Journal of Cell Science & Therapy
Open Access
ISSN: 2157-7013
ISSN: 2157-7013
Research Article - (2016) Volume 7, Issue 3
Purpose: To establish epithelial-mesenchymal transition (EMT) models in renal cell carcinoma cell lines by CoCl2-induced hypoxia.
Materials and methods: The renal cell carcinoma cell lines A498 and 786-O were used in the experiment and CoCl2 was used to simulate hypoxia. The cancer cells were cultured with different concentrations of CoCl2. Morphology and cyto-activity changes were detected to obtain the optimal concentration of CoCl2 for simulating hypoxia. After CoCl2 treatment, the cells were subjected to Western blot analysis to test the expression of HIF-1α and the changes of EMT-related molecules (E-cadherin, fibronectin).
Results: Cell conjunctions of CoCl2-treated groups were loose and scattered compared to the control. The effect of CoCl2 on A498 cell viability was not distinct at a low dosage, but when the concentration of CoCl2 reached 250 mM, cell activity gradually declined. In contrast, CoCl2 induced 786-O cell proliferation in the range of 50 mM- 200 mM, but it inhibited cell growth at dosages higher than 200 mM. The expression of E-cadherin was significantly down-regulated, and fibronectin was up-regulated in both A498 and 786-O cell lines under CoCl2-simulated hypoxia in comparison with normoxic conditions (P<0.01).
Conclusions: EMT models of the renal cell carcinoma cell lines were successfully established by CoCl2-induced hypoxia. The models will help us further study the mechanisms of EMT and investigate novel therapeutic targets to inhibit tumor invasion and metastasis.
Keywords: Renal cell carcinoma; Epithelial-mesenchymal transition; Hypoxia; E-cadherin; Fibronectin
Kidney cancer originating from the renal tubular epithelium system is one of the most common cancers of the urinary system. It accounts for approximately 3% of all malignant tumors. The incidence of newly diagnosed patients has increased by approximately 2.5-3% annually from 2001-2010 [1,2]. Metastatic renal cell carcinoma is detected in approximately 30% of patients at the time of diagnosis [3]. The overall distribution of metastatic sites is the lung, bone, lymph nodes, liver, adrenal gland and brain [4]. Metastatic renal cell carcinoma has a poor prognosis with a median overall survival of 12 months and a 5-year survival rate of less than 10%, which seriously affects patients’ quality of life [5]. Invasion and metastasis, the major characteristics of malignant tumors, lead to the poor prognosis and death of patients. At present, the intrinsic molecular mechanisms and the extrinsic microenvironment that enhance the metastatic potential of primary tumor cells are still hot topics in the field of cancer research. Remarkably, a multitude of studies have revealed that activation of epithelial-mesenchymal transition (EMT) endows metastatic properties upon cancer cells to promote invasion, migration, and subsequent dissemination [6-12]. EMT is a process known as epithelial phenotype transdifferentiation into mesenchymal phenotype. During the EMT, cellular morphology and adhering capacity will change and the expression of epithelial molecular markers will down-regulate while that of mesenchymal molecular markers will increase. Hypoxia, one of the various factors that can induce EMT in cancer cells, exists in many regions of solid tumors [13,14] because angiogenesis in tumors is heterogeneous, and cancer cells grow rapidly. If an EMT model can be successfully established with a hypoxic microenvironment in vitro, it will provide support to study the mechanisms of tumor invasion and metastasis. Currently, there is no relevant EMT model in the field of renal cell carcinoma. In this study, the renal cell carcinoma cell lines A498 and 786-O were incubated with cobaltous chloride (CoCl2). We monitored the process of the EMT from three aspects: characteristics of cell growth, the expression of hypoxia-inducible factor-1α (HIF-1α) and the expression changes of EMT-related molecules.
Cell lines and cell culture
Renal cell carcinoma A498 and 786-O cells were provided by Sun Yat-Sen University Laboratory (Guangzhou, China). Both are adherent epithelial human renal cell carcinoma cell lines with a generation time of 2-3 days. They were cultured in a humidified incubator (Thermo Scientific, USA) at 37°C, 5% CO2. A498 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, USA) supplemented with 10% fetal bovine serum (Hyclone, USA), 100 U/ mL penicillin and 100 μg/mL streptomycin (Gibco, USA); 786-O cells were cultured in RPMI-1640 medium (Hyclone, USA) supplemented with 10% fetal bovine serum (Hyclone, USA), 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco, USA). For experiments requiring hypoxia, CoCl2 (Sangon, Shanghai) was added to simulate a hypoxic microenvironment.
Effects of CoCl2 on cell growth
Cancer cells were harvested during the logarithmic growth period and counted by Countstar (Inno-Alliance Biotech, USA). The cell concentration was adjusted to 5.0 × 104/mL with the respective culture medium. Cell suspensions, approximately 100 μL/well, were seeded in 96-well plates (Corning Costar, USA) and cultured in a humidified incubator at 37°C, 5% CO2 for 24 h. Then, 10 μl of CoCl2 at different concentrations was added to each well, making final concentrations of 0 μM, 50 μM, 100 μM, 150 μM, 200 μM, 250 μM, 300 μM, 400 μM, 800 μM. During the process of culturing, cellular morphology was observed under an inverted microscope (Olympus CKX41SF, Japan), and cytoactivity was tested by Cell Counting Kit-8 (Dojindo, Japan) at 24 h, 48 h, 72 h, 96 h and 120 h.
Effects of CoCl2 on the expression of HIF-1α
Cancer cells were harvested during the logarithmic growth period, counted by Count star and adjusted to 1.0 × 105/mL with the respective culture medium. Cell suspensions, approximately 2 mL/well, were seeded in 6-well plates (Corning Costar, USA) and cultured in a humidified incubator at 37°C, 5% CO2 for 24 h. Then, to every well 200 μL of different concentrations of CoCl2 was added, making final concentrations of 50 μM, 100 μM, 200 μM, 400 μM, 800 μM. Each sample had set a blank control group. After treatment with CoCl2, the plates were incubated for 72 h and then the total protein was extracted for Western Blot assays to test the expression of HIF-1α in tumor cells.
The cells were lysed in lysis buffer for approximately 15 min, and the lysed cells were centrifuged for 15 min at 12,000 rpm. Protein quantification was determined using the bicinchoninic acid (BCA) colorimetric assay (Pierce BCA Protein Assay Kit, Thermo Scientific, USA). Proteins (10 μg) undergoing sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) were transferred to a PVDF membrane (Invitrogen, USA) by electroblotting. The membranes were incubated in TBST (10 mM TBS and 0.05% Tween 20) supplemented with 5% fat-free milk at room temperature for 1 h. Next, the membranes were placed in a bull serum albumin diluted solution containing the primary antibody HIF-1α (1:1000, #14179, cell signaling, USA) or α-Tubulin (1:1000, #2125, cell signaling, USA) and reacted at 4°C overnight. After repeated washing, the membranes were incubated with secondary Anti-Rabbit IgG, HRP-linked Antibody (1:2000, #7074, cell signaling, USA) and enhanced electrochemiluminescence (ECL) detection reagent. The above experiment was repeated at least 3 times. The bands were detected using the enhanced chemiluminescence system (Bio-Rad, USA).
Effects of CoCl2 on EMT-related molecular changes
Cancer cells were harvested during the logarithmic growth period, counted by Count star and adjusted to 1.0 × 105/mL with the respective culture medium. Cell suspensions, approximately 2 mL/well, were seeded in 6-well plates and cultured in a humidified incubator at 37°C, 5% CO2 for 24 h. Referring to the results of observing cell morphology, activity and expression of HIF-α in the experiment above, 200 μL of CoCl2 was added to every well to make a final concentration of 200 μM. The plates were incubated for 72 h, and the total protein was extracted for Western Blot assays to test the changes of EMT-related molecules.
The Western blotting procedure was the same as above. However, the primary antibodies were fibronectin (1:1000, ab6328, abcam, UK), E-cadherin (1:1000, 5296s, cell signaling, USA), and α-tubulin (1:1000, #2125, cell signaling, USA), and the secondary antibody was Anti- Rabbit IgG, HRP-linked Antibody (1:2000, #7074, cell signaling, USA). The above experiment was repeated at least 3 times. In each group, α-tubulin was regarded as the loading control. Image J software was used to calculate the luminance values of target Western blot protein bands and compare them with an internal reference to obtain the luminance ratio.
Statistical analysis
All of the statistical analysis was performed with SPSS 19.0 software. The measurement data were reported as the mean ± standard deviation 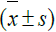 . Comparisons among data sets were made with Student’s t-test. Differences were considered to be statistically significant when the P value was <0.05.
. Comparisons among data sets were made with Student’s t-test. Differences were considered to be statistically significant when the P value was <0.05.
Characteristics of cell growth
A498 and 786-O cells were cultured in normal medium or supplemented with CoCl2. After 72 h, cell conjunctions between 786-O cells were much looser and more scattered in the groups with added CoCl2 than in the blank control group (Figure 1A and 1B). However, A498 cells showed the same characteristics in cell growth after 96 h (Figure 1C and 1D).
Figure 1: Morphological changes during the process of cell culture (X200).
A. Morphological changes in the blank control group of 786-O cells (72 h), B. Morphological changes in the CoCl2-treated group of 786-O cells (72 h), C. Morphological changes in the blank control group of A498 cells (96 h), D. Morphological changes in the CoCl2-treated group of A498 cells (96 h).
Effects of CoCl2 on cell viability
The effects of CoCl2 on the viability of A498 cells were not distinct at low dosage, but when the concentration increased to 250 μM, cell activity gradually declined. For example, when the concentration was 250 μM, relative cell viability decreased from 85%-50% from 24 h - 120 h (Figure 2). However, when the concentration of CoCl2 ranged from 50 μM-200 μM, it obviously stimulated 786-O cells to proliferate. When the concentration exceeded 250 μM, it produced inhibitory effects on cell viability. For example, relative cell viability remained below 100% from 24 h - 120 h at the concentration of 250 μM (Figure 3).
Expression of HIF-1α
When cultured with different concentrations of CoCl2 for 72 h, the expression quantity of HIF-1α in A498 cells was significantly increased with increasing CoCl2 concentration, and the difference was significant compared with the blank control group (Figure 4). Expression of HIF- 1α was not observed in 786-O cells whether cultured in normoxic conditions or treated with CoCl2 for 72 h.
Changes of EMT-related molecules
In the process of culture, we had observed that when the concentration of CoCl2 was 200 μM and cells were cultured for 72 h, the two cell lines grew well, and their intercellular structure changed significantly, such as cell conjunctions being looser and scattered. Therefore, we selected the culture conditions above to simulate a hypoxic microenvironment. The results indicated that for the blank control group and CoCl2-treated group in the A498 cell line, the luminance ratio of E-cadherin expression was 0.934 ± 0.402 and 0.234 ± 0.616, respectively, and the luminance ratio of fibronectin expression was 0.059 ± 0.168 and 0.175 ± 0.049, respectively. The differences between the two groups were considered to be statistically significant (P<0.01) (Figure 5). For the blank control group and the CoCl2- treated group in the 786-O cell line, the luminance ratio of E-cadherin expression was 1.250 ± 0.435 and 0.026 ± 0.004, respectively, and the luminance ratio of fibronectin expression was 0.104 ± 0.022 and 0.674 ± 0.253, respectively. The differences between the two groups were considered to be statistically significant (P<0.01) (Figure 6).
The EMT is a wide range of biological processes that allow a polarized epithelial cell to undergo multiple biochemical changes that enable it to assume a mesenchymal cell phenotype, which includes enhanced migratory capacity, invasiveness and resistance to apoptosis. In 1982, Greenburg and Hay [15] revealed that environmental conditions have profound effects on the epithelial phenotype, the cell shape and the polarity as expressed by the presence of apical and basal surfaces. The EMT exists in many basic physiological and pathological processes, divided into three subtypes: 1) association with implantation, embryo formation, and organ development; 2) association with tissue regeneration and organ fibrosis; and 3) existence in cancer progression and metastasis [16]. In recent years, the EMT was found in gastrointestinal cancer, pancreatic cancer, liver cancer, prostate cancer, breast cancer and other tumors and is associated with tumor infiltration and metastasis [17-23]. Zhang et al. [14] demonstrated that hypoxia was able to induce the EMT and enhance the ability of invasion and migration in HCC cells. Huang et al. [24] also reported that upregulation of HIF-1α, together with EMT alteration, resulted in increased migration and invasion of HepG2 cells under hypoxia. Chu et al. [25] showed that the underlying mechanism of tumor progression in breast cancer might be associated with the epithelial- mesenchymal transition (EMT). Therefore, further study of the EMT will help us understand the molecular mechanism of tumor metastasis and provide a potential therapeutic target for cancer treatment.
Hypoxia is an important factor inducing EMT in tumors. Solid tumors grow in hypoxic conditions, which exert critical effects on the gene phenotype of tumor cells. Tumor growth and metastasis are promoted by a variety of pathways involved in affecting metabolism, activating tumor growth factors, promoting drug or radiation resistance, and enhancing malignant biological behavior [26-28]. An important mechanism by which hypoxia promotes the invasion and metastasis of malignant tumors is according to the regulation of the EMT process. Under hypoxic conditions, a series of changes of cytokines occur in tumor cells. Studies have demonstrated that HIFs are key transcription factors that transmit hypoxic signals and mediate hypoxia effects [29-31]. They mediate many links within tumor invasion and metastasis by combining with hypoxia response elements and activating downstream target genes. Meanwhile, HIFs also play an important role in inducing the EMT in a variety of ways [32-36].
In this study, we applied CoCl2 to simulate hypoxia. CO2+ is a substrate of the iron chelating enzyme; it can replace the Fe2+ in the oxygen sensor of heme and prevent the oxygen sensor from combining with oxygen. Then, the tumor cells would "feel" a lack of oxygen in normoxia and consequently up-regulate the expression of hypoxia inducing factors [25,37-39]. During the process of culturing, the effect of CoCl2 on the viability of A498 cells was not distinct at lower dosage. When the concentration of CoCl2 reached 250 μM, cell activity significantly declined. This finding suggested that A498 cells were more tolerant of CoCl2-induced hypoxia. In contrast, 50 μM-200 μM of CoCl2 stimulated the proliferation of 786-O cells. However, dosages higher than 200 μM had an inhibitory effect on cell growth. Therefore, the different cell lines showed diverse tolerance profiles to CoCl2- induced hypoxia.
To identify the occurrence of the EMT, we monitored the changes of cell morphology and detected the expression of HIF-1α and EMTrelated molecular markers. CoCl2-treated tumor cells appeared looser and scattered from cell-to-cell junctions. These changes allowed the epithelial cells to break away from intercellular junctions and become migratory and aggressive. In addition, HIF-1α expression in A498 cells significantly increased in a CoCl2 -dose-dependent manner. However, HIF-1α expression was not observed in 786-O cells. It was reported that certain VHL-defective cells, 786-O, did not express detectable HIF-1α [40]. This again demonstrates that renal carcinoma cell lines with different biological characteristics have different responses to hypoxia. Hypoxic conditions could induce the EMT through different signaling pathways [35,36].
The epithelial phenotype turning into a mesenchymal phenotype is an important characteristic of the EMT. Another critical signature of the EMT is the reduction or loss of E-cadherin [41]. E-cadherin is an intercellular adhesion molecule that belongs to the type-I adhesive proteins and calcium-dependent trans membrane glycoproteins in epithelial tissues. It can promote connections between cells, maintaining the cytoskeleton and the stability of attachment brackets. A number of transcription factors directly or indirectly inhibit the expression of E-cadherin or prevent it from interacting with itself, which results in EMT [11,42-44]. Loss or reduction of E-cadherin has a significant influence on cellular functions, including loss of cell polarity and decreased cell adhesion ability. In recent years, many studies have shown that the aberrant activation of EMT promotes tumor metastasis by repressing cell adhesion molecules, such as E-cadherin [45,46]. Reduced intracellular adhesion allows tumor cells to disseminate and spread throughout the body. When the EMT process starts, the expression of mesenchymal phenotype related molecules will be upregulated. We found that the expression of E-cadherin was significantly down-regulated, and fibronectin was up-regulated in both A498 and 786-O cell lines under CoCl2-induced hypoxia.
In conclusion, we have successfully generated EMT models by incubation of the renal carcinoma cell lines A498 and 786-O with CoCl2. The models will help us and other researchers not only further study the mechanisms of the EMT but also investigate novel therapeutic targets to inhibit tumor invasion and metastasis.
This study was supported by the 2014 Beijing Natural Science Foundation (Grant No. 7142059). Sincere thanks to Dr. Ning Zhang and Qing Li for project development and manuscript editing; Dr. Baoan Hong for data management, analysis and manuscript writing; Dr. Changhua Zhou and Siqi Chen for help with completing the experiment and data collection; Dr. Yong Yang and Kan Gong for experiment instruction; and Dr. Xin Du for help with data management.