Internal Medicine
Open Access
ISSN: 2165-8048
ISSN: 2165-8048
Research Article - (2025)Volume 15, Issue 1
Background: Despite studies on trace metals and Chronic Respiratory Diseases (CRDs), their causal relationship in American adults is uncertain. Our research applied observational and bidirectional Mendelian Randomization (MR) methods to ascertain this association.
Objective: The aim of this study was to evaluate the potential linkages between serum levels of Copper (Cu), Zinc (Zn) and Selenium (Se) and the incidence of chronic respiratory diseases within the adult demographic of the United States.
Methods: Drawing on the National Health and Nutrition Examination Survey (NHANES) 2013-2016 data, our study incorporated 2807 adults to examine serum Cu, Zn and Se impacts on CRDs risks using logistic regression and cubic spline analysis. Subsequently, bidirectional MR assessments were conducted to ascertain the causative linkages pertaining to these associations.
Results: Employing adjusted-weighted logistic models, high serum Cu significantly escalated the risks for emphysema (OR:3.83) and Chronic Obstructive Pulmonary Disease (COPD) (OR:2.14-3.33), while elevated Cu and Zn levels corresponded to respective OR of 1.82 and 1.91 for chronic tracheitis and COPD. Conversely, moderate Se reduced tracheitis risk (OR:0.64). MR indicated no genetic causation between Cu and respiratory diseases (p>0.05), yet Cu exposure was causally linked to COPD (p=0.003), as was Zn (p=0.014). selenium’s protective genetic association with chronic tracheitis was confirmed (p=0.010).
Conclusion: High serum Cu links to increased emphysema, tracheitis and COPD risk; Zn equally raises COPD risk; Se may lessen tracheitis risk. This observation warrants confirmation through additional large-scale, prospective cohort studies with sufficient sample sizes and extended follow-up durations.
Cu; Zn; Se; Chronic Respiratory Diseases (CRDs); NHANES; Mendelian Randomization (MR)
COPD, asthma, chronic tracheitis and emphysema are prevalent CRDs. COPD is a pathologic condition characterized by persistent inflammation and constriction of the airways, serving as an umbrella term that encompasses long-term lung afflictions which impede airflow and induce respiratory difficulty, the most common of which are chronic tracheitis and emphysema. The condition is principally linked to risk factors like tobacco smoking and prolonged exposure to smoke, dust and chemical agents. In 2019, an estimated 391.9 million individuals aged 30-79 globally reported cases of COPD, posing a substantial burden on the international healthcare system. COPD is currently ranked as the fourth leading cause of death worldwide and is anticipated to rise to the third by 2020, with a prevalence rate of about 10%. Contrarily, asthma represents a distinct and typically fully reversible inflammatory airway condition, characterized by hyper-responsiveness and intermittent constraints on airflow, leading to wheezing, chest tightness, shortness of breath and exacerbated symptoms at night or early morning. The prevalence of asthma has witnessed an exponential increase in Western nations in recent decades. According to the World Health Organization, asthma was the most prevalent chronic respiratory disease globally in 2015, with twice the number of COPD cases, leading to an annual loss of 15 million disability-adjusted life years and accounting for approximately 1% of the worldwide disease burden, with an estimated 358 million individuals affected. The WHO also forecasts a rise in asthma patients by another 100 million by 2025 [1].
Essential trace metals are vital micronutrients in the human body, playing indispensable roles across various metabolic reactions and are key to maintaining normative physiological functions including anti-inflammatory actions, cellular signal modulation, participation in immune responses and fortification against oxidative damage. Cu, Zn and Se are significant trace metals for human health and have been associated with neurodegenerative disorders, various forms of cancer, thyroid functionality, risk of cardiovascular diseases and non-alcoholic fatty liver disease. Recent studies have underscored the potential contribution of trace elements like Cu, Zn and Se in combating respiratory viral infections. Prior investigations had delved into the interplay between serum Cu levels and COPD, albeit with inconclusive findings. Nonetheless, most studies on the nexus between trace metals and chronic respiratory diseases have focused predominantly on the ties between a solitary metal and a single disease, rather than examining the collective relationship between multiple trace metals and an assortment of chronic respiratory diseases.
Historically, investigations have shed light upon the potential mechanisms through which trace metals may influence chronic lung conditions. For instance, Cu transcends its role as a vital metal ubiquitously found in nature to being an essential constituent of numerous enzymes imperative for the maturation and functional efficacy of pulmonary immune cells, the respiratory tract's microbial composition and disease progression. It is pivotal for immune defense and antiinflammatory responses within the respiratory tract. Elevated concentrations of Cu have been linked to lung inflammation and chronic respiratory disorders. A substantial population study from Toronto, Canada revealed that long-term exposure to Cu may escalate the risk of various respiratory ailments, particularly COPD, pneumonia and incidents of respiratory system-associated mortality. Zn is critical for immune cell functionality and sustaining antioxidant defense mechanisms, with Zn deficiencies potentially leading to a weakened immune response, diminished antibody production and an increased vulnerability to pathogenic infections. Se serves as a principal component of glutathione peroxidases, enzymes that proficiently neutralize hydrogen peroxide in the organism, shielding cellular structures from oxidative injury. Given that the lungs are amongst the most exposed organs to external environmental factors, they are frequently susceptible to oxidative stress and deleterious substances. Hence, a robust antioxidant system is crucial for countering these stimuli and averting cell and tissue damage. Through its modulation of (Glutathione Peroxidase, GPx) activity, Se plays a role in preserving this defense mechanism. Insufficient Se levels might exacerbate inflammatory responses, leading to more severe pulmonary pathology and affecting the progression of various lung diseases.
The acute exacerbation of chronic lung conditions, hospitalizations, missed classes and the financial toll of healthcare costs have culminated in grave public health concerns. In light of the diseases' high prevalence and association with numerous detrimental health outcomes worldwide, elucidating the risk factors for chronic lung diseases is essential. Building upon the existing body of research, we utilized data from NHANES to explore the association between serum trace elements and chronic lung diseases among U.S. adults. Our objective was to advance our understanding of the role of serum trace elements in the pathophysiology of chronic pulmonary disorders.
Data source and participants
Our analysis was predicated on data derived from the NHANES, a stratified, multistage probability endeavor that encapsulates a representative sample of the non-institutionalized civilian population in the United States. Relevant information about NHANES can be accessed.
The methodological frameworks deployed for the NHANES received approval from the institutional review board of the national center for health statistics, which operates under the auspices of the centers for disease control and prevention. Prior to inclusion in the study, informed consent was duly acquired from all participants.
Employing bidirectional two-sample MR with genetic instrumental variables from GWAS data, this research assessed the causal relationship between trace metals and CRDs. Detailed biogenetic information on trace metals and CRDs can be found.
Figure 1 delineates the research design, sampling and exclusion procedures employed in the study. The research encompassed two publicly accessible datasets from the survey cycles of 2013-2014 and 2015-2016. The cycle for 2013-2014 included a total of 10,175 participants, while the 2015-2016 cycle had 9,971 participants. In each cycle, subjects were selected based on the following criteria:
1) Adolescents under the age of 20 were excluded; 2) Participants lacking serum trace metal analysis were omitted; 3) Individuals without a diagnosis of CRD were excluded; 4) Data missing multiple covariates, such as age, gender, race, education level, marital status, income, smoking, alcohol consumption, physical activity, hypertension and diabetes were also excluded from the study. Across both cycles, a combined total of 2807 participants were included in our analysis [2].
Figure 1: Flowchart of the population included in our final analysis.
Measures
Serum trace metals: Blood specimens from all participants were collected into the appropriate tubes and gently inverted five to six times promptly. The samples were allowed to coagulate at room temperature for 30-45 minutes before being centrifuged at 2,900 revolutions per minute for 15 minutes. Subsequently, the serum was stored under frozen conditions and transported to the division of laboratory sciences at the national center for environmental health, centers for disease control and prevention, for analysis. Serum concentrations of Zn, Cu and Se were determined using Inductively Coupled Plasma Dynamic Reaction Cell Mass Spectrometry (ICP-DRC-MS), following a comprehensive quality control procedure. A full overview of the laboratory procedures is available on the NHANES website.
The Limits of Detection (LOD) for serum Cu, Zn and Se were 2.5 µg/dL, 2.9 µg/dL and 4.5 µg/L, respectively, with all datasets for the three trace metals surpassing the LOD. In the logistic regression model, serum trace metals were categorized into three groups based on the tertiles of their measured values (
CRDs: The assessment of CRDs was contingent upon information ascertained from the questionnaire section of the national health interview survey conducted in the United States. For instance, to evaluate the presence of COPD, participants were questioned, "have you ever been diagnosed with COPD by a health care professional?" subsequently, participants were stratified based on their responses (yes or no), while answers denoted as "don't know" or "refusal" were deemed as missing data; this approach was analogously applied to the evaluation of emphysema, chronic tracheitis and asthma for inclusion in the study. A more comprehensive exposition of all the variables is accessible on the NHANES database website.
Covariates: Based on previously published research, potential covariates associated with CRDs have been identified. The presence of mediating effects among these covariates was evaluated using the method proposed by Baron and Kenny. These covariates encompass socio-demographic elements, behavioral traits and health characteristics [3].
The sociodemographic variables included age categories (20-39, 40-59 and 60 years and older), gender (female and male), racial groups (Non-Hispanic white, Mexican American, Non-Hispanic black and other/multiracial), marital status (married, widowed, divorced, separated, never married and living with partner), levels of educational attainment (below high school graduate, high school graduate or equivalent and some college education or higher) and tiers of family income (0-130% of the Federal Poverty Level (FPL), 131-350% of the FPL and above 350% of the FPL, where FPL is defined as the family income to poverty threshold ratio).
Behavioral characteristics were delineated by smoking status (Individuals with a lifetime consumption of 100 or more cigarettes were classified as smokers, with the designation being recorded as either 'yes' or 'no'), alcohol consumption (no or yes) and physical activity levels (inactive or active).
Health-related factors encompassed body mass index (underweight/normal, overweight and obese), presence of hypertension (no or yes) and diabetes status (no or yes).
Statistical analysis: Our study's primary strength lies in the use of an expansive, nationally representative sample of American adults, which enhances the reliability and precision of our findings. Moreover, we employed serum trace metal levels as markers, overcoming the biases of dietary recall surveys and confounding of individual metabolic factors. However, there are several limitations to our study that must be acknowledged. Firstly, the cross-sectional design of our study inevitably allows for confounding factors and precludes us from establishing a causal relationship between serum Cu levels and chronic respiratory diseases. Additionally, limited to questionnaire data from NHANES 2013-2016, we could not explore serum trace element profiles over time.
Analysis was conducted in accordance with the Centers for Disease Control and Prevention (CDC) NHANES data analysis guidelines. Mean ± SD and percentages were used to describe continuous and categorical variables separately. Baseline characteristic percentages between survey cycles or CRDs phenotypes were tested using the Rao and Scott adjusted χ 2 test. A stepwise binary logistic regression was then employed to analyze the Odds Ratios (ORs) and 95% Confidence Intervals (CIs) for the serum trace metals present at risk. In model I, sociodemographic characteristics were adopted, with behavioral characteristics further incorporated into model II and additional adjustments made for health factors in model III.
Furthermore, using Restricted Cubic Splines (RCS) models at the 5th, 50th and 95th percentiles of the distribution, we examined potential dose-response relationships between serum trace metals and sleep disorders.
Multiple SNPs served as Instrumental Variables (IVs) in our MR analysis. We computed pooled Wald ratios via the Inverse Variance Weighted (IVW) method, determining the trace metals-CRDs association. MR-Egger regression honoring distinct IV assumptions bolstered our findings' robustness, with the mean pleiotropic effect gauged by the MR-Egger intercept (P<0.05). Sensitivity was affirmed through the leave-one-out approach. Additionally, we analyzed the reverse causation of Cu on COPD, employing the two sample MR software package.
The leave-one-out method was conducted as an analysis of sensitivity to check whether the MR estimate is driven by a single SNP. We made a visible funnel plot for heterogeneity by showing the association of the inverse of the SE with the MR estimate. In regard to reverse MR, the procedures mentioned above were repeated via swapping trace metals and CRDs with the same database to assess the reverse-causal association of CRDs and trace metals.
Statistical analyses were performed using R software version 4.1.0 and Stata 16.0. All statistical tests were two-sided, with significance considered at p<0.05 [4].
Main analyses of NHANES
Delineates the baseline characteristics of the participants, stratified by survey wave. The overall prevalence of emphysema stands at 2.2%, showing a slight upward trend from 2.0% in 2013-2014 to 2.5% in 2015-2016, though this increase lacks statistical significance. The changes in the prevalence rates of chronic tracheitis, COPD and asthma over the two periods also have no statistical significance. The distribution of serum Se levels across the two periods showed a slight increase at the lowest (
Table displays the characteristics of participants, comparing those with CRDs to those without. From the perspective of univariate analysis, advanced age, race, level of education, marital status, alcohol consumption, smoking, hypertension, diabetes, income status and serum Cu levels may be risk factors for emphysema. Conversely, BMI, serum Zn levels, serum Se levels, physical activity and sex appear to be unassociated with the prevalence of emphysema (Supplementary Tables 1 and 2).
Advanced age, sex, race, educational attainment, marital status, smoking, hypertension, diabetes, serum Cu levels, income status and BMI may act as risk factors for chronic tracheitis. In contrast, alcohol consumption, serum Zn levels, serum Se levels and physical activity do not appear to correlate with the incidence of chronic tracheitis.
Advanced age, race, marital status, income status, alcohol consumption, smoking, hypertension, diabetes and serum Cu levels may be potential risk factors for COPD. Conversely, educational level, BMI, serum Zn levels, serum Se levels, physical activity and gender appear to be unrelated to the prevalence of COPD.
Advanced age, gender, level of education, marital status, alcohol consumption, smoking, hypertension, BMI and serum Cu levels may be potential risk factors for asthma. In contrast, race, income status, serum Zn levels, serum Se levels, physical activity and diabetes appear to be unrelated to asthma prevalence.
Outlines the findings from a binary logistic regression analysis assessing the impact of trace serum metals on the prevalence of CRDs in the U.S. adult population. Comprehensive adjustments were made to account for sociodemographic factors, behavioral patterns and health-related covariates in the analysis. The study observed no significant correlation between serum levels of Zn or Se and the likelihood of emphysema. Nevertheless, adults exhibiting the highest quartile of serum Cu concentration (>Q3) were associated with a substantially higher risk a 283.0% increase of emphysema compared with those within the lowest quartile (
Follow-up analyses applying Restricted Cubic Splines (RCS) were implemented to delineate the dose-response dynamics linking serum trace metal concentrations with the incidence risk of CRDs. Depicted in Figures 2A and 2B, a discernible increase in the risk for both emphysema and COPD was observed corresponding to elevated Cu exposure, with these elevations attaining statistical significance (p=0.023 for emphysema and p=0.016 for COPD, respectively). Nevertheless, the evidence currently available is inadequate to assert a nonlinear enhancement of risk in direct proportion to increased Cu exposure. Displayed in Figure 2C is a statistically significant positive association between exposure to Cu and the occurrence of chronic tracheitis (p=0.027), exhibiting a nonlinear pattern as exposure levels vary (p for nonlinearity=0.013). Figure 2D indicates that the association between Cu and asthma lacks statistical significance. Moreover, while Figures 2E to 2L showcase the potential interaction effects of Zn, Se and CRDs within the serum as analyzed by the logistic regression model, these relationships failed to attain statistical significance.
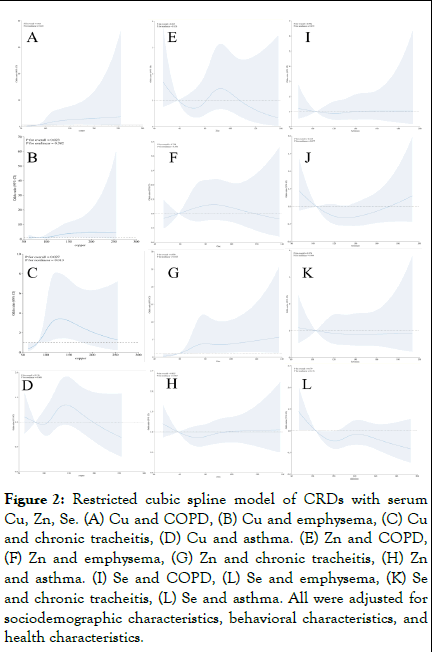
Figure 2: Restricted cubic spline model of CRDs with serum Cu, Zn, Se. (A) Cu and COPD, (B) Cu and emphysema, (C) Cu and chronic tracheitis, (D) Cu and asthma. (E) Zn and COPD, (F) Zn and emphysema, (G) Zn and chronic tracheitis, (H) Zn and asthma. (I) Se and COPD, (L) Se and emphysema, (K) Se and chronic tracheitis, (L) Se and asthma. All were adjusted for sociodemographic characteristics, behavioral characteristics, and health characteristics.
MR of trace metals and CRDs
We pinpointed SNPs robustly linked with Cu, Zn and Se exposure and subsequently excised any linked disequilibrium. Our analysis discerned nine SNPs correlated with Cu levels, four in relation to Zn and six pertaining to Se, all manifesting synergistic effects with studied health outcomes. It was determined that these SNPs were independent of both the outcomes themselves and potential confounding variables [6].
Figure 3 presents the results of MR analyses, including randomeffects IVW analysis, which indicated that serum Cu were not genetically causal for COPD (OR:1.000, 95% CI:0.998-1.001, p=0.544) nor for chronic tracheitis (OR:0.999, 95% CI: 0.998-1.000, p=0.085). Additional MR approaches, such as MREgger, weighted median, simple mode and weighted mode analyses, consistently supported the inference that Cu does not have a genetic causative role in either COPD or chronic tracheitis (p>0.05).
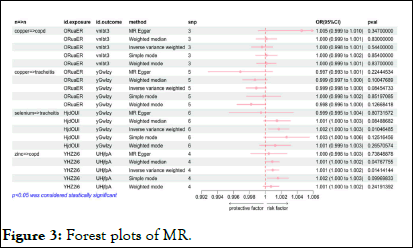
Figure 3: Forest plots of MR.
Conversely, in Figure 4, reverse MR analysis did not establish a causal effect of chronic tracheitis on Cu exposure, but it did reveal a causal association between COPD (OR:1.18241E-23, 95% CI:9.58E-39-1.46E-08, p=0.003) and Cu exposure. Similarly, Zn levels were found to be genetically causally related to COPD (OR:1.001, 95% CI:1.001-1.002, p=0.014), with reverse MR also indicating a causal link between COPD (OR: 2.718, 95% CI:2.475-2.985, p<0.001) and Zn exposure.
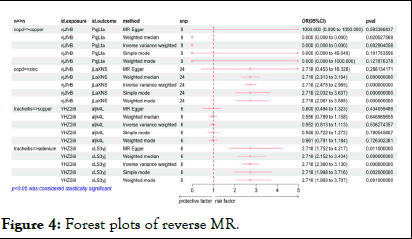
Figure 4: Forest plots of reverse MR.
Moreover, a genetic causal association was identified between Se and chronic tracheitis (OR:1.002, 95% CI:1.000-1.00 3, p=0.010), with reverse MR supporting a causal relationship between chronic tracheitis (OR:2.718, 95% CI:2.360-3.13, p<0.001) and se exposure. These findings prompt a re-evaluation of the roles that these trace elements may play in respiratory health and disease mechanisms.
Sensitivity analyses
The outcomes of logistic regression analyses across three distinct models, examining the correlation between serum trace metal levels and CRDs in U.S. adult populations. Figure 2 illustrates that the trajectories discerned within the RCS plots align well with the magnitude and direction of effect sizes ascertained by the logistic regression analysis [7].
Regarding inverse MR, we repeated the above steps by exchanging exposures and CRDs in the same database to assess the inverse causality of CRDs with exposures. Figure S1 and S2 show the results of bidirectional MR. As a sensitivity analysis, we used a "leave-one-out" approach to check whether the MR estimates were driven by a single SNP. Figure S3 and S4 illustrates the results.
In the context of mounting occurrences of chronic respiratory ailments in recent years, there has been a pronounced surge in the interest regarding the potential interconnections between micronutrients and these conditions. Zn, Cu and Se rank amongst the crucial trace metals imperative for human health. They are pivotal in catalyzing key biological functions across diverse processes specifically, mitigating oxidative stress in the respiratory system, bolstering immune defenses and facilitating anti-inflammatory responses. Prior studies have demonstrated that Zn, Cu and Se function as regulators of cellular plasticity, influencing both oxidative stress and the inflammatory cascade.
Consequently, we appraised the association between serum levels of Zn, Cu and Se and the prevalence of chronic respiratory diseases among 2807 adults who partook in the NHANES survey in the United States from 2013 to 2016. To the best of our knowledge, our survey represents the inaugural discourse exploring the connections between serum concentrations of Zn, Cu and Se and chronic respiratory conditions and stands as one of the most extensive crosssectional studies in this domain. By incorporating sample weights, stratification and clustering in our analysis, we were positioned to evaluate the relationship between serum trace metals and chronic respiratory diseases within a representative sample of the general adult population in the United States. Our regression analysis reveals that increased serum Cu levels within the adult US demographic are positively associated with the prevalence of emphysema, chronic tracheitis and COPD. However, this association was not evident in the context of asthma risk. Elevated serum Zn concentrations have been observed to exhibit a direct correlation with an increased risk of developing COPD. Conversely, elevated serum Se levels demonstrate a negative correlation with the incidence of chronic tracheitis.
Nonetheless, observational studies inherently carry limitations such as potential confounding factors and the issue of reverse causality, which restrict our capability to establish a direct causal linkage. To address this concern and build upon the initial observations, MR was employed as a means to investigate a causal relationship between metal exposure and CRDs. Within our MR framework, no genetic causality was observed between Cu exposure and the incidence of either COPD or chronic tracheitis, suggesting that elevated Cu intake is not genetically implicated in the etiology of these conditions. Conversely, a reverse MR analysis indicated an association between COPD and heightened Cu levels, hinting at possible alterations in Cu metabolism or intake secondary to the presence of COPD. Furthermore, a forward MR analysis revealed a genetic causal link between Zn exposure and COPD, a finding corroborated by reverse MR analysis, thereby strengthening the hypothesis that Zn may be a contributory genetic factor in COPD susceptibility. The observed genetic causality between Se and chronic tracheitis aligns with the epidemiological data extracted from the NHANES database, supporting the notion that fluctuations in Se levels could be relevant to the pathogenesis of chronic tracheitis [8].
Our research findings on the implications of serum Cu levels in CRDs corroborate extant literature. Evidence implicates Cudependent lysyl oxidase in the etiology of emphysema, while Cu deficiency has been observed subsequent to chronic TNF-αmediated pulmonary inflammation, which may be pivotal in the genesis of inflammation-driven lung pathologies. An extensive examination of 1,097 individuals employed in the metallurgy sector revealed a higher prevalence of chronic tracheitis among those engaged in Cu production. Moreover, data indicate an elevated risk of COPD in men with Cu exposure. In a casecontrol study, asthmatic patients exhibited a 20% increase in serum Cu levels compared to the control group (p<0.01). Preliminary basic research also suggests that Cu exposure may contribute to pulmonary inflammation, which potentially correlates with the pathogenic mechanisms of asthma. However, our investigation did not establish a linkage between Cu levels and the risk of asthma, indicating the need for additional research to determine the precise association between serum Cu and asthma in a larger population.
In contrast, our findings concerning Zn and COPD present some incongruities with prior research. Despite the intriguing properties of Zn, including anti-inflammatory, antioxidant, immune-modulatory and metabolic effects, which have gained attention for the management of pulmonary disorders, the interruption of autophagy by cigarette smoke exposure and subsequent Zn deficiency have been shown to provoke cellular apoptosis. Nutritional strategies involving reduced Zn intake followed by supplemental restitution to normalize respiratory Zn levels have demonstrated potential in mitigating the severity of pulmonary inflammation. It is noteworthy that elevated doses of Zn do not invariably exert negative consequences in individuals with COPD; however, the limitations of this study, which included a modest cohort of 76 participants from the Stara Zagora region, underscore the necessity for expanded research in this area to reconcile the consistency of outcomes.
When addressing Se and chronic tracheitis, our findings are in harmony with preceding research trajectories. In the American adult demographic, enhanced consumption of dietary antioxidants is observed to be inversely associated with the incidence of CRDs, especially emphysema and chronic tracheitis. Selenium 's defensive capacity against pulmonary inflammation is further buttressed by its antagonistic action on the deleterious pulmonary impacts of cigarette-borne toxins such as cadmium. Additionally, Se is essential for sustaining immune defenses by modulating the activity of GPx. The collective insights from these studies advocate the continuation of inquisitive scrutiny into the nuanced roles of trace elements in CRDs.
Mendelian randomization analyses propose a bidirectional relationship wherein Zn and Se levels may both influence and be affected by CRD status implying their dual role as potential biomarkers for CRD pathophysiology and outcomes. These insights contribute to a nuanced understanding of micronutrient interactions in CRDs, emphasizing the importance of considering bioavailability, metabolic interactions and disease etiology. The implications of this research extend to advocating for personalized nutrition approaches in CRD prevention and treatment strategies and underscore the need for future studies to incorporate multifaceted approaches to decipher the precise relationship between micronutrients like Zn and Se with CRDs.
The data accumulated in this study suggest a significant correlation between elevated serum Cu levels and heightened odds ratios for pulmonary conditions such as emphysema, chronic tracheitis and COPD, underscoring a potential contributory role of Cu exposure in the pathogenesis of these respiratory disorders. Regulatory strategies targeting serum Cu concentrations, which may include modulating dietary Cu intake, could emerge as feasible preventive measures against the development of emphysema, chronic tracheitis and COPD. Accordingly, it is imperative that future investigational endeavors orient towards elucidating the influence of serum Cu on CRDs to underpin the formulation of evidence-based dietary and public health recommendations.
It merits attention that a condition known as Asthma-COPD Overlap Syndrome (ACOS) has garnered clinical intrigue delineating a cohort of patients with characteristics of both asthma and COPD. They exhibit clinical features of both afflictions incomplete reversibility of airways (associated with COPD) and variability or acute exacerbations (associated with asthma). Relative to individuals with solely asthma or COPD, those with ACOS have an inferior quality of life, are subject to more complications and command a significant share of healthcare resources. Nonetheless, a French COPD cohort study reported that ACOS patients with asthma diagnosed before the age of 40, as compared with "pure" COPD patients, had a lower cumulative smoking rate, a higher prevalence of obesity and atopic disorders and utilized more asthma therapies. In terms of disease severity (dyspnea, QoL, exacerbations, comorbidities) and prognosis (mortality), they exhibited no difference from "pure" COPD patients. Contrasting these studies, the cohort study underscored, to some extent, the significant impact of early diagnosis and treatment of ACOS on patient outcomes. Patients diagnosed early with asthma and treated more aggressively with asthma therapies showed better quality of life than those receiving COPD treatments. This poses a diagnostic and therapeutic challenge for physicians, as there are no specific biomarkers to distinguish ACOS from asthma or COPD, the approach to diagnosing ACOS depends on the population from which the patient originates. We are keen on further exploring the relationship between serum Cu and ACOS to aid in clinical diagnosis; treatment for the two also diverges, identifying ACOS patients has significant therapeutic implications, particularly the need for early usage of inhaled corticosteroids and the avoidance of using long-acting bronchodilators alone in such patients; of course, this necessitates additional clinical and fundamental research to corroborate the specific impact of serum Cu on ACOS patients.
For an extended period, an association between persistent chronic inflammation and cancer has been acknowledged, with implications that a convoluted inflammatory process involving multiple types of immune cells may lead to tissue damage and remodeling, culminating in CRDs and the nefarious lung cancer. It is noteworthy that COPD and emphysema have been identified as risk factors for lung cancer, which is the leading cause of cancer-related mortality worldwide, accounting for 18% of all cancer fatalities with over 1.8 million deaths annually. In recent studies, the aberrant metabolism of Cu has been discovered to correlate not only with chronic pulmonary diseases but also intimately with carcinogenic mechanisms. For instance, anomalous expression of genes associated with Cu metabolism in the study of lung adenocarcinoma unveils the role of Cu homeostasis in tumor development. Similarly, both Cu deficiency and excess may impact the synthesis of pulmonary elastin, thus affecting lung structure and functionality. Investigations into pulmonary Cu metabolism are pivotal for deciphering the pathogenesis of these diseases and also offer potential interventional avenues for the prevention and treatment of pulmonary disorders.
In the realm of fundamental research, several studies are investigating the molecular mechanisms through which Cu chelators might be leveraged in treating CRDs. Notably, Cuproptosis, a recently uncovered form of regulated cell death induced by excessive Cu2+, is distinct from all other known pathways behind cellular demise; it transpires in the Tricarboxylic Acid (TCA) cycle through the binding of Cu with lipoylated components, leading to subsequent protein aggregation, proteotoxic stress and ultimately cell death. The discovery of cuproptosis provides further impetus to explore molecular mechanisms within pulmonary diseases. Research has highlighted 18 key genes connected to Cu metabolism, including 5 cuproptosis-associated genes, to be significantly enriched in signal pathways and biological processes related to the progression of COPD. LH et al. have documented that the cuproptosis-associated gene glutaminase escalates Cu ion accumulation in alveolar macrophages in COPD. In asthma, scholars have reported "Identification of cuproptosis-related asthma diagnostic genes by WGCNA analysis and machine learning," and in vitro experiments have shown increased expression of the cuproptosis-linked genes DYSF and CXCR1 in asthma. In lung cancer, it has been conveyed that mice harboring BRAFV600E -driven lung cancer exhibit an enhanced tumor proliferation when supplemented with elevated levels of Cu. Future investigations are requisite to delve into the exact role of Cu in pulmonary-related diseases and how modulation of Cu metabolism and cuproptosis can effectively control or prevent pulmonary diseases [9].
The significant strength of our investigation lies in the establishment upon a comprehensive and demographically heterogeneous U.S. adult cohort, endowing our results with considerable veracity and solidity. Our analytical approach leveraged the scrutiny of serum trace metals, effectively bypassing the biases intrinsically linked with dietary recall inquiries and the confounding factors pertinent to individual metabolic peculiarities. A distinct advantage of our research is the application of bidirectional analysis alongside observational studies and MR, thereby reinforcing the scholarly integrity of our results. Solo observational studies stand susceptible to reverse causality and inscrutable confounding influences. Although MR strategies can control for such confounders, they are frequently prone to an increased incidence of false negatives. The integration of these investigative techniques within our framework, which delivered concordant findings, asserts the dependability of our conclusions. The capacious dataset foundational to our MR analysis significantly bolsters our statistical prowess in elucidating the association between Cu intensities and COPD [10].
Nonetheless, certain constraints within our approach warrant acknowledgment. The cross-sectional essence of our study intrinsically includes confounding factors and hinders the definitive ascertainment of causative ties between serum trace elements and chronic respiratory disorders. Moreover, the scope of analysis was delineated to NHANES questionnaire data collected between 2013 and 2016, thus not facilitating an investigation into the nexus between serum trace metals and particularized clinical expressions of CRDs, such as dyspnea and reduced pulmonary function. Furthermore, our deliberations are predicated exclusively on European and American subjects, curtailing the extension of our conclusions to diverse populations due to anticipated heterogeneity and the pleiotropic propensities of MR.
The NHANES dataset demonstrates a correlation that suggests elevated Cu levels are linked with an increased risk of chronic tracheitis and COPD, more so at higher concentrations. Mendelian randomization analysis corroborates a similar positive association for Zn levels with COPD risk. In contrast, Se is suggested to have a protective effect against chronic tracheitis, an inference supported by reverse MR analysis, which also postulates an influence of chronic tracheitis on serum Se levels. These insights underline the importance of further investigative efforts to elucidate the impact of trace elements on respiratory disease outcomes and inform preventative healthcare strategies.
We extend our heartfelt appreciation to the dedicated team at the Centers for Disease Control and Prevention (CDC) and the staff at the National Center for Health Statistics (NCHS) for their invaluable support. We also express our gratitude to the participants of the national health and nutrition examination survey, whose engagement has been instrumental to this research. Furthermore, the authors are grateful for the contributions of the Neale lab consortium and the UK biobank consortium for providing high-quality GWAS data that has greatly facilitated the research community.
The dataset underpinning this investigation was sourced from the National Health and Nutrition Examination Survey (NHANES), publicly available at the official NHANES web portal.
In compliance with the approved protocol by the National Center for Health Statistics Research Ethics Review Board, participants have provided their written informed consent for participation in NHANES. The dataset employed in this study is anonymized, in the public domain and was utilized without the need for further ethical clearance from the Swedish ethical review authority, adhering to the national guidelines for research.
(I) Conception and design: HM Yu; (II) Administrative support: X Gu, L Ma; (III) Provision of study materials or patients: HM Yu, TH Lv, ZS Ji; (IV) Collection and assembly of data: TH Lv, S Wang, S Liu; (V) Data analysis and interpretation: YR Chen, S Wang, Q Zhao; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Yu H, Lv T, Ji Z, Wang S, Liu S, Chen Y, Zhao Q, et al. (2025) Associations of Serum Cu, Zn and Se with Chronic Respiratory Diseases in the American Adults: Data from NHANES 2013–2016 and a Bidirectional Mendelian Randomization Analysis. Intern Med. 15:503.
Received: 24-Apr-2024, Manuscript No. ime-24-32904; Editor assigned: 29-Apr-2024, Pre QC No. ime-24-32904 (PQ); Reviewed: 13-May-2024, QC No. ime-24-32904; Revised: 11-Jan-2025, Manuscript No. ime-24-32904 (R); Published: 18-Jan-2025 , DOI: 10.35248/2165-8048.25.15.503
Copyright: © 2025 Yu H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.