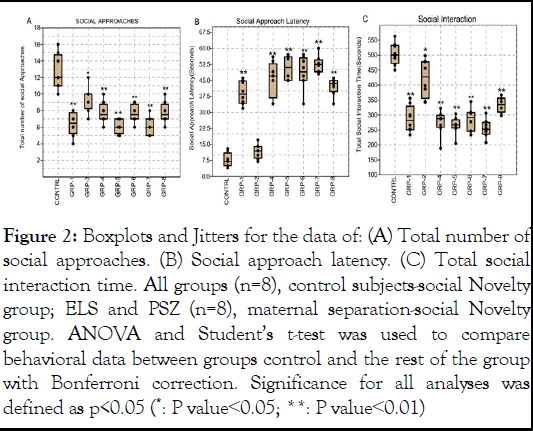
Figure 2: Boxplots and Jitters for the data of: (A) Total number of social approaches. (B) Social approach latency. (C) Total social interaction time. All groups (n=8), control subjects-social Novelty group; ELS and PSZ (n=8), maternal separation-social Novelty group. ANOVA and Student’s t-test was used to compare behavioral data between groups control and the rest of the group with Bonferroni correction. Significance for all analyses was defined as p<0.05 (*: P value<0.05; **: P value<0.01)
Impact of ELS and PSZ on Neutrophil Lymphocytes Ratio (NLR) and the Monocyte-Lymphocyte Ratio (MLR)
The ANOVA on the NLR revealed a significant difference in means between the groups (F (7, 56)=13.36 df=23.85, p<0.001 ω²=0.5748 Figure 3A). Furthermore, the post hoc test revealed significant differences between the control group and several experimental groups control group and GRP-1 (p<0.001), GRP-2 (p=0.0001), GRP-4 (p<0.001), GRP-5 (p<0.001), GRP-6 (p<0.001), GRP-7 (p<0.001), and GRP-8 (p<0.001).
Also, the post hoc showed significant differences between the control group and several experimental groups on the MLR, group 1 (p=0.029), GRP-2 (p=0.022), GRP-4 (p=0.003), GRP-5 (p=0.003), GRP-6 (p=0.002), and GRP-7 (p=0.009 Figure 3B) all exhibited significantly different social interaction patterns compared to the control group. However, no significant difference was found between the control group and GRP-8 (p=0.075).
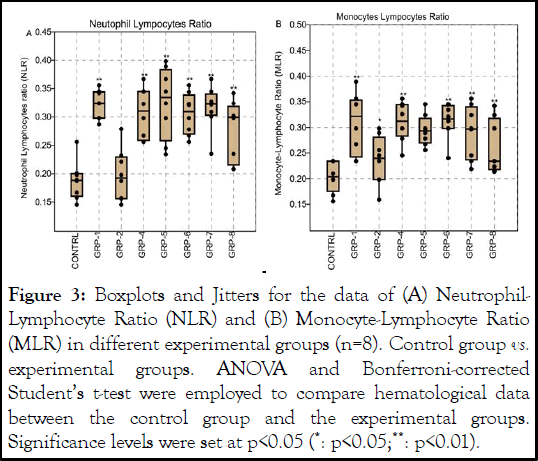
Figure 3: Boxplots and Jitters for the data of (A) Neutrophil- Lymphocyte Ratio (NLR) and (B) Monocyte-Lymphocyte Ratio (MLR) in different experimental groups (n=8). Control group vs. experimental groups. ANOVA and Bonferroni-corrected Student’s t-test were employed to compare hematological data between the control group and the experimental groups. Significance levels were set at p<0.05 (*: p<0.05;**: p<0.01).
Impact of ELS and PSZ on inflammatory biomarkers such as interleukin 1b and 6, MMP-9 and BDNF
The ANOVA on interleukin beta showed a significant difference in means between the groups, (F (7, 56)=20.96, df=23.43 p<0.001, ω²=0.6858 Figure 4A). The post hoc analysis showed significant differences between the Control group and several experimental groups (GRP-1 (p<0.001), GRP-2 (p<0.001), GRP-4 (p=0.021), GRP-5 (p=0.192), GRP-6 (p=0.065), and GRP-7 (p=0.009, Figure 4A)). However, it's worth noting that no significant difference was observed between the control group and GRP-8 (p=.075), suggesting comparable outcomes between these two groups. On the interleukin 6, the ANOVA also indicated significant differences in means among the groups but with a larger effect size (F (7, 56)=28.05, p<0.001, ω²=0.7474 Figure 4B). Furthermore, ANOVA for MMP-9 plasma levels showed a significant difference in means among the groups (F (7, 56)=28.05, p<0.001, ω²=0.7474 Figure 4C). Finally, post hoc comparisons on the BDNF revealed several noteworthy findings Figure 4D. First, group 1 vs. control group (p<0.001). Similarly, group 2 vs. control group (p<0.001). Group 4 vs. control group (p=0.003). Group 5 (GRP-5) showed a significant difference (p=0.050), indicating variations in outcomes. Group 6 (GRP-6) displayed a significant difference (p=0.044) compared to the control group. Finally, group 7 vs. control group (p=0.000). However, group 8 vs. control group (p=0.997).

Figure 4: Plasma biomarker levels in response to ELS and PSZ (A) Interleukin-1β (IL-1β) levels, (B) Interleukin-6 (IL-6) levels, (C) Matrix Metalloproteinase-9 (MMP-9) levels, and (D) Brain- Derived Neurotrophic Factor (BDNF) levels in plasma samples from rats subjected to chronic stress (n=8) compared to control rats (n=8). Data are presented as violin boxplots with individual data points (jitters) superimposed. Statistical analyses were performed using ANOVA followed by Bonferroni-corrected Student’s t-test to assess differences between the control group and the experimental group. Significance levels were set at p<0.05 (*: p<0.05;**: p<0.01).
ELS and PSZ impact on PFC and hippocampus astrocytes processes intersections
The ANOVA on central astrocytes processes intersection results indicated a significant difference in means among the groups (F (7, 56)=26.62, p<0.001, ω²=0.737 Figure 5A). Similarly, the ANOVA results on the lateral processes intersections revealed a significant difference in means among the groups however with a smaller effect size (F (7, 56)=5.273, p=0.0001148, ω²=0.3185 Figure 5B). Post hoc comparisons on the total astrocytes processes revealed several specific group differences (Figure 7C). Group 1 showed significant differences compared to the control group (p=0.000538), indicating distinct outcomes. Group 2 displayed significant differences when compared to the control group (p=0.000538) and group 5 (p=0.02277). Group 4 exhibited significant differences when compared to the control group (p<0.001) and group 5 (p=0.01489), suggesting variations in outcomes. Group 7 showed significant differences compared to the control group (p<0.001), group 5 (p>0.05), and group 8 (p>0.05), indicating distinct outcomes in these comparisons. However, no significant differences were found between the control group and group 6 (p=0.1048) or group 8 (p>0.05).
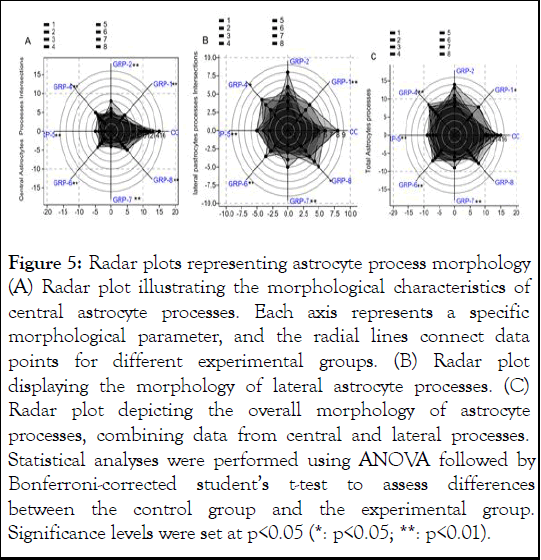
Figure 5: Radar plots representing astrocyte process morphology (A) Radar plot illustrating the morphological characteristics of central astrocyte processes. Each axis represents a specific morphological parameter, and the radial lines connect data points for different experimental groups. (B) Radar plot displaying the morphology of lateral astrocyte processes. (C) Radar plot depicting the overall morphology of astrocyte processes, combining data from central and lateral processes. Statistical analyses were performed using ANOVA followed by Bonferroni-corrected student’s t-test to assess differences between the control group and the experimental group. Significance levels were set at p<0.05 (*: p<0.05; **: p<0.01).
The immune system is a vital mechanism of surveillance and defense, not unlike the nervous system. The development of the immune system is not completed at birth, but rather continues throughout childhood, environmental stimulation in childhood years can have profound effects on the immune system. The findings of this study revealed that stressors such as Maternal Separation (MS), Gestational Stress (GS), and parental schizophrenia or their interactive nature shapes vulnerability to develop social cognition, anxiety and depression through inflammatory damages and astrocyte processes pruning in SD rats later in life. Consistent with our findings, ELS or PSZ such as MS has been reported to reduce social behaviour, increase anxiety and depression through social interaction behaviour and Elevated plus Maze in rats. The reduced social cognition and increased anxiety and depression could be due dysregulation of the immune system in rodents. Stress and psychopathological factors can trigger a chronic state of low-grade inflammation. In light of this, several studies have subsequently investigated the association between early-life stress and later immune functioning in rodents and non-human primates. Assessments of immune function have encompassed a diverse array of indicators, spanning from naturally occurring pro-inflammatory cytokines in the bloodstream to immune cell proliferation triggered by antigens in the spleen, to the expression levels of genes associated with inflammation in the brain, and the composition of the gut microbiota. Understandably, due to this combination of various study designs and measurement methods, the findings have yielded inconsistent outcomes concerning the connections between early-life stress and the immune system.
Consistent with substantial evidence demonstrating a decline in social cognition and an elevation in anxiety resulting from the influence of Early-Life Stress (ELS) and Parental Schizophrenia (PSZ), there is also notable research indicating alterations in immune cell proliferation, particularly concerning the Neutrophils-to-Leucocytes ratio and Monocytes-to-Lymphocytes ratio. We also observed a significant increase in the Neutrophilsto- Leucocytes Ratio (NLR) and Monocytes-to-Lymphocytes Ratio (MLR) in the groups exposed to ELS, PSZ, or both, in comparison to the control group, except for group 2 (exposed to prenatal stress only). Additionally, group 8 (comprising normal parents and schizophrenia pups) exhibited a statistically significant difference in both NLR and MLR when compared to group 7 (consisting of schizophrenia parents and pups) (p<0.05). The current findings align with previous research on neuropsychiatric conditions, specifically regarding Neutrophilsto- Leucocytes Ratio (NLR), Platelets-to-Lymphocytes Ratio (PLR), and Monocytes-to-Lymphocytes Ratio (MLR). For instance, one study suggested that individuals with Alzheimer's disease exhibited elevated NLR compared to Healthy Controls (HC), although this outcome appears to be more closely associated with age. Similarly, other investigations have reported increased NLR, PLR, and MLR values in individuals with Schizophrenia when compared to HC. Additionally, a metaanalysis revealed that individuals diagnosed with Major Depressive Disorder (MDD) displayed higher NLR levels compared to HC. In cases of chronic stress or exposure to psychopathological factors such as ELS or PSZ like aforementioned, the balance between these cell types may be disrupted. An elevated Neutrophil-to-Lymphocyte Ratio (NLR) is often associated with chronic inflammation and stress. Moreover, in line with a recent systematic review and metaanalysis focusing on individuals with Major Depressive Disorder (MDD) in China, it was observed that individuals with depression had elevated Neutrophils-to-Leucocytes Ratio (NLR), Platelets-to-Lymphocytes Ratio (PLR), and Monocytes-to- Lymphocytes Ratio (MLR) when compared to individuals without the condition.
In addition to immune cells, pro-inflammatory biomarkers have been a significant topic of discussion as potential contributors to impaired social cognition and pre-onset anxiety in the context of Major Depressive Disorder (MDD). This has been an ongoing focus of research and discussion in the field of psychiatry for some time now. Pro-inflammatory cytokines are predominantly produced by activated macrophages and are involved in the upregulation of inflammatory reactions. There is abundant evidence that certain pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α are affected by ELS and PSZ, and likely the first to causes anxiety and depression before the onset of MDD. In a manner akin in this research, we observed a significant increase in the IL-1β and IL-6 across the treatment groups compared to the control. Although the ontogenesis of the immune system starts well before birth there was no significant difference between the control and group 2 (Prenatal stress). Several separate studies have reported the impact of ELS and PSZ on the aforementioned interleukins; research has linked Early-Life Stress (ELS) to a more pronounced increase in circulating Interleukin (IL)-6 levels when individuals are subjected to tasks like delivering a speech about their job qualifications and performing mental arithmetic in front of impassive evaluators. Furthermore, a recent meta-analysis indicated that individuals with a history of childhood trauma exhibit notably elevated levels of circulating IL-6, Tumor Necrosis Factor-Alpha (TNF-α), and C-Reactive Protein (CRP), even in the absence of specific laboratory stressors, although not necessarily in the absence of the ordinary stressors encountered in daily adult life. In addition to the two pro-inflammatory biomarkers, in the current study we observed significant increase on the Matrix Metalloproteinase-9 (MMP-9) in the groups exposed to ELS and PSZ compared to the control. Indeed, elevated MMP-9 levels are often associated with inflammation. In cases of chronic stress or psychopathological exposure, MMP-9 levels may increase, potentially affecting neural plasticity and contributing to reduced social cognition and anxiety. Furthermore, MMP-9, Brain-Derived Neurotrophic Factor (BDNF) plays a pivotal role in neuronal survival, growth, and neurotransmitter regulation. It actively contributes to shaping neuronal plasticity, a fundamental factor for learning and memory processes. In our current study, we observed a significant reduction in BDNF levels in the groups exposed to Early Life Stress (ELS) and rats with schizophrenia (PSZ) compared to the control group. Notably, positive parenting interventions led to elevated BDNF levels, as evidenced by the significant differences between group 8 (comprising normal parents and schizophrenia pups) and group 7 (comprising schizophrenia parents and their offspring).
Originally considered an important regulator of early neuron development and survival, BDNF has more recently been implicated in various processes within the mature brain, including synaptic plasticity. Early studies have shown that BDNF mRNA is upregulated by stimulation paradigms that induce Long-Term Potentiation (LTP) in regions such as the hippocampus and prefrontal cortex. Historically, the presynaptic effects of BDNF have been attributed to its modulation of vesicular glutamate release efficiency in mammalian synapses, including those in the hippocampus. Finally, as a result of low BDNF in the brain in the current study we also observed significant reduction in astrocytes processes in the hippocampus and PFC between the groups exposed to ELS and PSZ compared to the control, with also significant differences between the group 8 and 7. While there is no direct, singular relationship between these MMP-9, BDNF and astrocytes processes, they are all components of a broader network of factors that can influence social cognition in various ways. While BDNF has been linked to learning and memory processes, which are integral to social cognition. It is possible that BDNF levels and activity could influence an individual's ability to learn and remember social cues, leading to changes in social cognition. Abnormal MMP-9 activity has been associated with neurological and neuropsychiatric disorders, some of which involve deficits in social cognition, anxiety and depression. Taken together as shown by our current study, changes in astrocyte function could potentially impact the neural circuits involved in social cognition, learning and memory.
In conclusion, the present study sheds light on the intricate web of interactions between early life stress, schizophrenia, neuroinflammation, and their collective impact on cognitive and motor functioning in a rat model. Our findings emphasize the multifaceted nature of these factors and their potential role in shaping neurodevelopmental outcomes. Our study underscores the importance of considering early life experiences as significant contributors to the risk and manifestation of psychiatric conditions like schizophrenia. Early life stress may serve as a critical trigger, potentiating the development of cognitive impairments and motor dysfunction often associated with this complex disorder. Moreover, the involvement of neuro-inflammation as a mediating factor underscores the need for a more comprehensive understanding of the immune-brain interface in the context of mental health.
While our findings provide valuable insights into the interplay between these factors, it is crucial to acknowledge the limitations of this rat model and the inherent complexities of translating these findings to human populations. Nevertheless, our work highlights the need for further investigation into the mechanisms by which early life stress and neuro-inflammation contribute to the heterogeneity of schizophrenia symptomatology, including cognitive and motor deficits.
There are no Acknowledgments
Fredrick Otieno Oginga (FOO) was responsible for managing all literature searches. FOO and Thabisile Mpofana (TM) collaborated on the initial draft of the paper, providing substantial input in the form of text passages and revisions for significant intellectual content. All authors have participated in, reviewed, and granted approval of the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing interests.
College of Health Science scholarship at The University of KwaZulu Natal. Department of Physiology, School of Medicine Nelson Mandela University Summerstrand, Gqeberha, South Africa Developing Research, Innovation, Localization and Leadership in South Africa (DRILL) Fund.
The funder will support the collection of data by the original investigators, data management, and data analysis. The funder is not involved in the design of the projects, protocol and the analysis plan. The funder will have no input on the interpretation or publication of the study results.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Oginga FO, Mpofana T (2025) Beyond Psychopathology: Examining the Complex Interactions of Early Life Stress, Schizophrenia, and Neuro-Inflammation on Cognitive and Motor Functioning. J Dep Anxiety. 14:560.
Received: 10-Sep-2023, Manuscript No. JDA-23-27491; Editor assigned: 12-Oct-2023, Pre QC No. JDA-23-27491 (PQ); Reviewed: 26-Oct-2023, QC No. JDA-23-27491; Revised: 18-Jan-2025, Manuscript No. JDA-23-27491 (R); Published: 25-Jan-2025 , DOI: 10.35248/2167-1044.25.14.560
Copyright: © 2025 Oginga FO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.