
Biochemistry & Pharmacology: Open Access
Open Access
ISSN: 2167-0501

ISSN: 2167-0501
Research Article - (2020)Volume 9, Issue 1
This study was carried out to investigate the anti-plasmodial effect of Moringa oleifera seeds in Plasmodium berghei infected albino rats to affirm its traditional use in the treatment of malaria in Nigeria and some African countries. The pods of this plant were collected from a garden in Port Harcourt, Nigeria and authenticated by a taxonomist. The seed were dried under shade, deshelled, crushed into powder and extracted with 99% ethanol using rotary evaporator and water bath. Phytochemical investigation was conducted on the powdered seeds using standard method to determine the presence of the bioactive compounds and oral acute toxicity test was also carried out using the modified method of Karber. The in-vivo anti-plasmodial effect of the plant extract of Moringa oleifera (200, 300 and 500 mg/kg), Artemether/Lumefantrine (A/L), Artesunate/Mefloquine (A/M) and Chloroquine (CQ) against chloroquine sensitive strain of Plasmodium berghei in infected albino rats (120-130 g) were investigated using a 4 day range test for curative effect and prophylactic model. Standard methods were used to determine the percentage parasitemia. A non-dose dependent significant (P<0.05) parasitemia suppression was discovered after an oral treatment with the ethanol extract of the seed plant at different dose levels as compared to the control group. However, 300 mg-kg-1 (72.56 ± 1.75%) of the extracts exhibited a relatively higher chemosupression compared to 200 mg-kg-1 (78.55 ± 2.42%), 300 mg-kg-1 (79.15 ± 0.72%) and 500 mg-kg-1 (77.60 ± 1.53%). The standard anti-malarial used in this study showed the higher chemosupression. The phytochemical analysis also revealed the presence of the following bioactive components such as Saponins, Alkaloid and Flavonoids etc. The ethanol extract of Moringa oleifera seeds demonstrated a high anti--plasmodial activity against P. berghei in albino rats and affirms Moringa oleifera as a potential source of effective and safe herbal therapeutic agent in trado-medical practice for the treatment of fever, pain and malaria in Nigeria.
Plasmodium berghei; Chemosupression; Moringa oleifera; Anti-plasmodial effect; Curative effect; Prophylactic model; Malaria
Malaria is a global concern in African countries and has become one of the oldest human diseases in the past few decades. Each year, there are over 300 million acute cases of the disease, giving rise to more than a million deaths, mostly in the sub-Sahara Africa where the disease is endemic [1]. Recently, it has been estimated that about 3.3 million people of the total global population reside in malaria endemic zones and Africans are the most afflicted with about 90% of all malaria death [2,3].
Among the World Health Organization (WHO) report in 2015, it was observed that the most cases of malaria (88%) and malaria deaths (90%) took place in the WHO African region, thereafter 10% deaths in the region of the South East Asia and 2% in the region of Eastern Mediterranean, also the two African countries, Democratic Republic of Congo and Nigeria, made up of more than 35% of global malaria deaths [4]. Artemisinin, the major component of the most available anti-malarial drugs, artemisinin has been observed to have developed resistance to parasite in Thailand, Cambodia, Myanmar, China and Viet Nam.
Effective case management of malaria with potent anti-malarial agents and the control of the malaria vector, female anopheles’ mosquitoes, remain the major tools in eradicating malaria [5]. The WHO has extensively recommended the use of Artemisinin-based Combination Therapies (ACTs) as first line drugs for an effective management of uncomplicated Plasmodium falciparum and multipledrug resistant malaria in order to contain the disease in endemic areas [6]. ACTs act through decomposition of the endoperoxide bridge mediated by the heme to produce free radicals with carboncenters that are selectively toxic to Plasmodium parasites [7].
Among the therapies found in Artemisinin-based Combinations are:
a. Artemether + Lumenfantrine
b. Artesunate + Amodiaquine
c. Artesunate + Mefloquine
d. Artesunate + Sulfadoxine + Pyrimethamine
e. Dihydroartemisin + Piperaquine etc.
The ACTs are potent blood schizonticidal anti-malarial agents which are used increasingly in the treatment of Plasmodium infections [8]. ACTs have high efficacy against malaria parasite because of their high killing rates [9]. Most natural products are important sources of biologically active compounds, which are generally safer to mammals than many synthetic drugs against malaria. The ACTs have mild side effects such as nausea, vomiting, abdominal pains and dizziness.
Artemisinins were discovered from medicinal plants, Artemisia annua, in providing a solution to the resistance of Plasmodium falciparum by anti-malarial agents such as Chloroquine, Primaquine in the treatment of uncomplicated malaria [10]. The discovery of Artemisinins gave rise to several documented medicinal plants that have been found to be effective for fighting malaria [11].
It has been reported that modern medicines, traditional medicines, drugs, pharmaceutical intermediates, food supplements, constituents of synthetic drugs all derive their source from Moringa plants and this has been demonstrated through various studies, thus their use as bioactive medicines can be linked since the beginning of human civilization [12]. In line with the discovery of the potency of some medicinal plants for the treatment of malaria and other ailments, this investigation was carried out to determine the possible relevance of Moringa oleifera as a future anti-plasmodial drug for the management of malaria infection, to compare the activity of those two ACTs, and its possible relevance as an antiplasmodial drug.
Moringa, also called drumstick tree or Ben tree is used widely for its edible fruits, leaves, flowers, roots, and seed oil that have been proven to be useful sources of food, medicinal products, fuel wood and active ingredients for synthetic drugs [13-15]. Moringa oleifera seeds contain about 38.00% to 42.00% hexane extracted oil [16]. The oil is light yellow in colour with mild taste and odourless. Its potential properties includes: anti-oxidant/anti--plasmodial, anti-- inflammatory, anti--aging, anti--microbial, disinfectant, excellent carrier oil, hepato-protective, skin moisturizer and preservative [17]. Recent studies have shown that Moringa oleifera “miracle tree” has both in vivo and in vitro anti-plasmodial and mosquito repellent activities [17].
This study was aimed at examining the in-vivo anti-plasmodial effect of ethanol extract of Moringa oleifera seeds in albino rats infected with Plasmodium berghei using the curative and prophylactic models described in the methodology below. The objectives of the study are: To validate the traditional use of Moringa oleifera seeds for the treatment of Plasmodium infection in Nigeria and in other countries, to compare its anti-malarial effects the standard drugs Artesunate/Mefloquine and Artemether/Lumefantrine, which are the first line anti-malarial drugs recommended in Artemisininbased Combination therapies (ACTs), to evaluate the toxicity effect of the seed extract of Moringa oleifera on albino rats using the modified arithmetic method of Karber and to determine the phytochemical component of the seeds of Moringa oleifera using standard methods.
Purchase, identification and extraction of plant (M. oleifera)
Moringa oleifera seed was purchased at Moringa House, 14, McAkini Road off Ada-George Road, Port Harcourt, Nigeria. The plant seed was identified and authenticated by a taxonomist. The seeds of M. oleifera were properly processed, grinded to powder and subjected to ethanol soxhlet extraction. The seed extracts were evaporated to near dryness on rotary evaporator (60°C), weighed and preserved at 4°C in a refrigerator before use.
Determination of phytochemical components of the Moringa oleifera seeds
Preliminary phytochemical screening of Moringa oleifera seeds were carried out following the standard methods described by Sofowora, Evans and Ajayi [18-20].
Test organism
A total of 100 healthy male and female albino rats of about 6-8 weeks weighing (30-40 grams) were used for the research. They were handled and cared for according to the standard guidelines for laboratory animal care. Further, they were housed in regular animal cages with maximum of 5 rats per cage under specific pathogen free condition at room temperature and feed with standard Recommended Dietary Allowance (RDA) and water. The animals were allowed to acclimatize to the environment for one week before experimentation according to the Institute for laboratory Animal Research [21]. The cages were cleaned and beddings changed every 24 hours.
Acute toxicity test (LD50) of Moringa Seed oil extract A pilot study was carried out using the oil extract of Moringa seed to establish the range of toxicity using 6 rats in order to establish the proper dose level for LD50 determination. In the study, it was observed that none of the doses (150. 450 and 950 mg/kg body weight) of the extract of Moringa seed could cause mortality of the rats after a period of 48 hours.
Using the modified Arithmetic method of Karber, the oral acute toxicity test for the ethanol extract of Moringa seed was determined in this study [22]. Thirty rats were randomly divided into six groups (group A-F) of five animals per cage. They were provided with free access to standard animal fed and water. Group B-F were administered with the different doses of the Moringa extract (180, 270, 405, 608 and 911 mg/kg body weight) intraperitoneally, while Group A was orally administered 0.1 ml of vegetable oil, the solvent in which the extract was miscible. All the rats were observed for 48 hours for behavioural and physiological signs of acute toxicity [23].
Phytochemical investigation of Moringa oleifera seeds revealed the presence of Carbohydrates, Saponins, Alkaloids, Cardiac glycosides, Flavonoids, Terpenoids and Terpenes as shown below (Table 1) of which some of them are known to provide an antiplasmodial effects based on other studies, while Anthraquinones, Carotenoids, Tannins and Cyanogenic glycosides were absent [24]. This result of the phytochemical evaluation of Moringa oleifera seeds supports the findings of other authors on the phytochemical analysis of Moringa oleifera plant parts [14,15,25,26], although in contrast with the study performed by Kasolo which indicated the presence of Anthraquinones in Moringa oleifera seeds [27].
| Phytochemicals | Remarks |
|---|---|
| Triterpenes | Present |
| Phlobatannins | Absent |
| Triterpenoids | Present |
| Alkaloids | Present |
| Carbohydrate | Present |
| Carotenoids | Absent |
| Saponins | Present |
| Cardiac glycosides | Present |
| Tannins | Absent |
| Flavonoids | Present |
| Anthraquinones | Absent |
| Fixed oil | Present |
| Cyanongenic glycoside | Absent |
Table 1: Phytochemical composition of Moringa oleiferaseeds.
Based on some literatures, flavonoids which were found to be present in Moringa oleifera seeds could be responsible for most antioxidant and anti--radical effects of Moringa oleifera that produced the anti-plasmodial activity [28-30].
Table 2 revealed that ethanol extract of Moringa oleifera seeds were non-toxic even at 911 mg/kg body weight (highest dose administered) in albino rats. At each dose administered, no mortality was recorded in any of the groups of albino rats and no significant changes in behaviours such as diarrhoea, motor activity, convulsion and coma in the albino rats was observed. It can be suggested that the average LD50 ethanol extract of Moringa oleifera seeds may be greater than 911 mg/kg body weight and slightly toxic based on the toxicity scale defined by Hodge and Sterner [31].
| Group | Dose (mg/kg) | Dose Difference | No. of albino rats | No. Death | Mean Death | Dose diff X Mean death |
|---|---|---|---|---|---|---|
| A | 0 | 0 | 5 | 0/5 | - | - |
| B | 180 | 0 | 5 | 0/5 | - | - |
| C | 270 | 90 | 5 | 0/5 | - | - |
| D | 405 | 135 | 5 | 0/5 | - | - |
| E | 608 | 203 | 5 | 0/5 | - | - |
| F | 911 | 303 | 5 | 0/5 | - | - |
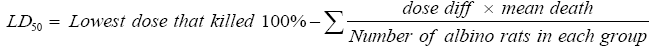
Table 2: LD50 calculation by modified Arithmetic method of Karber for ethanol extract of Moringa oleiferaseeds.
The results obtained showed that the anti--plasmodial activity of ethanol seed extract of Moringa oleifera used as an orthodox medicine in African region and elsewhere, against Plasmodium berghei malaria parasite, exerted a chemosupression effect at different concentrations over 72 hours supporting its folk use in the treatment of uncomplicated malaria. The extract gave a significant (P<0.05) non dose dependent reduction in the mean percentage parasitemia levels. This current finding contradicts the research result reported by Olashinde [32] involving Moringa oleifera seed which showed that the in vivo anti-malarial reduction of the mean % parasitemia was dose dependent for the n-hexane and ethanol extracts (Table 3).
| Toxicity class | LD50 (rat, mouse) mg/kg |
|---|---|
| Extremely toxic | <1 |
| Highly toxic | 1 – 50 |
| Moderately toxic | 50 – 500 |
| Slightly toxic | 500 – 5000 |
| Practically no toxicity | 5000 – 15000 |
Table 3: Hodge and Sterner toxicity scale.
The doses of 200 mgkg-1 (68.93%) and 500 mgkg-1 (67.01%) and 300 mgkg-1 (72.56%) exhibited a good chemosupression of Plasmodium multiplication compared to the negative control. According to a study conducted by Deharo [33], the in-vivo anti-plasmodial effect of plant extracts with chemosupression effect of up to 50% or more was seen to be very good. This implies that the Moringa oleifera seed extract is a very good chemosuppressive. The chemosupression of the Plasmodium parasite was higher with the administration of A/L (94.27%), A/M (88.33%), while Chloroquine 10 mg/kg exhibited the highest suppression (95.26%). The anti-plasmodial mode of action of the Artemisinins have been proposed by Robert [34] to be through the generation of free radicals reactive oxygen which could be similar to the effect of Moringa constituents possibly due to an increase in the concentration of its active components, which could acts separately to exhibit varying effect from each other (functional antagonism) or due to the competitive occupation for the receptor sites on the Plasmodium parasite as each concentration increases in the body system of the Swiss albino rats (competitive antagonism) resulting to a reduced anti-plasmodial activity (Table 4) [35].
| Treatments | Mean % parasitemia | % Chemosupression | |
|---|---|---|---|
| Day 3 | Day 6 | ||
| Distilled water | 19.00 ± 2.05 | 38.04 ± 1.50 | 0 |
| Artem/ Lum | 16.68 ± 4.87 | 02.18 ± 0.42* | 94.27 |
| Artes/Meflo | 16.68 ± 5.30 | 04.44 ± 0.73* | 88.33 |
| Chloroquine | 17.50 ± 3.11 | 01.80 ± 0.29* | 95.27 |
| Moringa 200 mgkg-1bw | 17.68 ± 2.00 | 11.82 ± 2.57* | 68.93 |
| Moringa 300 mgkg-1bw | 14.92 ± 1.51 | 10.44 ± 1.75* | 72.56 |
| Moringa 500 mgkg-1bw | 19.88 ± 1.40 | 12.55 ±0.84* | 67.01 |
Table 4: Mean percentage parasitemia and chemosupression in Plasmodium berghei inoculated albino rats when treated with ethanol extract of Moringa oleiferaseed and standard antimalarial drugs in the 4-days curative test.
The result of the study suggests that the Moringa extract has more of plasmodistatic effect than plasmodicidal as the parasites were not completely cleared by the seventh day and also a higher significant increase (P ≤ 0.05) in the erythrocyte per field was observed in the CQ, A.L and AM treated groups when compared with the negative control which implies that the reduction in mean percentage parasitemia caused a rise in the red blood cell counting of the infected albino rats.
The prophylactic test result showed a non-dose dependent significant (p<0.05) reduction of parasitemia level in Plasmodium berghei infected albino rats for ethanol extract of Moringa oleifera seed. The highest experimental dose 500 mg/kg (77.60%) exhibited a lower anti--plasmodial effect, while the dose 300 mg/ kg (79.18%) gave the highest chemosupression when compared to Moringa treated groups as shown in Table 4. The standard drugs, A/L. A/M and CQ produced a much higher chemosupression of 96.72%, 96.21% and 97.16% respectively.
The ethanol extract of Moringa oleifera seeds is relatively safe at the doses used for the acute toxicity test and its large safety margin is responsible for the wide use in Nigeria and other countries for the treatment of different ailment without acute toxic complications or symptoms. The finding also shows that the ethanol extract of Moringa oleifera seeds possesses chemosuppressive and chemoprophylactic anti--plasmodial effects against Plasmodium berghei infection in experimental rats. Since the percentage chemosupression in parasitemia is more than 50%, it can be recommended as a potential source of effective, affordable and safe herbal plant which can be used as herbal medicine for treatment of fever, pain and malaria.
1. This study has proven that Moringa oleifera seeds possess an anti- -plasmodial activity against Plasmodium parasite in albino rats and has contributed towards further use in the management of malaria infection.
2. This study has provided a baseline for further investigation on the different plant parts of Moringa oleifera.
3. This study has provided reference for further estimation of the actual LD50 of ethanol extract of Moringa oleifera seeds.
4. This study has added to the validation of the phytochemical components of Moringa oleifera seeds which have been suggested to be responsible for its anti--plasmodial activity.
Citation: Obediah GA, Obi NC (2020) Anti-plasmodial Effect of Moringa oleifera Seeds in Plasmodium berghei Infected Albino Rats. Biochem Pharmacol (Los Angel) 9:268. doi: 10.35248/2167-0501.20.9.268.
Received: 02-Dec-2019 Accepted: 10-Jan-2020 Published: 17-Jan-2020 , DOI: 10.35248/2167-0501.20.9.268
Copyright: © 2020 Obediah GA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.