Internal Medicine
Open Access
ISSN: 2165-8048
ISSN: 2165-8048
Research Article - (2019)Volume 9, Issue 1
Introduction: Type-1 diabetes (T1D) is a disease that leads to pancreatic beta cells death, resulting in complete insulin loss.
T1D occurs as a result of autoimmunity which takes insulin producing β cells as a target and is considered as a metabolic disorder causing hyperglycemia resulting from a serious loss of endogenous insulin roduction. Using cadaveric islets as a replacement therapy was done to reverse diabetes. Many various progenitor cell types have been found in the peripheral blood mononuclear cell (PBMC) fraction denoting hose PBMCs may have the ability to differentiate into many mature functional cell types in certain microenvironments. It is settled that circulating peripheral blood mononuclear cells (PBMC) contain germinal cell population which can share in the regeneration of tissues in different organs.
Materials and Methods: 2 groups of type I diabetes patients had been monitored in a private clinic, number 40 each group, with 35 females and 45 males. It lied between 8-25 years old with 4-7 years of diabetes onset.
The first group was on insulin therapy and they received peripheral blood mononuclear cells concentrate directly in the dorsal pancreatic artery. The second group was only on insulin injection.
Results: The results show a significant increase in c peptide levels after direct injection of peripheral blood mononuclear cells in the dorsal pancreatic artery with p-value less than 0.0001.
Conclusion: The peripheral blood mononuclear cells can induce beta cell regeneration and increase beta cell mass which is detected by an increase in c peptide levels.
SGLT-2-i; Non-alcoholic fatty liver; Steato-hepatitis; Hepatic fibrosis
T1D is a disease that leads to pancreatic beta cells death, resulting in complete insulin loss. Autoimmune-mediated destruction of beta cells (type 1a) represents most type 1 diabetes cases whereas idiopathic destruction of beta cells (type 1b) represents only a minority of cases. T1D accounts for 5%-10% of the total cases of diabetes worldwide [1].
The human leukocyte antigen (HLA) complex on chromosome 6 is the most serious of the genes involved in susceptibility (and resistance) to T1D, specifically, the HLA class II. Two susceptibility haplotypes in the HLA class II region are now considered the principal susceptibility markers for T1D41 [2]. Whereas about 5% or less of peoples with HLA-conferred genetic susceptibility actually develop clinical disease, it’s interesting to know that about 95% of children with T1D have one or both vulnerability haplotypes [3].
There is a strong co-relation between T1D and other autoimmune diseases, like autoimmune thyroid disease [4]. It is settled that these autoimmune diseases are caused by genes within the major histocompatibility complex [5].
It is considered that Genes alone couldn’t result in T1D; there is a role also for the environment. There is strong evidence that not all persons who have high-risk genes get T1D. It is also evidenced that, most people with high-risk HLA class II genes (DR4/DQ8 and DR3/DQ2) do not get T1D. There must be at least one environmental factor exciting the autoimmune process before a clinical diagnosis of hyperglycemia and T1D. It is considered that islet autoantibodies the first laboratory evidence of beta cell autoimmunity) in high-risk individuals happen below 2 years of age [6]. Denoting that environmental factor is present in early life. Viral infection is considered as the most studied environmental factor causing type-1 diabetes [7].
Till this moment, insulin is considered the only therapy for the treatment of T1D. Until now, there is no treatment option to keep endogenous insulin production. All clinical trials failed to slow the progression of the disease or inhibit its onset. It is thought that specific therapies targeting the immune system are needed to prevent type 1 diabetes [8]. It is considered that HLA DQ8 which is present in about 60% of all T1D patients leads to considerable risk for disease development [9].
Using cadaveric islets as a replacement therapy was done to reverse diabetes [10] with some patients preserving insulin independence at 5 years. But, healthy pancreatic islets are not available for common use [11].
It is evidenced that rodent βcell mass could change in response to various circumstances. For example, βcell mass increases during pregnancy, due to both βcell hyperplasia and hypertrophy [12]. But after delivery, β cell apoptosis rate increases resulting in the return of β cell mass to normal [13]. It is thought that changes occurring in pregnancy could help the pregnant female to adapt with hyperglycemia by increasing insulin. Also, various conditions of insulin resistance resulting in increased β cell mass [14].
It is evident that new insulin-producing β cells are created from endocrine progenitor cells during embryonic development of the pancreas [15]. These progenitor cells arise from the pancreatic epithelium and then differentiate into various hormone‐producing cells to make the islets of Langerhans [16].
It is considered that β cell formation which is shifted postnatally from an embryonic mode based on progenitor cells to simple duplication’ mode is reversible and consequently under certain conditions, the embryonic mode is motivated. Which give a good therapeutic option, even for type 1 diabetes patients in spite of completely losing their β cells, by keeping the possibility of stimulating the embryonic mode for β cell genesis? It has been thought that adult pancreatic ducts could act as ‘facultative progenitor cells’ in which, they could maturate into new β cells through the embryonic pathway when subjected to certain injury circumstances, a process named [17]. It is also thought that new β cells could originate from acinar cell Trans differentiation [18], or bone marrow-derived cells [19].
Stem cells are cells that are able to construct any human tissue, and so these cells have a great possibility for tissue regeneration. To say that the cells are “stem cells,” they should have a fundamental character. In which, stem cells must be able to selfrenew in an unlimited way to give cells as same as the original cell. What happens is like cancer cells which split in an organized way but the difference is that stem cell division is highly organized. They can result in a specialized cell type [20].
Stem cells are sorted into two categories relied on their biologic character - pluripotent and multipotent stem cells.
Pluripotent stem cells can give rise to all cell types in the body. In natural development, pluripotent stem cells are existed just in the embryo before giving rise to the more specialized multipotent stem cells that could differentiate into particularized cells. In the other side, more limited multipotent stem cells have several subtypes: some could be only cells of a particular germ line and the other cells, could be of a special character. We can say that pluripotent cells could give rise to any cell of the body by differentiating into multipotent stem cells that could pass with a series of divisions into more limited specialized cells [21].
It is considered that most adult tissues contain stem cells which are able to differentiate into a specific tissue. Many various progenitor cell types have been found in the peripheral blood mononuclear cell (PBMC) fraction denoting that PBMCs may have the ability to differentiate into many mature functional cell types in certain microenvironments [22]. It is considered that PBMCs can differentiate along different lineages relying on culture conditions or where it will be transplanted [23].
Blood is considered the most appropriate source in which can get stem cells and we can get from patients in whom it can be saved for future utilization. Fortunately, stem cells can be developed in culture and can be stimulated to convert to pluripotent stem cells (iPSCs) [24].
Endothelial progenitor cells which are present in BM and peripheral blood have the ability to convert to mature endothelial cells (ECs) for revascularization, and so present a substantial stimulatory pathway for angiogenesis [25].
Granulocyte colony-stimulating factor multiple injections can assemble progenitor cells from the BM and moving them to peripheral blood circulation [26]. Mesenchymal stem cells which are derived from peripheral blood mononuclear cells could stimulate angiogenesis at sites with ischemia, and decrease the ischemic deterioration [27].
It is considered that the peripheral blood mononuclear cell population contains various progenitor cells. Some studies declared that comparatively few hematopoietic stem cells are present in the peripheral blood sharing in the conservation of hematopoietic function [28]. Fortunately, fibrocytes, which is considered as a Mesenchymal cell, were found in cultures derived from the peripheral blood mononuclear cell of the peripheral blood [29]. Fibrocytes in the peripheral blood were considered to have great differentiation ability under certain conditions. Tissue Injury is considered to be the cause for moving these progenitor cells to injury sites in which sharing in the regeneration of these tissues [30].
It is settled that circulating peripheral blood mononuclear cells [PBMC] contain germinal cell population which can share in the regeneration of tissues in different organs [31].
PBMCs contain Cells which comprise CD14+ monocytes which are created from hematopoietic stem cells in the bone marrow and consist of about 10% of white blood cells in the peripheral circulation. It also considered that Monocytes could differentiate and convert to multiple phagocytes [32]. It is suggested that mononuclear cells could differentiate into other cell types beside phagocytes, like skeletal muscles and bone, which making them a possible choice for tissue repair [33].
To get new insulin-producing cells, we can use peripheral blood mononuclear cells as an origin because these cells may be capable of conversion into unrelated cell types. There are only two studies so far which used monocytes as the source for insulin-producing cells [34]. Based on previous studies, Monocytes could be de-differentiated in vitro when Interleukin-3 (IL-3) and Macrophage Colony-stimulating Factor (M-CSF) are present, and so we can say that this process can transform peripheral blood mononuclear cells to Progenitor Cells of Monocytic Origin (PCMOs) . Fortunately, when Epidermal Growth Factor (EGF) and Hepatocyte Growth Factor (HGF) are present, PCMOs could be moreover cultured in vitro in for 4-6 days, consequently, they were able to express pancreatic genes yielding little amounts of insulin [35].
Peripheral blood mononuclear cells could be converted to cells that produce sufficient insulin to correct blood glucose [36], and so we can say that Peripheral blood mononuclear cells could be used for regeneration of pancreatic beta cells as autologous transplants in which are removed from a person, stored, and later given back to that same person [37].
It was seen that when autologous bone marrow stem cells are injected directly into the pancreas via the gastro duodenal artery in T2DM, there was a proven success [38].
2 groups of type I diabetes patients had been monitored in a private clinic, number 40 each group, with 35 females and 45 males after written consent of all patients. All the patients Lied between 18-25 years old with 8-12 years of diabetes onset.
The first group was on insulin therapy in the form of Lantus once daily and apidra 3 times daily and they received auto peripheral blood mononuclear cells concentrate directly in the dorsal pancreatic artery.
The second group was only on insulin injection in the form of Lantus once daily and Apidra 3 times daily.
In the first group; we measured postprandial c peptide levels before and after 3 months of direct injection of peripheral blood mononuclear cells in the dorsal pancreatic artery.
In the second group, we measured c peptide levels at the beginning of the trial and after 3 months.
Preparation of peripheral blood mononuclear cells
All the patients in the 2 groups received granulocyte colony stimulating factor drug (Filgrastim) daily for 5 days to stimulate the bone marrow to produce granulocytes and stem cells and release them into the bloodstream.
Filgrastim is a human granulocyte colony stimulating factor (GCSF) produced by recombinant DNA technology, which regulates the production of neutrophils within the bone marrow.
Filgrastim was given by subcutaneous injection.
Filgrastim was given on a daily basis for 5 days.
Filgrastim was refrigerated and removed from refrigerator 30 minutes before injection. And not shaken and Protected from light.
Was given by a dose of 10 mcg/kg/day SC.
No side effects were detected in the patients.
The blood was collected and manipulated in ibn Sina laboratory centre.
About 60 cm blood from a peripheral vein was collected in a heparinized tube.
After that mixed with equal volume of phosphate buffered saline.
Ficoll-Hypaque solution was used for separation of monocytes.
It was placed at the bottom of the tube.
Using 5 ml Ficoll-Hypaque for 10 ml blood/PBS mixture.
Centrifuging for 30 min at 2000 rpm.
The upper layer that contains the plasma and most of the platelets were removed by a pipet.
The mononuclear cell layer was transmitted to another centrifuge tube.
Then cells were washed by adding phosphate buffered saline and centrifuging 10 min at 1300 rpm.
And then mononuclear cells were re-suspended with plateletrich plasma and counted.
The injection was done in tanta radiology centre by direct injection in the dorsal pancreatic artery of about 30 cm concentrate of mononuclear cells with prp.
For analysis of data we used unpaired t-test.
In the first group of patients (Figures 1-3, Tables 1 and 2) on insulin and mononuclear cells injection directly in dorsal pancreatic artery; p-value; <0.0001 which is sign.
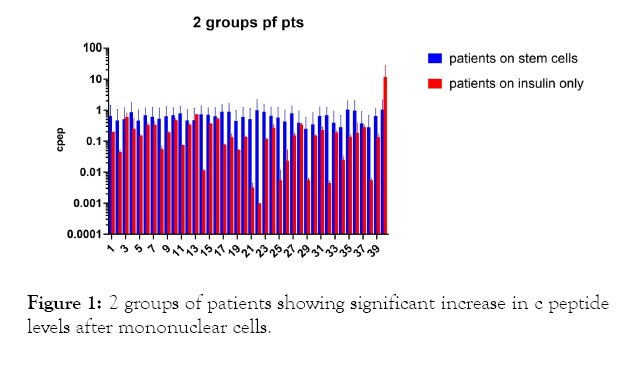
Figure 1: 2 groups of patients showing significant increase in c peptide levels after mononuclear cells.
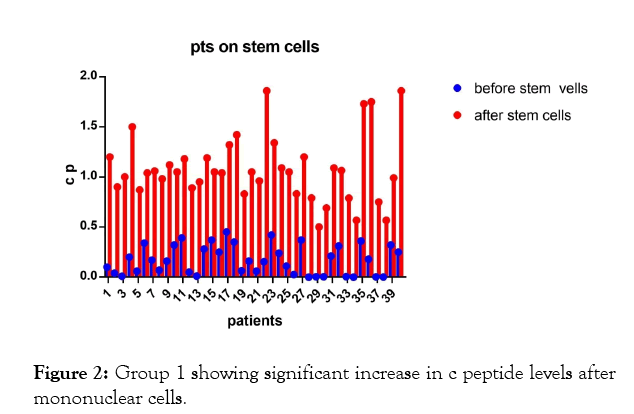
Figure 2: Group 1 showing significant increase in c peptide levels after mononuclear cells.
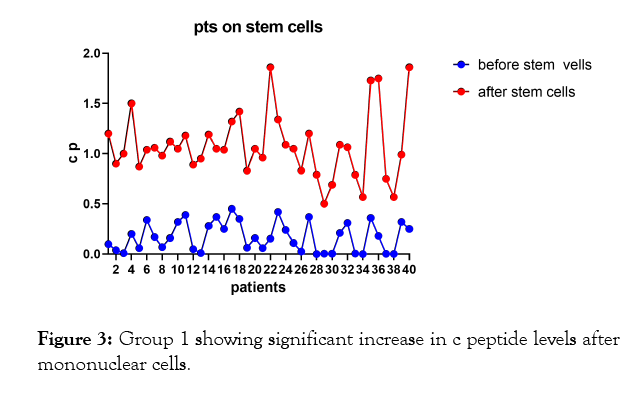
Figure 3: Group 1 showing significant increase in c peptide levels after mononuclear cells.
| x | Group A | Group B | ||
|---|---|---|---|---|
| X-Tile | Patients on stem cells | Patients on Insulin only | ||
| x | Before Stem | After Stem c' | Before | After |
| 0.1 | 1.2 | 0.2 | 0.2 | |
| 0.04 | 0.9 | 0.05 | 0.04 | |
| 0.01 | 1 | 0.75 | 0.46 | |
| 0.2 | 1.5 | 0.05' | 0.45 | |
| 0.06 | 0.87 | 0.16 | 0.14 | |
| 0.34 | 1.04 | 0.37 | 0.29 | |
| 0.17 | 1.08 | 0.41 | 0.27 | |
| 0.07 | 0.98 | 0.06! | 0.047 | |
| 0.16 | 1.12 | 0.2 | 0.18 | |
| 0.32 | 1.05 | 0.53 | 0.43 | |
| 0.39 | 1.18 | 0.076 | 0.075 | |
| 0.05 | 0.89 | 0.36 | 0.32 | |
| 0.011 | 0.95 | 0.74 | 0.72 | |
| 0.28 | 1.19 | 0.011 | 0.012 | |
| 0.37 | 1.05 | 0.38 | 0.36 | |
| 0.25 | 1.04 | 0.54 | 0.52 | |
| 0.45 | 1.32 | 0.08 | 0.076 | |
| 0.35 | 1.42 | 0.16 | 0.11 | |
| 0.063 | 0.83 | 0.054 | 0.049 | |
| 0.16 | 1.05 | 0.14 | 0.13 | |
| 0.06 | 0.96 | 0.004 | 0.002 | |
| 0.154 | 1.86 | 0.001 | 0.001 | |
| 0.42 | 1.34 | 0.12 | 0.11 | |
| 0.24 | 1.09 | 0.32 | 0.22 | |
| 0.11 | 1.05 | 0.01 | 0.001 | |
| 0.026 | 0.832 | 0.044 | 0.003 | |
| 0.37 | 1.2 | 0.17 | 0.13 | |
| 0.001 | 0.79 | 0.37 | 0.32 | |
| 0.004 | 0.5 | 0.006 | 0.005 | |
| 0.005 | 0.69 | 0.16 | 0.14 | |
| 0.21 | 1.09 | 0.27 | 0.19 | |
| 0.31 | 1.065 | 0.00! | 0.004 | |
| 0.006 | 0.789 | 0.21 | 0.17 | |
| 0.001 | 0.567 | 0.03 | 0.02 | |
| 0.36 | 1.73 | 0.16 | 0.12 | |
| 0.18 | 1.75 | 0.038 | 0.33 | |
| 0.003 | 0.75 | 0.32 | 0.25 | |
| 0.002 | 0.567 | 0.006 | 0.005 | |
| 0.32 | 0.99 | 0.16 | 0.11 | |
| 0.25 | 1.86 | 0.43 | 23 | |
Table 1: Group 1 and group 2 of patients.
| Unpaired t test | |
|---|---|
| Table Analyzed | |
| Column B | After Stem Cell |
| Vs. | Vs. |
| Column A | Before Stem Cell |
| Unpaired t test | |
| p-value | <0.0001 |
| p-value summary | **** |
| Significantly different (p<0.05) ? | Yes |
| One- or two-tailed p-value? | Two-tailed |
| t,df | t=16.05, df=78 |
| How big is the difference? | |
| Mean ± SEM of column A | 0.1719 ± 0.02287, n=40 |
| Mean ± SEM of column B | 1.078 ± 0.05158, n=40 |
| Difference between means | 0.9059 ± 0.05642 |
| 95% confidence interval | 0.7935 to 1.018 |
| R squared (eta squared) | 0.7677 |
| F test to compare variances | |
| F,DFn,Dfd | 5.086, 39, 39 |
| p-value | <0.0001 |
| p-value summary | **** |
| Significantly different (p<0.05) ? | Yes |
Table 2: Patients on stem cells.
| Unpaired T-test | |
|---|---|
| Table Analyzed | P is only on Insulin |
| Column B | on Insulin after |
| Vs. | Vs. |
| Column A | On Insulin before 6 |
| Unpaired T-test | |
| p-value | 0.3427 |
| p-value summary | ns |
| Significantly different (p<0.05) ? | No |
| One- or two-tailed p-value? | Two-tailed |
| t, df | t-0.9547, df=78 |
| How big is the difference? | |
| Mean ± SEM of column A | 0.2042 ± 0.03141, n=40 |
| Mean ± SEM of column B | 0.7503 ± 0.5711, n=40 |
| Difference between means | 0.5461 ± 0.572 |
| 95% confidence interval | -0.5927 to 1.685 |
| R squared (eta squared) | 0.01155 |
| F test to compare variances | |
| F, DFn, Dfd | 330.6,39, 39 |
| p-value | <0.0001 |
| p-value summary | **** |
| Significantly different (p<0.05) ? | Yes |
Table 3: Patients on insulin only.
In the second group of patients (Figure 1, 4, 5, Tables 1 and 3) who were on insulin only; p-value 0.3427 which is nonsignificant.
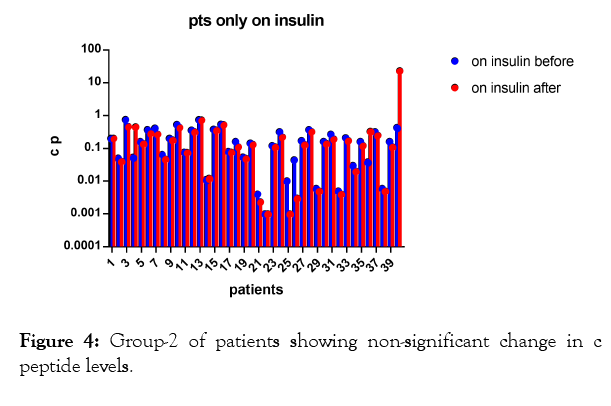
Figure 4: Group-2 of patients showing non-significant change in c peptide levels.
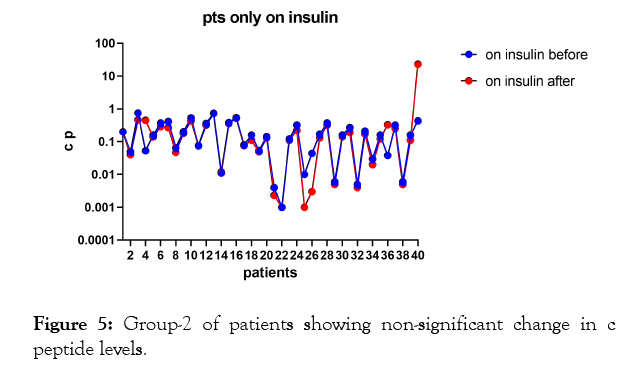
Figure 5: Group-2 of patients showing non-significant change in c peptide levels.
The results shows significant increase in c peptide levels after direct injection of peripheral blood mononuclear cells in dorsal pancreatic artery after stimulation by granulocyte colonystimulating factor (G-CSF) with p-value less than 0.0001 as seen in Figures 1-3 with non-significant change in c peptide levels in the second group who were on insulin only and who received granulocyte colony-stimulating factor (G-CSF) also with p-value; 0.3427 which is non-significant.
Type 1 diabetes (T1D) occurs as a result of autoimmunity which takes insulin producing β cells as a target and is considered as a metabolic disorder causing hyperglycemia resulting from a serious loss of endogenous insulin production in the past type 1 diabetes was considered as a disease of children but now it is evidenced that type 1 diabetes could occur even in older peoples [39].
It is settled that more than 30,000 persons getting type 1 diabetes per year in US. and the incidence of type 1 diabetes all over the world is elevated to about 28% per week [40]. Unfortunately, most patients with type 1 diabetes don't get glycemic control in spite of insulin therapy and complications of type 1 diabetes occur frequently. Type 1 diabetes put great physical and physiological stress on patients and their relatives also lead to an elevated risk of morbidity and mortality. for all of these reasons, there must be new therapies to cure type 1 diabetes, targeting beta cell regeneration and stopping the immune system from attacking beta cells [40].
It is evidenced that peripheral blood mononuclear cells (PBMCs), are able to liberate considerable amounts of active paracrine factors that make advantageous repair effects. The PBMC were used in a successful way for the treatment of some serious diseases like myocardial infarction, heart failure, and stroke.
In this study, we used peripheral blood mononuclear cells to evaluate their effect on pancreatic beta cells regeneration and on c peptide levels, by infusion these cells directly in the dorsal pancreatic artery and comparing them with the group of patients on insulin therapy only.
There are 2 limitations of this study; the first the small number of patients but we can explore the results to the general population, the second limitation is whether the results are transient or persistent.
As type 1 diabetes is a serious disease and there are many stresses on patients and their relatives, we hope to find a new conclusive therapy.
The results of our study show that autologous peripheral blood mononuclear cells which are easy to get may be a good choice for pancreatic beta cells regeneration and consequently for type-1 diabetes cure.
Further large-scale studies and clinical trials are required to verify our results.
As stem cells are considered as being able to construct any human tissue, and have a great possibility for tissue regeneration, and as Peripheral blood is a large accessible source of adult stem cells. peripheral blood mononuclear cells which are created from hematopoietic stem cells in the bone marrow and consist of about 10% of white blood cells in the peripheral circulation could differentiate into other cell types beside phagocytes, like skeletal muscles and bone, which making them a possible choice for tissue repair. The peripheral blood mononuclear cells can induce beta cell regeneration and increase beta cell mass which is detected by an increase in c peptide levels which may add a new choice for type 1 diabetes treatment.
Citation: Younis M (2019) Autologous Peripheral Blood Mononuclear Cells Can Lead to Pancreatic Beta Cell Regeneration. Intern Med 9:305. doi: 10.35248/2165-8048.19.9.305.
Received: 16-Feb-2019 Accepted: 04-Mar-2019 Published: 11-Mar-2019 , DOI: 10.35248/2165-8048.19.9.305
Copyright: ©2019 Younis M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.