Journal of Cell Signaling
Open Access
ISSN: 2576-1471
ISSN: 2576-1471
Review Article - (2022)Volume 7, Issue 7
The complexity of signalling pathways in cancers is often responsible for the failure of therapy or the emergence of therapy resistance. Cancer can no longer be viewed as an outcome of one driver gene, as multiple factors are involved. Activation of oncogenic signalling pathways can provide answers regarding disease pathogenesis. The cross-talk between signalling pathways, network re-wiring, feedback mechanisms, and gene redundancy functions are major contributors to therapy resistance which need to be considered to predict effective therapy. In this article, we provide a comprehensive overview of the intricacies of 9 signalling pathways commonly found to be deregulated in several cancer types.
Cell signaling; Resistance; Cross-talk; Cancer; Therapy
Cancer is a complex disease that involves interplay of several genes. Some of these genes have an oncogenic function, which eventually activates a signalling pathway, allowing tumour cells to survive and proliferate. Each cancer type is divided into subtypes based on the underlying mechanism of pathogenesis. Each subtype is characterized by specific genomic alterations that contribute to the activation of certain signalling pathways, which culminate in clinical outcomes. Molecular classification of tumours has enabled clinicians to decide the appropriate targeted therapy. Despite the availability of large amounts of multi-omics data from each patient, the predictive power of accurate prognosis of tumour progression and response to treatment is poor. In some cases, we have witnessed failures of carefully designed clinical trials which were based on genomic profiles of patient samples. These failures can be attributed to the complexity of signalling pathways in tumours of patients having the same cancer type. Some patients respond to targeted therapy and show improved survival, whereas some respond poorly. Moreover, some patients show good responses initially but eventually develop therapy resistance. These outcomes are a result of intrinsic resistance or acquired resistance involving intricate signalling cross talks, feedback mechanisms, and network re-wiring. For instance, activation of Mitogen-Activated Protein Kinase Kinase (MAPKs) signalling is commonly observed in BRAF-driven melanoma patients treated with BRAF inhibitors because of compensatory signalling mechanisms [1]. Patients with Non-Small Cell Lung Cancer (NSCLC) activating EGFR mutations showed a better response to gefitinib than standard chemotherapy; however, 90% of these patients developed relapse after 2 years [2]. One of the reasons behind these observations can be genetic redundancy between the genes of the same family. These genes have similar functions, and they sustain cellular signalling when one of the genes is inhibited by targeted therapy. For example, MAPK signalling is sustained even when one of the genes belonging to the RAF family (ARAF, BRAF, and CRAF) is inhibited because other genes of the RAF family perform partial compensatory functions [3]. Considering only genetic dependency of tumours would be inadequate while planning targeted treatments. It is essential to consider potential signalling cross-talks, network re-wiring mechanisms, gene redundancy, and tumour heterogeneity to determine the outcome of clinical treatments. The functional complexity of signalling pathways can be studied through synthetic lethality screens using RNAi and CRISPR-Cas9 approaches. Such studies can identify genes with compensatory functions, which can be targeted simultaneously for enhanced therapeutic effects.
Signalling complexity makes cancer an unconquerable disease. Hundreds of signalling pathways operate with complex inter-pathway communication in different types of cancer. This review provides a bird’s eye view to understand the complexity of major 9 signalling pathways operating in several cancer types as shown in Figure 1.
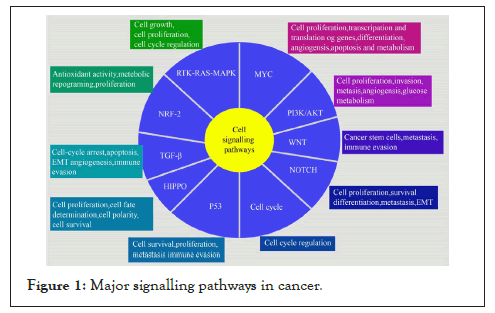
Figure 1: Major signalling pathways in cancer.
The PI3K-AKT signalling pathway
PI3K-AKT is one of the most common deregulated signalling pathways in several cancers, particularly in gynaecological cancers [4]. PI3K is activated by phosphorylation by different Receptor Tyrosine Kinases (RTKs) on ligand binding. PI3K phosphorylates phosphatidylinositol-4,4-bisphosphate [4,5] P2 (PIP2) and converts it into phosphatidylinositol [3-5] P3 (PIP3) in the plasma membrane. Of the several types, type I PI3K is a heterodimer comprising of a catalytic subunit p110 and a regulatory subunit p85. PIP3 then recruits several proteins containing Pleckstrin Homology (PH) domains such as Protein-Dependent Kinase 1 (PDK1) and Akt/Protein Kinase B (PKB) at the membrane [4]. AKT activation occurs by phosphorylation of Thr308 and Ser473 residues by PDK1 and mTORC2 or DNA-PK, respectively [4]. The phosphorylated AKT translocate to phosphorylate downstream targets. Phosphorylation of tumour suppressor TSC2 by AKT causes the release of inhibitory activity of TSC2 on mTOR, leading to the activation of mTOR signalling [5]. The mTORC1 complex, comprising mTOR, Raptor, mLST8, and PRAS40, activates downstream effector targets 4EBP1 and P70S kinase (S6K), resulting in increased protein synthesis to promote cell growth and proliferation [5]. PTEN, a tumour suppressor, is one of the important negative regulators of PI3K-AKT signalling. PTEN dephosphorylates PIP3 to PIP2 to inhibit signalling [6]. Other negative regulators include PPP2R1A, which inhibits AKT [7]; INPP4B and PTPN12, which inhibit PIK3CA [8]; and TSC1/2, which inhibits mTOR activity [5]. The activated PI3K-AKT pathway promotes cell proliferation, angiogenesis, invasion and metastasis, ribosome biogenesis, mRNA translation, cell cycle entry, and glucose metabolism and inhibits apoptosis (Figure 2) [5,9].
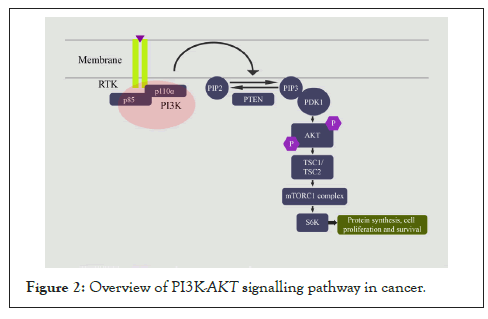
Figure 2: Overview of PI3K-AKT signalling pathway in cancer.
Alterations in cancer: Several members of the PI3K-AKT signalling pathway harbour genomic alterations in several cancers. Loss of PTEN is commonly observed in cancers of the central nervous system, endometrium, colon and rectum, skin, breast, prostate [10], and melanoma [11]. PIK3CA alterations are frequent in breast, endometrial, colorectal, urinary tract, cervical, ovarian, gastric, HNSCC, squamous cell lung cancer [10], GBM, AML, and hepatocellular [11] cancers. Amplification of AKT is observed in ovarian, pancreatic, HNSCC, gastric, and sporadic melanomas, whereas breast, bladder, and colorectal cancers display E17K AKT mutation [10].
Signalling cross-talks: A cross-talk between the PI3K-AKT pathway and other signalling pathways is commonly observed in several cancer types. Ras is known to interact with PIK3CA (p110) [12], and ERBB4 interacts with the p85 subunit of PI3K, resulting in the activation of the downstream MAPK signalling pathway [13]. AKT phosphorylates MDM2, which then binds and ubiquitinylates P53, promoting its proteasomal degradation, thus inhibiting p53-dependent apoptosis [14]. A cross-talk with NFƘB signalling is observed through AKT activation of IKβ kinase (IKK). IKK activates the p65 subunit of NFκB by phosphorylation and prevents apoptosis in colorectal cancer [5,15]. AKT also inhibits GSK3 by phosphorylation, thus promoting glucose metabolism [16]. Angiogenesis is facilitated by the activation of HIF1α and VEGF through AKT in cancers [5]. Several signalling pathways enhance PI3K-AKT signalling. The NOTCH pathway cross-talks through the inhibition of PTEN by Hes-1 [17] and hippo signalling through YAP-mediated miR-29 upregulation that targets PTEN [18]. TGF-β activates this pathway through SMAD-dependent and non-SMAD mechanisms [19]. LKB1 negatively regulates mTORC1 through TSC2 and Raptor phosphorylation [20].
Resistance mechanisms: The PI3K-AKT-mTOR pathway is one of the major contributors to resistance in several anti-cancer therapies. Studies in breast cancer cell lines have shown that PI3K and AKT can phosphorylate Estrogen receptor (Erα) at Ser167 to activate estrogen signalling in the absence of estrogen, thus contributing to endocrine therapy resistance. PI3K activates S6K in some breast cancer cell lines after prolonged estrogen deprivation [21]. PIK3CA mutations are recurrent in ER+ breast cancer patients, contributing to the activation of the signalling pathway and consequently, to the emergence of resistance [21]. From clinical studies, it has been proven that PI3K contributes to endocrine therapy resistance and that treatment with both everolimus (mTOR inhibitor) and exemestane (aromatase inhibitor) improved progression-free survival in breast cancer patients [22]. Resistance to anti-HER2 monotherapy is also commonly reported in breast cancer due to HER2-mediated activation of the PI3K-AKT-mTOR pathway [23]. The tumours and cells treated with a BRAF inhibitor, dabrafenib, developed resistance and often showed overexpression of IGF-1R, which activated the PI3K/AKT/mTOR pathway [24]. MET amplification in NSCLC results in the activation of the PI3K/AKT/mTOR pathway via ERBB3 phosphorylation [25]. Patients resistant to MET inhibition by crizotinib are likely to exhibit activation of the PI3K/AKT/mTOR pathway as demonstrated by studies using cell lines [25]. Moreover, the PI3K/AKT/mTOR pathway is the culprit behind chemotherapy resistance in ovarian cancer. Post chemotherapy, increased expression and activation as assessed by phosphorylation of EBP1, p70S6K, and S6K was observed in ovarian cancer tissues, suggesting the activation of the PI3K-AKT pathway. Thus, a certain subset of patients can benefit from the combination treatment of PI3K pathway inhibitors and chemotherapy.
Assays: The activation of the PI3K-AKT signalling pathway in tumour samples can be studied using IHC for pAKT (Ser473 and Thr308), pP70S6K (Thr421, Thr389, and Ser424), p-mTOR (Ser2448), p-4EBP1 (Thr37/46), PTEN, p110γ and p85α subunits of PI3K [26-28]. Proteome analysis of tumour tissues or the use of phospho-proteome screens/phosphorylation arrays for these proteins can predict pathway activation. In cell lines, western blotting to detect the phosphorylated and total forms of these proteins is usually performed. Some of the commercially available cell-based assays such as FOXO reporter kit (BPS Bioscience) or Cell Sensor reporter assays are robust methods to study pathway activity. Another comprehensive method to detect the activity of several players of the pathway and study the effect of the inhibitors on phosphorylation status and activity is the Lantha Screen Cellular Assay (Thermo Fischer Scientific), a FRET-based assay. This cell-based assay identifies phosphorylation on the proline-rich AKT substrate PRAS40 (Ser183 and Thr246), programmed cell death protein 4 (PDCD4)(Ser457), and AKT (Thr308 and Ser473), and it has utility in high throughput screening applications [29-35]. FDA approved therapy targeting members of PI3K/ AKT pathway is shown in Table 1.
| Gene | Inhibitors | Monotherapy or combination therapy | FDA approval for use in cancer types |
|---|---|---|---|
| PI3K δ | Idelalisib | Monotherapy | Relapsed small lymphocytic lymphoma and follicular lymphoma (30) |
| PI3K δ | Idelalisib | Combination with rituximab | Relapsed or refractory chronic lymphocytic leukaemia (30) |
| PI3K α | Alpelisib | Monotherapy | PIK3CA-mutated, advanced, or metastatic breast cancer (NCT02437318) |
| PI3K α/δ | Copanlisib | Monotherapy | Relapsed follicular lymphoma (31) |
| PI3K δ/CK1ε | Umbralisib | Monotherapy | Marginal zone lymphoma and follicular lymphoma post-therapy (31) |
| PI3K δ/γ | Duvelisib | Monotherapy | Relapsed or refractory Chronic Lymphocytic Leukaemia (CLL)/ Small Lymphocytic Lymphoma (SLL) and follicular lymphoma post two prior therapies (32, 33) (NCT02004522) |
| mTOR | Sirolimus | Monotherapy | Patients with lymphangioleiomyomatosis with mutations in the TSC2 gene in Renal Cell Carcinoma (RCC) (34) |
| mTOR | Everolimus | Monotherapy | Renal cell carcinoma and pancreatic cancer (34) |
| mTOR | Everolimus | Used in combination with the aromatase inhibitor exemestane | HR+, HER2-breast cancer (35) (NCT01743560) |
| mTOR | Everolimus | Monotherapy | Advanced neuroendocrine tumours (NCT01595009) |
| mTOR | Everolimus | Used in combination with paclitaxel and carboplatin | Advanced large cell lung cancer with neuroendocrine differentiation (NCT01317615) |
| mTOR | Everolimus | Monotherapy | Metastatic renal cell carcinoma patients after failure of first-line therapy with sunitinib or pazopanib (NCT01514448) |
| mTOR | Everolimus | Monotherapy | Advanced renal cell carcinoma (NCT01206764) |
| mTOR | Temsirolimus | Monotherapy | Advanced renal cell carcinoma patients with metastatic recurrent and/or unresectable tumours (NCT01206764) |
| mTOR | Temsirolimus | Monotherapy | Patients with relapsed, refractory mantle cell lymphoma (NCT01180049) |
Table 1: FDA approved therapy targeting members of PI3K/ AKT pathway.
The NOTCH signalling pathway
The Notch signalling pathway plays both oncogenic and tumour suppressive roles in different cancer types [36]. The ligands DLL-1,2,4, Jagged 1, and Jagged 2 bind to Notch receptors (Notch 1-4) to activate signalling, which is mediated by a process involving two-step proteolytic cleavage. The first cleavage removes the extracellular domain of Notch by ADAM family metalloproteinases. The second cleavage by the γ-secretase complex (PSEN2, APH1, PEN-2, and Nicastrin) releases the intracellular domain (NICD) from the transmembrane domain, which is then translocated to the nucleus. NICD associates with MAML and DNA-binding protein CSL to form an activator complex, which further binds to CREBBP and EP300 inside the nucleus to facilitate the transcription of Notch targets such as HES (HES1, HES5, HERP) and HEY (HEY1, HEY2) family genes [37]. Other Notch target genes include NRARP, a feedback inhibitor of Notch signalling [38], and cell cycle regulators like p21, cyclin D1, C-MYC, and NF-κB [39]. The Notch pathway is negatively regulated by co-repressors NCOR1, NCOR2, SPEN, and NUMB [40]. Activated Notch signalling promotes cell proliferation, survival, and differentiation [41]. It also promotes EMT phenotype and metastasis [36] in cancers (Figure 3).
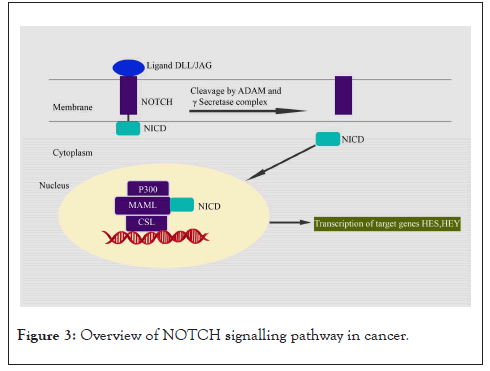
Figure 3: Overview of NOTCH signalling pathway in cancer.
Alterations in cancer: Genomic alterations in the members of the Notch signalling pathway play context-specific roles as oncogenes and Tumour-Suppressor Genes (TSGs). Notch1 acts as an oncogene in breast, colorectal, gastric, ovarian, Head and Neck Squamous Cell Carcinoma (HNSCC), lung, and adenoid cystic carcinoma, whereas it acts as a TSG in cervical, colorectal, pancreatic, prostate, hepatocellular, skin, brain, B-cell malignancies and HNSCC [42,43]. Notch2 functions as an oncogene in cervical and gastric cancers and as a TSG in breast, colorectal, and B-cell malignancies [42]. Notch3 works as an oncogene in breast, cervical, colorectal, ovarian, and lung cancers, and as a TSG in B cell malignancies [42,43]. Further, Notch4 works as an oncogene in breast cancer and as a TSG in B cell malignancies [42]. Ligands such as JAG-1 play an oncogenic role in breast, colorectal, ovarian, and gastric cancers and work as TSG in prostate and B cell malignancies [42]. JAG-2 works as an oncogene in colorectal cancer and as a TSG in B cell malignancies [42]. The reason for the dual role of Notch in different cancers is still unknown. Even within the same organ, contrasting Notch pathway functions have been reported. Overall, Notch has tumours suppressive role in HNSCC but in oral cancer, gain-of-function activating mutations are reported. Similarly, Notch as an oncogene is known in T-ALL and CLL cancers and as a tumour suppressor in AML and CML [44]. This dual function is associated with cell fate determination [44,45].
Signalling cross-talks: Notch signalling is involved in cross-talks with other oncogenic signalling pathways in cancer. Notch-1 contributes to resistance to EGFR TKI therapy in lung cancer by promoting the EMT phenotype [46]. The cross-talk occurs via MAPK/MEK/ERK pathway. This cross-talk also causes an increase in pAKT in TNBC cells [47]. Inhibition of HER2 causes upregulation of Notch-1 expression and downstream target genes in breast cancer [47]. Notch-1 mediates cell cycle progression by enhancing C-MYC expression and suppresses P53 activity in T-ALL cells [39]. Notch signalling mediated by JAG-1 increases phosphorylation of AKT in cervical cells [47]. TGF-β upregulates JAG-1 expression. TGF-β and Notch regulate the same target genes such as SMAD3. JAG-2 ligand expression is reported to promote hedgehog signalling in breast cancer [48]. HES-1 represses PTEN expression, thus contributing to PI3K signalling in T-ALL [47]. HES1 inhibits DUSP1, thereby activating ERK [49].
Resistance mechanisms: One of the resistance mechanisms in endocrine therapy in ER-positive breast cancer is contributed by notch signalling. Studies have shown that breast cancer cells (ER-positive) treated with tamoxifen or fulvestrant show increased expression of activated Notch1 and Notch4 [50]. Combination treatment with γ-Secretase Inhibitors (GSI) and tamoxifen reduced tumour growth in ERα-cells [51]. In breast cancer, Cancer Stem Cells (CSC) play an important role in developing resistance to treatment, and CSC activity is mediated by Notch4. Further, ERα-Y537S mutation enhances the activity of breast CSC via notch signalling [51]. Genetic and pharmacological inhibition of Notch4 reduced CSC activity in in-vitro and in-vivo models [52]. Treatment of HER2-positive breast cancer cells by trastuzumab is known to increase the expression of Notch1, thus contributing to resistance [50]. Notch signalling also contributes to chemoresistance to agents such as cisplatin, doxorubicin, gemcitabine, and paclitaxel in several cancer types [53]. Notch signalling also enhances radio-resistance in glioma stem and progenitor cells through the activation of Notch1 and Notch2. Glioma stem cells treated with GSI were more sensitive to radiation therapy, as evidenced by increased cell death and reduced clonogenic survival [54,55]. Chemoresistance in ovarian cancer is also mediated by Notch3 activation. Activated Notch3 results in increased expression of CSC markers and ALDH1 activity in ovarian cancer cells, contributing to resistance [56]. Radiation resistance in EGFR mutated NSCLC can be overcome with combined treatment targeting EGFR and Notch as erlotinib-mediated inhibition of EGFR increased ALDH and Notch transcriptional activity in cells [57].
Assays: In patient samples, activated Notch1 activity is assessed using IHC with anti-NICD1 monoclonal antibody [58,59] and antibodies against Notch1-3 [60]. In a recent study, researchers detected the expression levels of a set of direct target genes of Notch to calculate a pathway score that corresponds to the activity of notch signalling in different cancer types [61]. Western blotting in cell lines is performed for full forms and activated form (NICD) of Notch, Notch target gene HEY-1, HES1, and pathway repressor NUMB [62-64] and ligands Jagged-1, Jagged-2, and DLL1 [65]. A Notch reporter assay comprising of Notch1-expressing vector and a Notch pathway-responsive reporter vector CSL (CBF1/RBP-Jk) expressing luciferase aids in quantitating notch signalling activity under given treatment conditions [65,66].
Therapy targeting NOTCH genes: Notch-targeted therapy is not yet approved for treatment in patients. However, several γ-secretase inhibitors (AL101, RO4929097) are currently in clinical trials, and certain antibody‑based biologics (ABT‑165, AMG 119, Rova‑T) are under investigation [67].
The MYC pathway
The MYC oncogene family comprises of transcription factors C-MYC, N-MYC, and L-MYC, which are encoded by genes MYCC, MYCN, and MYCL respectively [68]. Of the three, C-MYC is expressed ubiquitously in proliferating cells [69]. C-MYC, a member of the bHLH family, heterodimerize with another bHLH-LZ domain protein, MAX, and binds to E-box at consensus sequence CAC(G/A)TG present at target gene promoters to regulate their expression [69]. MAX also plays a transcription repressor role by forming a complex with other proteins like MAX gene–associated 9 (MGA), MXD family proteins (MXD1-4), and MYC/MAX/MAX-binding protein (MNT) and by blocking E-box sequence DNA-binding sites [70]. The MAX-MXD complex recruits transcription repressor Sin3 and Histone Deacetylase (HDAC) to downregulate gene expression [69]. MXL is another dimerization partner of MXD1 through which it interacts with MAX. MXL binds to E-box DNA and contributes to repressive activity [69]. MXL interacts with MLXIP (MondoA) and MLXIPL (MondoB) and mediates the cellular transcriptional response to changes in metabolites such as glucose and glutamine levels [71]. The interaction of Myc with its interacting partners is responsible for different biological activities in tumours. Deregulated Myc activity promotes transcription and translation of genes, cell proliferation, differentiation, angiogenesis, apoptosis, and metabolism [72] in cancers (Figure 4).
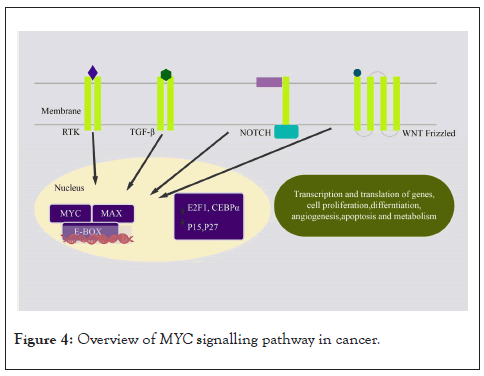
Figure 4: Overview of MYC signalling pathway in cancer.
Alterations in cancer: Alterations in MYC genes are reported in 70% of human cancers [73]. Copy gains are the most common alterations in MYC oncogenes. Pan-cancer analysis reports amplification in 21% samples for MYCC, whereas MYCN and MYCL show 7% focal amplification [74]. CMYC amplification is frequent in ovarian cancers, endometrial cancer, breast cancer, colorectal cancer, prostate cancer, pancreatic cancer, renal clear cell carcinoma, squamous lung cancer, and oesophagal cancers. Similarly, N-MYC amplification or overexpression is observed in neuroblastoma, retinoblastoma, medulloblastoma, small-cell lung cancer, and prostate cancer, whereas L-MYC amplification is found in small-cell lung cancer [73,75]. Another common alteration is C-MYC translocation into the immunoglobulin heavy chain locus, which is found in 80% of cases of Burkitt lymphomas. Similar translocation into the T-cell receptor loci is observed in T-cell acute leukaemia, and translocation into different immunoglobulin chain loci is found in multiple myeloma and diffused large cell lymphoma [76,77].
Signalling cross-talks: Several ligand-based receptor pathways like WNT, receptor tyrosine kinases (RTK), TGF-β, and T-cell receptor (TCR) positively regulate the expression of MYC, which in turn facilitates transcription of target genes promoting cell proliferation. MYC expression is regulated by several of the cancer signalling pathways, such as WNT, EGFR/HER2, and Ras [78]. Deregulated MYC activates checkpoints p53 and ARF. However, the loss-of-function mutations in these genes mediate Myc-induced tumorigenesis [79]. The STAT3/C-MYC pathway along with mTOR/PKM2 plays a role in regulating energy metabolism in gastric cancers [80]. The cross-talk of MYC and HIF pathways contributes to cancer metabolism, resulting in increased tumour growth, angiogenesis, and metastasis [81]. In breast cancer, C-MYC is activated by Notch and ER-α, eventually contributing to tumour progression and endocrine resistance [78,82].
Resistance mechanisms: C-MYC expression in several cancer types are correlated with resistance to chemotherapy [83]. Resistance to platinum-based chemotherapy in ovarian cancer, head and neck squamous cell carcinoma, lung cancer, and Acute Myeloid Leukaemia (AML) can be attributed to increased C-MYC expression after treatment [84], which is associated with poor disease-free survival and overall survival [85,86]. In ER-positive breast cancer, increased C-MYC expression is known to contribute to tamoxifen resistance [87]. MYC expression is associated with the renewal of CSCs, which contributes to therapy resistance in triple-negative breast cancers [88]. Given that C-MYC is a common downstream activator of PI3K/AKT, MAPK, and Notch signalling, it is a prognostic marker and therapeutic target in cancer treatment [89].
Assays: In patient tissue samples, IHC for MYC is routinely performed to detect MYC activation [90,91]. MYC amplification is detected using fluorescent in-situ hybridization (FISH) [92], comparative genome hybridization [93], and digital PCR (dPCR) [94]. MYC mRNA levels can be quantified for copy number change using quantitative reverse transcription PCR using TaqMan probes [95]. Pathway activation is studied in cell lines by western blotting for Myc proteins and their downstream targets cdk1, cdk2, and cyclins (A2, B1 and E); it is also assessed by detecting the loss of p27 [96]. Since C-MYC forms complexes with several other proteins to perform diverse biological functions, immunoprecipitation and western blotting can be done for proteins Max, Mxd1, and Mad1 [97,98]. Commercially available kits to study MYC pathway activation are MYC Reporter Kit (BP Biosciences) and a luciferase reporter assay kit. Kits such as the cMyc transcription factor assay kit or CHIP-Seq high sensitivity kit (Abcam) are used to study the transcriptional activity of C-MYC. C-MYC levels in human tissue or cell lysates can be detected using a C-MYC (total) Human ELISA kit (Thermo Fischer Scientific).
Therapy targeting MYC pathway genes: There are no FDA-approved MYC inhibitors [99]. However, research on a novel approach to inhibiting MYC target genes using MYC inhibitor Omomyc is ongoing. Omomyc binds to box sequences in the promoter region of MYC target genes and prevents transcription. In this approach, Myc-Max heterodimers are replaced by Omomyc-max heterodimers [100].
The transforming growth factor-β (TGF-β) pathway
The TGF-β superfamily includes TGF-β1, TGF-β2, TGF-β3, Bone Morphogenetic Proteins (BMPs), activins, and other related proteins. TGF-β signalling plays important roles in different cellular processes, including cell cycle regulation, cell migration, apoptosis, angiogenesis, and immune modulation [101]. TGF-β family members act as tumour suppressor during the early stages of cancer development; however, during later stages, it enhances the invasion and metastasis of carcinoma cells [102]. The active members of the TGF-β family form homodimers and signals by bringing together receptor serine/threonine kinases known as the type I (TGFBR1) and type II (TGFBR2) receptors. Upon TGF-β binding, the type II receptors phosphorylate and activate the type I receptors, which then phosphorylate receptor-activated Smad proteins that work as transcription factors. A pair of activated Smad proteins (SMAD2 or SMAD3) form a heterotrimeric complex with SMAD4 and translocate to the nucleus where the complex associates with additional DNA-binding cofactors to induce or repress the transcription of target genes. Activation of R-Smads by TGFBR1 is inhibited by Smad6 or Smad7 [103]. Smad-Ubiquitination-Regulatory Factors (Smurf1 and Smurf2) and E3 ubiquitin ligases control the levels R-Smads [103]. TGF-β, independent of Smads, can modulate the activity of other signalling pathways including MAPK pathways, Rho-like GTPase signalling pathways, and PI3K/AKT pathways in a ligand-receptor manner [104]. These noncanonical (Smad-independent) TGF-β signalling pathways play an important role in cell migration and Epithelial-Mesenchymal Transition (EMT) (Figure 5) [105].
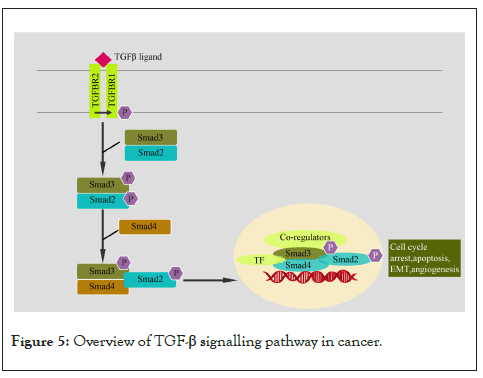
Figure 5: Overview of TGF-β signalling pathway in cancer.
Alterations in cancer: Inactivating mutations in the TGFBR2 gene are reported in the colon, gastric, biliary, pulmonary, ovarian, esophageal, and head and neck carcinomas [106]. Elevated TGF-β levels are reported in many cancers including breast, colon, esophageal, gastric, hepatocellular, and lung [106]. Inactivation of the TGFBR1 gene is majorly mediated by promoter methylation or intragenic mutation in different types of cancers including breast, gastric, HNSCC, and ovarian cancers [106]. Inactivating mutation and deletion of SMAD4 and SMAD2 genes are frequently reported in pancreatic and colon cancers, although they are also reported in other cancers [106]. Mutations of SMAD3 genes are less frequently reported [107].
Resistance mechanisms: Activation of TGF-β signalling has been associated with resistance against targeted and conventional chemotherapeutic drugs [108]. EMT induction via activation of TGF-β signalling is shown to be the major player in imparting resistance to EGFR-targeted therapies in NSCLC [109-111] and the dual IGF-I/IR inhibitor therapy in hepatocellular carcinoma cells [112]. Activation of TGF-β signalling is also associated with resistance to BRAF and MEK inhibitors via upregulation of EGFR and Platelet-Derived Growth Factor Receptor-β (PDGFRB) in melanoma [113]. TGF-β also promotes heterogeneity in squamous cell carcinoma stem cells cisplatin resistance [114]. TGF-β signalling contributes to drug resistance in liver cancer cells by inducing the expression of xenobiotic nuclear receptor pregnane X receptor, which increases the expression of drug efflux transporters [115].
Signalling cross-talks: The TGF-β signalling pathway communicates with other signalling pathways in a synergistic or antagonistic manner and regulates cellular functions. It has been reported that in the early stages of cancer, PI3K/AKT pathway activation antagonizes the TGF-β induced cytostatic or apoptotic response; however, in advanced cancers, these pathways act synergistically and promote cancer cell aggressiveness [116]. The cross-talk between TGF-β and Ras-MAPK pathway is reported by many research groups. Several reports have suggested a synergistic association between TGF-β and HER2 in accelerating metastatic breast cancer progression [117,118]. TGF-β facilitates cancer cell survival by repressing p53 transcription and translation, thereby impairing the proapoptotic actions of p53 [119]. Furthermore, Smad proteins associate with mutant p53 to deregulate p63-mediated transcription and enhance metastasis [120].
Assays: In tissue samples, activated TGF-β activity can be assessed using antibodies against phosphorylated SMAD2 or SMAD3 or by estimating SMAD4 or TGF-β protein levels using IHC or western blotting [121-123]. ELISA can be used to check the levels of TGF-β [122,124]. Drugs targeting different members of TGF-β signalling pathway is shown in Table 2.
| Gene | Inhibitor | Therapy type | Clinical trial |
|---|---|---|---|
| TGFBR1 | Galunisertib (LY2157299) | In combination with enzalutamide | Metastatic castration-resistant prostate cancer (NCT02452008) |
| TGFBR1 | LY2157299 | In combination with carboplatin/paclitaxel | Carcinosarcoma of the uterus or ovary (NCT03206177) |
| TGFBR1 | LY2157299 | In combination with nivolumab | Advanced refractory solid tumours and recurrent or refractory NSCLC or hepatocellular carcinoma (NCT02423343) |
| TGFBR1 | LY2157299 | In combination with neoadjuvant chemoradiation | Rectal cancer (NCT02688712) |
| TGFBR1 | LY2157299 | Monotherapy and in combination with sorafenib or ramucirumab. | Hepatocellular carcinoma (NCT01246986) |
| TGFBR1 | Vactosertib (TEW-7197) | Monotherapy | Anaemic patients with myeloproliferative neoplasms (NCT04103645) |
| TGFBR1 | Vactosertib | In combination with pomalidomide | Relapsed or relapsed and refractory multiple myeloma (NCT03143985) |
| TGF-βisoforms | SAR439459 (Pan-mAb) | In combination with cemiplimab | Advanced solid tumours (NCT04729725) |
| TGF-β 1 and 3 | AVID200 | Monotherapy | Treatment-refractory advanced and metastatic cancers (NCT03834662) TGF-βRII a |
| TGF-β | SHR-1701 | In combination with SHR2554 (solid tumours) monotherapy (lymphoma) | Advanced solid tumours and B-cell lymphomas (NCT04407741) |
| TGF-β | Bintrafusp alfa (M7824) | In combination with chemoradiation | Oesophagal squamous cell carcinoma (NCT04595149) |
| TGF-β | M7824 | Monotherapy | Advanced or metastatic biliary tract cancer (NCT03833661) |
| TGF-β | M7824 | In combination with topotecan or temozolomide | Relapsed small cell lung cancers (NCT03554473) |
| TGF-β | NIS793 | Monotherapy or in combination with PDR001 | Advanced/metastatic solid tumours (NCT02947165) |
Table 2: Drugs targeting different members of TGF-β signalling pathway.
The nuclear factor erythroid 2-related factor 2 (Nrf2) pathway
The Nrf2 pathway is a master regulator of the antioxidant program. It also plays a role in metabolic reprogramming, protein homeostasis, proliferation, and regulation of inflammation [125,126]. Nrf2 has cancer-preventive effects in normal cells; however, its constitutive expression contributes to cancer progression, metastasis, and therapy resistance [127,128]. Kelch-like ECH-associated Protein 1 (Keap1) is a component of the E3 ubiquitin ligase complex and a major negative regulator of Nrf2 [129]. When the levels of Reactive Oxygen Species (ROS) rise, Nrf2 disassociates from Keap1 and enters the nucleus to form a heterodimer with small MAF proteins (sMAF). Subsequently, it regulates the expression of genes with promoter regions containing Antioxidant Response Elements (AREs), which include genes involved in endogenous antioxidant protection and detoxification of ROS [130]. Apart from ROS levels, Nrf2 activity is also regulated by Protein Kinase (PKC, PI3K/Akt, GSK-3β, JNK); interaction with other protein partners (p21, caveolin-1) and epigenetic factors (micro-RNAs -144, -28 and -200a, and promoter methylation) (Figure 6) [131].
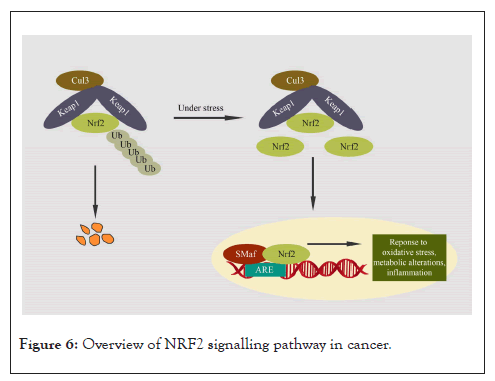
Figure 6: Overview of NRF2 signalling pathway in cancer.
Alterations in cancer: Cancer cells occasionally acquire aberrant Nrf2 activation [132]. Increased Nrf2 (NFE2L2) gene copy number is reported in lung cancer [133]. Inactivating mutations in KEAP 1 are reported in many cancers including breast, gallbladder, liver, ovarian, clear renal cell, lung cancers and prostate cancer [134]. Hypermethylation of the KEAP 1 gene promoter is reported in lung cancer [135]. Mutations in the Nrf2 gene are reported in head and neck cancers [134], lung cancers [136], esophageal cancers, and skin cancers [137]. Overexpression of Nrf2 is observed in several cancers including NSCLC, AML, pancreatic cancers, and breast cancer [138].
Signalling cross-talks: Oncogenic pathways like EGFR, RAS, C-MYC and B-RAF can increase Nrf2 transcription, thereby promoting ROS detoxification, tumorigenesis, and therapy resistance [139,140]. The PI3K/Akt pathway increases the levels of nuclear Nrf2, possibly by inhibiting GSK3-β, leading to cell proliferation and cytoprotection observed in lung cancer [141-143]. NF-kB can also induce Nrf2 expression in Acute Myeloid Leukaemia (AML) [144]. Studies have shown that activated p62 can directly bind to Keap1, leading to Nrf2 displacement and stabilization [145]. Nrf2 is shown to enhance cell migration and invasion by regulating RhoA-ROCK1 signalling in breast cancer [146] and lung cancer [147]. The HIF1α gene promoter contains ARE, and it is a direct transcription target of Nrf2 [148]. Overexpression of Nrf2 regulates angiogenesis by inducing the expression of HIF1α in different cancers, leading to tumour progression [149-151]. Cross-talk between Nrf2 and TGF-β1 promotes tumorigenic potential and invasive phenotype in pancreatic ductal cells and pancreatic ductal adenocarcinoma cells [152]. Nrf2 is shown to promote the proliferation of cancer cells by metabolic reprogramming [143,153]. A cross-talk between Nrf2 and Notch signalling is reported to mediate radioresistance in NSCLC cells [154-156].
Resistance mechanisms: KEAP1 gene mutations lead to constitutive activation of Nrf2, which imparts resistance to EGFR-TKI in NSCLC [140]. In lung cancer, KRAS-mediated cisplatin resistance occurs via upregulation of Nrf2 gene transcription by KRAS [157,158]. NF-κB–driven constitutive expression of Nrf2 is associated with tumour progression and resistance to cytarabine and daunorubicin in AML [144]. Constitutive Nrf2 expression is associated with resistance to chemotherapeutic drugs in lung cancer, breast cancer, and neuroblastoma cells [159]. Nrf2 and Notch signalling cross-talk are involved in mediating radio resistance in NSCLC cells [154-156]. Several reports as shown that aberrant Nrf2 activation can lead to the overexpression of multidrug resistance proteins, leading to chemoresistance in different cancer types [160-163].
Assays: TransAM® Nrf2 kits can be used to detect and quantify Nrf2 activation [144]. Activation of the Nrf2 pathway can be studied by measuring the mRNA expression profile of its downstream target genes using real-time PCR [159]. Knockdown and overexpression of the Nrf2 gene can also be performed to study the functional aspects [159]. Protein levels of Nrf2 and Keap1 can be studied using western blotting [159] or IHC [164].
Therapy targeting Nrf2 pathway genes: There are no FDA-approved Nrf2 pathway inhibitors for the treatment of cancers. Several natural compounds with Nrf2 inhibitory effects are being evaluated [165,166].
The Wnt pathway
Wnt signalling is involved in stem cell renewal, proliferation, and differentiation. It plays an important role in embryonic development and tissue homeostasis. In cancer, it promotes CSC, metastasis, and immune evasion [167]. The Wnt signalling pathway is divided into canonical (β-catenin-dependent) and non-canonical (β-catenin-independent) pathways. Production of Wnt ligands in secreting cells is a complex process in Wnt signalling [168]. Canonical Wnt pathway gets activated when secreted Wnt ligands (glycolipoproteins) bind to seven-pass transmembrane frizzed receptors and low-density lipoprotein receptor-related protein 6 or 5 (LRP6/LRP5) co-receptors. This complex formation recruits Dishevelled (Dvl) proteins to the plasma membrane. This results in LRP6 phosphorylation and activation and the recruitment of the Axin, which leads to the inactivation of the β-catenin destruction complex. This complex comprises scaffold protein Axin, Adenomatous Polyposis Coli gene product (APC), Glycogen Synthase Kinase 3 (GSK3β), and Casein Kinase 1α (CK1). CK1 and GSK3 phosphorylate β-catenin and targets for ubiquitination by β-Trcp and subsequent degradation. In the absence of an active destruction complex, β-catenin gets stabilized and translocates into the nucleus. β-catenin forms an active complex with LEF/TCF family transcription factors by displacing repressive TLE/Groucho complexes. The recruitment of histone-modifying co-activators leads to the transcription of target genes [169]. Wnt signalling is negatively regulated by RNF43/ZNRF3 [170,171], Wnt inhibitory factor 1 (WIF-1) [172], Cerberus [173], secreted Fzd-related proteins (sFRPs) [174], and Dickkopf proteins (Dkks) [175]. R-Spondin (RSPO) family of secreted proteins are activators of the canonical Wnt pathway [176]. Compared to the canonical Wnt pathway, the non-canonical pathways are more diverse but not well characterized. Wnt ligands Wnt5a and Wnt11 can bind to a panel of different receptors (Fzd family receptors, ROR2, ROR1, or RYK) and can activate non-canonical Wnt signalling. The binding of these Wnt ligands can activate multiple intracellular pathways of which the planar cell polarity and calcium signalling pathways are most widely studied [177,178]. The dysregulation of Wnt signalling pathways is studied in different cancers (Figure 7) [179].
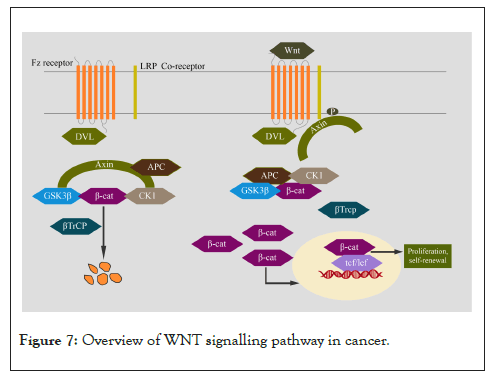
Figure 7: Overview of WNT signalling pathway in cancer.
Alterations in cancer: Truncation mutations of the APC gene are very common in colorectal cancer [180]. Gene amplification and overexpression of DVL1 are reported in lung cancer [181] and breast cancer [182]. Activating CTNNB1 gene mutations are observed in hepatocellular carcinoma, hepatoblastoma [183], ovarian cancer [184], and medulloblastoma [185]. Loss-of-function mutations in AXIN1 gene are reported in hepatocellular carcinoma [186], hepatoblastoma [183], and colorectal cancer [187]. Inactivating mutations of RNF43 and ZNRF3 have been reported in colorectal cancers [188]. Recurrent gain-of-function gene fusions involving RSPO2 and RSPO3 have been reported in colon cancers [189]. Overexpression of frizzed, LRP receptors and Wnt ligands is observed in breast cancers [190,191].
Signalling cross-talks: Several growth factors including hepatocyte growth factor [192-194], epidermal growth factor [195-197], insulin-like growth factors [198,199], vascular endothelial growth factor [200,201], and fibroblast growth factors [202,203] are involved in the stabilization and activation of β-catenin. Studies have shown cross-talk between Wnt and TGF-β pathways. For example, TGFBR2 gene inhibition in the stromal cells shows increased expression of WNT3a, leading to the development of prostatic cancer [204]. Another study showed a physical association between β-catenin and Smad7, which leads to β-catenin accumulation in prostate cancer cells [205]. Different groups have reported synergistic effects of Wnt/β-catenin and Notch signalling in tumorigenesis; for example in melanoma [206], tumours of the intestine [207-209], as well as in breast carcinomas [210]. In contrast, Notch1 activation downregulates β-catenin-mediated Wnt signalling in tongue squamous cell carcinoma, leading to the inhibition of proliferation [211] and induction of differentiation [212]. Several studies have reported cooperative effects of Wnt/β-catenin and hedgehog signalling pathways in different cancers [213-217]. Some studies, however, have reported an inverse association between Wnt and hedgehog [218,219]. In breast cancer, loss of p53 induces Wnt ligand secretion that stimulates IL-1β production by tumours-associated macrophages, leading to systemic inflammation which drives metastasis [220]. Wnt/β-catenin signalling is involved in rendering stem cell properties to cancer cells in prostate cancer via regulating H3K27me3 levels [221].
Resistance mechanisms: Wnt signalling is also reported in imparting resistance to radiation treatment in pancreatic cancer [222]. Inhibition of Wnt/β-catenin signalling downregulates O6-Methylguanine-DNA Methyltransferase (MGMT) expression, thereby restoring chemosensitivity in mouse models [223]. In hepatocellular carcinoma, upregulation of miR-130a inhibits the expression of tumours suppressor gene RUNX3, leading to the activation of Wnt/β-catenin signalling and cisplatin resistance [224]. In breast cancer cells, inhibition of FZD1 induces down-regulation of multi-drug resistance 1 gene and restores sensitivity to four chemotherapy drugs [225]. In pancreatic cancer cell lines, miR-29a mediates resistance to gemcitabine by the activation of the Wnt/β-catenin signalling pathway [226]. In mixed lineage leukaemia, β-catenin is required for development and drug resistance in leukemic stem cells [227]. Wnt signalling enhances DNA damage repair and confirms resistance to PARP inhibitor olaparib in ovarian cancer [228]. The activation of canonical Wnt signalling is associated with resistance to abiraterone acetate/prednisone treatment in patients with metastatic castration-resistant prostate cancer [229]. An RNA-Seq study on circulating tumour cells from patients with prostate cancer has shown that non-canonical Wnt signalling is associated with resistance to androgen receptor inhibitors [230].
Assays: mRNA expression of pathway components can be studied using qRT PCR [192] and IHC [192]. Commercially available TOP/FOP reporter assay is widely used for detecting active Wnt/β-catenin signalling [231]. Functional studies using β-catenin knockout mice generated using the Cre/loxP system can be performed [192]. Drugs targeting different members of WNT signalling pathway is shown in Table 3.
| Gene | Inhibitor | Therapy type | Clinical trial |
|---|---|---|---|
| DKK1 | DKN-01 | In combination with tislelizumab, oxaliplatin, capecitabine | Gastric cancer (NCT04363801) |
| DKK1 | DKN-01 | In combination with paclitaxel | Endometrial cancer, uterine cancer, ovarian cancer, carcinosarcoma (NCT03395080) |
| APC | Atezolizumab | In combination with bevacizumab | Colorectal cancer (NCT02982694) |
Table 3: Drugs targeting different members of WNT signalling pathway.
The p53 pathway
The p53 pathway consists of genes involved in responding to a variety of intrinsic and extrinsic stress factors by modulating DNA replication, chromosome segregation, and cell division. TP53 is a major tumour suppressor gene, and it functions as a transcription factor [232]. MDM2 is an E3 ubiquitin ligase involved in p53 degradation and regulation through a negative feedback loop, as the MDM2 gene is one of the target genes transcribed by p53 [233]. DNA damage sensors Replication Protein A (RPA) and the M/R/N (MRE11/Rad50/Nbs1) complex activate ATR and ATM kinases, respectively, when they detect ssDNA regions/replication-blocking DNA lesions or dsDNA breaks (DSBs). These kinases phosphorylate and activate p53 directly or via Chk1 and Chk2 [234-236]. This phosphorylation of p53 prevents the binding of MDM2 and leads to the activation of the p53 pathway. ATM kinases and p14ARF negatively regulate MDM2. Different mitogenic signals can activate MDM2 via phosphorylation mediated by AKT/PKB kinase and can increase MDM2 transcription via the RAS/MAPK pathway. Mutant p53 with a gain of novel functions is associated with tumour progression and therapy resistance (Figure 8) [237].
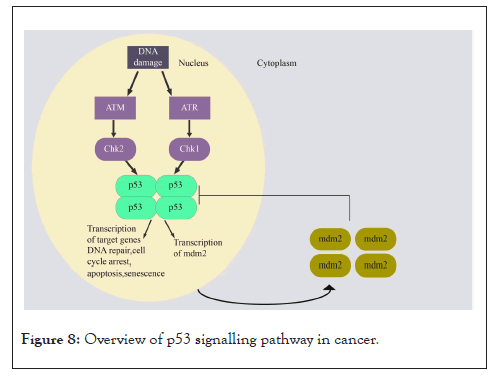
Figure 8: Overview of p53 signalling pathway in cancer.
Alterations in cancer: TP53 is the most frequently mutated gene in over 50% of human cancers [238,239]. Unlike other tumour suppressor genes, TP53 is majorly altered by missense mutations, leading to the expression of a mutant p53 protein, which might have dominant-negative effects and gain of novel functions [240,241]. The remaining cancers show MDM2 gene amplification and mutations in activating kinases like ATM and Chk2 [242-244].
Signalling cross-talks: NF-kB regulates p53 stability via IkB kinase 2, which phosphorylates p53 and targets it for ubiquitination [245,246]. p53 is important for NF-kB activity, and mutant p53 also interacts and activates NF-kB-dependent gene expression [247,248]. Notch signalling can suppress p53 activity directly or by increasing MDM2 activity [249]. p53 regulates the expression of focal adhesion kinase (FAK) in tumour cells, which plays an important role in the survival of tumour cells [250]. FAK interacts with p53 and regulates its transcription activity as well as degradation [251,252]. The interplay between p53 hypoxia-inducible factor 1α (HIF1α) is also reported by many researchers [253].
Resistance mechanisms: A mutant p53 exerts its gain of function by interacting with different transcription factors, thereby regulating the expression of several genes in a context-dependent manner [254,255]. A mutant p53 imparts chemoresistance in several cancers including breast cancer [256], ovarian cancer [257], colorectal cancer [258], and hematologic malignancies [259], which possibly via MDR1 upregulation [260]. A mutant p53 is also associated with radio-resistance in head and neck cancer [261] and colorectal cancer [258].
Assays: IHC or western blotting can be used to detect p53 accumulation [262]. Chromatin Immunoprecipitation (ChIP) assay can be used to determine target genes [263]. Amplification and protein expression of MDM2 can be studied using FISH and western blotting, respectively [264]. Drugs targeting different members of p53 signalling pathway is shown in Table 4.
| Gene | Inhibitor | Therapy type | Clinical trial |
|---|---|---|---|
| MDM2 | Idasanutlin | Monotherapy | Relapsed or refractory AML (NCT02545283) |
| MDM2 | APG-115+Cytarabine | Combination therapy | Relapse/refractory AML (NCT04275518) |
| MDM2 | APG-115+ Azacitidine | Combination therapy | Relapsed/progressed high-risk MDS (NCT04275518) |
| MDM4 | Docetaxel | Combination therapy in combination with doxorubicin and cyclophosphamide | Breast cancer (NCT00312208) |
| CDKN2A | IDE397 | Monotherapy | Solid tumours harbouring MTAP deletion (NCT04794699) |
| ATR | AZD6738 | In combination with acalabrutinib | Relapsed or refractory aggressive non-hodgkin's lymphoma (NCT03527147) |
| ATR | AZD6738 | In combination with gemcitabine | Advanced solid tumours (NCT03669601) |
| ATM | Docetaxel | In combination with carboplatin, rucaparib mesylate | Metastatic castration-resistant prostate cancer with homologous recombination DNA repair deficiency (NCT03442556) |
Table 4: Drugs targeting different members of p53 signalling pathway.
| Gene | Inhibitor | Therapy type | Clinical trial |
|---|---|---|---|
| MDM2 | Idasanutlin | Monotherapy | Relapsed or refractory AML (NCT02545283) |
| MDM2 | APG-115+Cytarabine | Combination therapy | Relapse/refractory AML (NCT04275518) |
| MDM2 | APG-115+ Azacitidine | Combination therapy | Relapsed/progressed high-risk MDS (NCT04275518) |
| MDM4 | Docetaxel | Combination therapy in combination with doxorubicin and cyclophosphamide | Breast cancer (NCT00312208) |
| CDKN2A | IDE397 | Monotherapy | Solid tumours harbouring MTAP deletion (NCT04794699) |
| ATR | AZD6738 | In combination with acalabrutinib | Relapsed or refractory aggressive non-hodgkin's lymphoma (NCT03527147) |
| ATR | AZD6738 | In combination with gemcitabine | Advanced solid tumours (NCT03669601) |
| ATM | Docetaxel | In combination with carboplatin, rucaparib mesylate | Metastatic castration-resistant prostate cancer with homologous recombination DNA repair deficiency (NCT03442556) |
Table 4: Drugs targeting different members of p53 signalling pathway.
The cell cycle pathway
Cell cycle pathway components play a vital role in promoting or inhibiting cell division. Retinoblastoma protein (pRb) represses the E2F family of transcription factors in the G1 phase of the cell cycle [265]. pRb is phosphorylated and inactivated by cyclin D and the cyclin-dependent kinases 4 or 6 (Cdk4/6) complex in the early and mid G1 phase and by the cyclin E-Cdk2 complex in the late G1 phase. Cyclin D-Cdk4/6 complex in turn is negatively regulated by the INK4 proteins (inhibitors of Cdk4), which include p16INK4A, p15INK4B, and p18INK4C [265]. The cyclin E-CDK2 complex is negatively regulated by Cdk inhibitors: p21Cip1 and p27Kip1 [265]. In addition, non-canonical functions of pRb have been reported, and they have implications for cancer therapy resistance (Figure 9) [266].
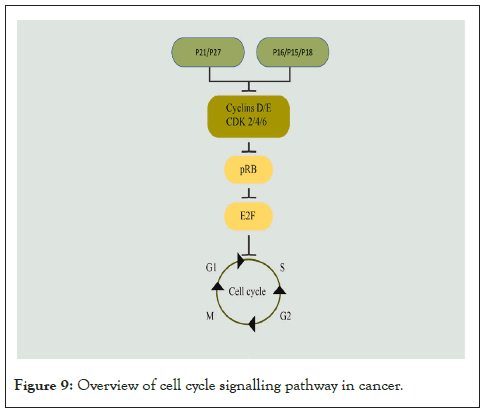
Figure 9: Overview of cell cycle signalling pathway in cancer.
Alterations in cancer: RB1 gene mutations are frequently observed in retinoblastoma [267], osteosarcoma [268], small-cell lung carcinoma [269], prostate cancer [270,271], breast cancer [272], and glioblastoma [273]. Further, CDKN2A gene deletion or promoter methylation is frequently seen in different cancers [274], including prostate cancer [270], head and neck cancers [275] and lung adenocarcinomas [276], and glioblastomas [273]. CCND1 gene overexpression and amplification were reported in many cancers including breast cancers [277], NSCLC [278], head and neck cancers [279], and pancreatic carcinomas [280].
Signalling cross-talk: Cyclin D1 in Cdk-independent manner can bind to nuclear receptors such as ERα, AR, Peroxisome Proliferator-activated Receptor (PPAR) γ, and Thyroid Hormone Receptor Beta (TR-β), thereby regulating cell cycle, growth, and differentiation [281]. cyclin D1-Cdk4 complex phosphorylates and inactivates SMAD3 in cyclin D1-overexpressing breast cancer cells [282]. PI3K pathway can enhance S-phase Kinase-associated Protein 2 (SKP-2) expression, which negatively regulates p27kip1 via mTORC2 signalling in RCC cells [283]. Notch signalling has also been reported to be positively regulating SKP-2 levels and thereby suppressing the levels of p27kip1 [284]. It has also been reported that ErbB2 and Fgfr-4 together regulate cyclin D1 levels [285].
Resistance mechanisms: Overexpression of Cdk4/6 is the main mechanism of resistance to Cdk4/6 inhibitors [286]. Cyclin D contributes to chemoresistance [287-289] and radio-resistance [290]. p16 loss is associated with chemoresistance by conferring CSC-like properties in breast cancers [291]. Reports suggest the role of p21 in promoting cell survival and cell cycle progression, and overexpression of p21, contributes to chemoresistance [292,293]. Inhibition of Cdk2 restores chemosensitivity in triple-negative breast cancers [294].
Assays: Protein expression of p16, pRb, Cdk4/6, Cdk2, cyclin E, and cyclin D can be studied using IHC or western blotting [295-298]. Drugs targeting different members of cell cycle signalling pathway is shown in Table 5.
| Gene | Inhibitor | Therapy type | Clinical trial |
|---|---|---|---|
| CDKN2A | IDE397 | Monotherapy | Solid tumours harbouring MTAP deletion (NCT04794699) |
| CDK4/6 | Palbociclib | Monotherapy | Oligodendroglioma (NCT02530320) |
| CDK4/6 | Palbociclib | Monotherapy | Recurrent ovarian cancer (NCT01536743) |
| CDK4/6 | Palbociclib | Monotherapy | Breast cancer (NCT03609047) |
| CDK4/6 | Trilaciclib | Monotherapy | Triple-negative breast cancer (NCT05112536) |
| CCNE1 | SRA737 | In combination with gemcitabine and cisplatin | Advanced solid tumours (NCT02797977) |
| CCND1 | Acemaciclib | Monotherapy | Head and neck cancer (NCT03356223) |
Table 5: Drugs targeting different members of cell cycle signalling pathway.
The RTK-RAS-MAPK Pathway
The activation of RTK-Ras-MAPK signalling is facilitated by the binding of receptor-specific growth factors or cytokines (IGF, EGF, FGF) with the Receptor Tyrosine Kinases (RTKs) such IGF-1R, EGFR and FGFR on the cell membrane [299], which results in ligand-induced dimerization of RTKs and subsequent activation by trans-auto phosphorylation events [300]. Post receptor activation several downstream proteins and adaptors like Src Homology-2 (SH-2), or Phosphotyrosine-Binding (PTB) domains are recruited to activate downstream signalling [301]. The next players involved are cytosolic proteins RAS GTPase family (HRAS, NRAS, KRAS) which are activated by association of SOS1. SOS enables the conversion of inactive Ras-GDP to the active form RAS-GTP. The active RAS in turn activates downstream effector RAF (ARAF, BRAF, CRAF) by phosphorylation. RAF subsequently activates MEK (MEK1, MEK2) and ERK (ERK1, ERK2) [299] by phosphorylation. In the nuclease, active ERK translocates and activates several transcription factors like CREB, C-MYC and NF-ƘB [299] to promote cell proliferation, differentiation and survival of cancer cells [302], invasion and metastasis (Figure 10) [303].
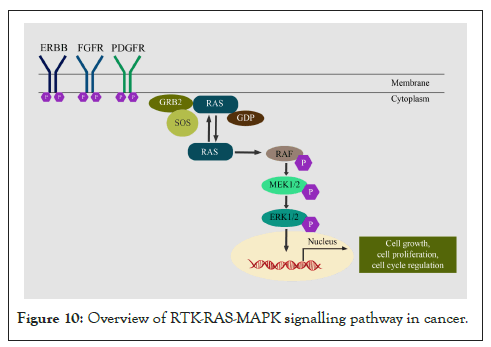
Figure 10: Overview of RTK-RAS-MAPK signalling pathway in cancer.
Alterations in cancer: The pan-cancer genomic alterations revealed frequent mutations of RTK-RAS-RAF in at least 50% of the tumours. KRAS mutations are more common in pancreatic adenocarcinomas and colorectal cancers, whereas NRAS mutations occur dominantly in melanomas, thyroid cancers, and leukaemia [304]. EGFR exon 19 deletion or L858R mutation is commonly reported in lung adenocarcinoma whereas EGFR over-expression is found in NSCLC patients. Copy amplification of EGFR is reported in colorectal cancer and HNSCC [305]. HER2 amplification is observed in 20% of the breast cancer cases [306]; 10–30% of gastric/gastroesophageal cancers; 10-20% of ovarian cancers; 2–29% of pancreatic cancer and 5-10% of bladder cancer (5–15%) [305,307]. EML4-ALK gene translocations are reported in 4-5% of NSCLC [308]. Genomic alterations in FGFR are reported in 5-10% of all human cancers and predominantly in 10–30% of urothelial carcinoma. In FGFR altered cancers of 4853 tumours, the most common alteration was amplification (66%), followed by SNVs (26%) and genomic rearrangements (8%) [309]. FGFR alterations are higher in urothelial (31.7%), breast (17.4%), endometrial (11.3%), and endometrial/ovarian carcinomas (8.1%) [310]. The oncogenic FGFR3-TACC3 fusion is reported in glioblastoma, bladder cancer, nasopharyngeal carcinoma, lung adenocarcinoma and cervical cancer [311].
Signalling crosstalk: Signalling crosstalk takes place at the receptor level, mediator level and effector levels. Crosstalk at the receptor level involves other tyrosine kinase receptors that activate the same downstream signalling whereas crosstalk at the mediator level involves activators of downstream signalling, which are activated through genomic alterations that no longer are receptor-dependent. Effector level activation involves molecules that favour cancer growth and survival [49]. In NSCLC, EGFR TKI resistance is imparted due to activation of FGFR and IGF-1R whereas cetuximab resistance in colon cancer is contributed to MET, IGF-1R and HER2 amplification [49]. Point mutations in KRAS are a causative factor for resistance to cetuximab [312]. EGFR and BRAF mutant lung tumours confer resistance to EGFR TKI, which is overcome by combination treatment with MEK inhibitor [313]. Crosstalk between PI3K/AKT/mTOR pathway and RTK/RAS/MAPK pathways are commonly observed in several cancer types. Inhibition of one of the pathways leads to activation of other pathways as a compensatory mechanism. For example, PI3K is activated by binding of RAS-GTP, inhibition with MEK inhibitors results in EGF-induced AKT activation and ERK is known to activate mTORC1, thus activating PI3K-AKT signalling in Cancers [314]. NOTCH induces acquired resistance to EGFR TKIs through upregulation of EMT markers like vimentin and snail in NSCLC. Similarly, treatment of HER2-positive breast cancer with trastuzumab elicited acquired resistance through an increase in Notch-1 and its target genes [47].
Resistance mechanisms: Resistance has been observed in HER2 over-expressing breast cancer patients treated with trastuzumab [315]. Targeting of ERBB2 in breast cancer results in upregulation of ERBB3 expression along with increased expression of IGF-1R, FGFR2, MET, FAK and Src family kinases to confer resistance to lapatinib, through a mechanism known as adaptive kinome reprogramming [316]. ALK translocation-based crizotinib-resistant lung cancers upregulate EGFR or KIT [317], whereas MET amplification is observed in lung cancer patients with EGFR activating mutations resulting in the development of resistance to gefitinib or erlotinib by activating ERBB3 mediated AKT signalling [318]. Such bypass mechanisms form the second most important resistance mechanism wherein tumour cells activate parallel pathways to continue the signalling pathway when one of the RTK is inhibited. Bypass mechanisms not only use alternative RTKs but also may involve upregulation of certain ligands. For example, elevated levels of the MET ligand HGF have been shown to rescue lung cancer cells from sensitivity to EGFR inhibition [319]. Secondary acquired mutations are one of the common mechanisms of resistance. In NSCLC, patients with EGFR L858R, or exon 19 deletion develop resistance to erlotinib and gefitinib [316]. EGFR T790M is one of the acquired mutations in most of lung cancer patients that confer resistance [320] after treatment with first-generation inhibitors.
Assays: Western blot analysis for phosphorylated and total proteins for RTKs like ERBB (1-4), FGFR (1-4), PDGFR, IGF-R, KIT and MEK, ERK, other RTKs and Ras in cell lines. IHC on tumours sections to evaluate over-expression of EGFR, ERBB2, KIT and MAPK is routinely done [321]. Antibodies against several RTKs are available to perform IHC and western blot. A proteome profiler antibody array enables the detection of up to 100 proteins (both total and phosphorylated forms) from a cell lysate (R&D systems). Other kits such as The Protein Tyrosine Kinase (PTK) Assay Kit (Sigma) measures kinase activity from a crude cell lysate or purified proteins by an ELISA assay. Since RTK ligand binding leads to dimerization of receptors for activation of the signalling pathway, some kits such as PathHunter® Dimerization assay (Eurofins) measure this interaction and are useful to study receptor activation. The FDA approved inhibitors targeting members of RTK-RASMAPK pathway is shown in Supplementary Table 1.
This review is a brief overview describing 9 major signalling pathways commonly deregulated in several tumours of different cancer types. We have defined the role of important signalling molecules in each signalling pathway and explained their relevance in cancer. Further, we have emphasized resistance mechanisms and potential signalling cross-talks that complicate treatment efficacy. We have provided insights on several cellular complexities that need to be considered when studying a signalling pathway in cancers. We emphasize that it is necessary to look beyond genomic alteration data. This is mainly because signalling pathways in cancer are complex networks, which initiate cross-talk with other cancer-promoting signalling pathways on treatment and re-wire the signalling network to sustain the survival of tumour cells and promote growth. Hence, there is a need for an integrative approach to study tumours on genomic levels as well as protein levels to determine dominant signalling pathways and observe a change in signalling patterns when treated with an inhibitor. However, every tumour is different and is governed by different signalling mechanisms. The computational models and tools, omics data, and inhibitor studies predict therapeutic targets but cannot guarantee clinical success. Therefore, not all patients respond alike to similar treatment. With the development of advanced computational tools, prediction models, and high-throughput functional assays, we anticipate that we will have a better understanding of signalling mechanisms and can choose the right targets for therapy.
• Ethics approval and consent to participate: Not Applicable
• Consent for publication: All authors have provided the consent for publication
• Availability of data and materials: Not applicable
• Competing interests: The authors declare that they have no conflict of interest.
• Funding: Not applicable
• Authors' contributions: TT, UP, SR and NC has designed the framework of the manuscript and have written the draft. All authors have contributed and reviewed the manuscript.
We acknowledge Smritie Sheth and Smita Harne for their help with the figures.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Togar T, Patel U, Ramchandrula S, Chirmule N (2022) All Roads Lead to Rome: Complexity of Signalling Pathways in Cancer. J Cell Signal. 7:288.
Received: 27-Jul-2022, Manuscript No. JCS-22-18581; Editor assigned: 01-Aug-2022, Pre QC No. JCS-22-18581 (PQ); Reviewed: 15-Aug-2022, QC No. JCS-22-18581; Revised: 22-Aug-2022, Manuscript No. JCS-22-18581 (R); Published: 29-Aug-2022 , DOI: 10.35248/2576-1471.22.07.288
Copyright: © 2022 Togar T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.