Entomology, Ornithology & Herpetology: Current Research
Open Access
ISSN: 2161-0983
ISSN: 2161-0983
Research Article - (2020)Volume 9, Issue 1
Males of stag beetles have a pair of enlarged mandibles and combat with other males to defend a food site to where females will come to feed. The winner can monopolize mating with females came. However, to combat with an opponent, a male must show aggressiveness to the opponent. Females also take a similar aggressive posture with males. Recently, it has been shown that the degree of aggressiveness in insects is mediated by biogenic amines in the brain. Here, we report that the different amine pathways mediate the aggressiveness of males and females of a stag beetle, Prosopocoilus inclinatus. In males, dopamine rose up the degree of aggressiveness with concentration of the chemical, and a dopamine antagonist suppressed this effect with its concentration, indicating the mediation of the aggressiveness through the dopamine pathway. Whereas, aggressiveness of females was rose up by octopamine and was lowered by thyramine with the agent's concentrations. An octopamine antagonist showed a concentrationdependent suppression of octopamine's effect. Our results suggest that aggressiveness mediation of the males and the females had evolved independently for different benefits, and thus both the sexes have different mediation pathways for their aggressiveness.
Stag Beetle; Prosopocoilus inclinatus; Biogenic-amines; Aggressiveness
Males of several coleopteran insects bear large weapons by which they combat with other males to get mates [1-3]. In the stag beetles, a male has a pair of enlarged mandibles, and combats aggressively with other males using this weapon to defend a sapexuding site to where females come to feed [4-6]. The winners can monopolize mating with females came, and thus gains a greater fitness than the losers. Therefore, how does a male get win in a combat is important for his reproductive success [4-8].
In order to win in a combat, a male must combat aggressively with the opponent. For this purpose, they must show a high degree of aggressiveness to the opponent at first. However, we frequently observed that aggressiveness of males varies considerably in nature and under rearing. How does their aggressiveness control?
Recently, degrees of aggressiveness in insects have been shown to mediate by biogenic amines in the brain [9,10] For example, aggressiveness of ant species is controlled by different biogenic amines species by Hasegawa [11,12]. Thus, the aggressiveness of stag beetles may be mediated in biogenic amines. If we know the kind of amine that mediates the aggressiveness of a stag beetle species, we can examine which traits of males in that species affect win or lose in a combat, and will obtain new insights into sexual selection in this fascinate insect taxa.
The aim of this study is to specify the kind of biogenic amine that mediates the aggressiveness of individuals of a stag beetle, Prosopocoilus inclinatus (Coleoptera: Lucanidae). To this purpose, we give the common four biogenic amines to both the sexes of P. inclinatus and compared changes in the degree of aggressiveness before and after of the taking of the chemicals. After finding of the target amines, we also examine the effect of antagonists to those amines’ receptors on the effect of those amines. We also investigated an effect of concentrations of these chemicals on the degree of the effect to confirm the observed changes in the response are due to chemical pathways for those amines.
Study organism and the preparation of samples. P. inclinatus is a common stag beetle in Japan (Figure 1).
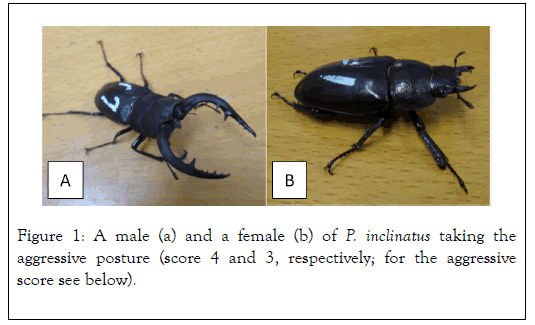
Figure 1. A male (a) and a female (b) of P. inclinatus taking the aggressive posture (score 4 and 3, respectively; for the aggressive score see below).
From 11-15 July 2016, we collected a total of 26 wild females of P. inclinatus during 20:00-22:00 under the city lights at Tomiuchi town in Hokkaido Japan. Each female was reared in a plastic box (16 × 9 × 10 cm in width × depth × height with a mesh cover stuffed by a rearing mat for stag beetles (a crushed fermented KUNUGI tree; Mushi-Hakase, Sapporo, Japan). The females were fed with a half-cut insect jelly (Pro JellyTM, KB farm, Saitama, Japan) once in 3 days. The food was replaced to new one in each three days. We gave 20 ml of water in each 3 days to each box to prevent the mat from drying. After one month, living females were removed from the boxes, and laid eggs were reared to hatchings under 23°C under the natural light period. After the most eggs hatched, all the larvae were excavated, and each larva was transferred to a rearing bottle for stag and rhinoceros beetles, which were stuffed with the rearing material (10 cm in the diameter with 11 cm depth; SRD500TM, Osaka, Japan). The rearing bottles were kept at 23°C in natural D/L cycle. As a result a total of 248 larvae were reared.
The most larvae (more than 90%) had been grown to adults within the year. However, as this stag beetle appears active during the summer, we started the experiment after adults appeared on the surface of the material (early July). We examined effects of four biogenic amines to an aggressiveness score (see below section for the definitions) of both the females and males. Each of 4 amines (dopamine (DA), octopamine (OA), thyramine (TA) and serotonin (5HT)) was adjusted to 0.001, 0.1, 1.0, 5.0 and 10 mM, and each 9-14 males and females were examined for each amine. Individuals for the experiments were separately kept in a plastic cup (10 cm in diameter and 5 cm in depth, and was floored by a filter paper) with a cap. A half-cut insect jelly was putted in the box and replaced each 3 days until the experiment. The aggressiveness of each individual was scored by the following criterions. When an individual hide on the floor was tapped it thorax from the backside by a wood bar of 2 g, its response was classified into the following 4 classes.
1) No response
2) Open the mandibles but not stand up
3) Open the mandibles and stand up but did not lift the tip of abdomen from the floor and
4) Open the mandibles fully and stand up with lifted up the tip of abdomen from the floor.
We regarded that the order of the categories represents the degree of aggressiveness. Before administration of an amine, we measured the aggressive score of each individual. Then, individuals were given a concentration of the examined amine, and kept 24 hours. Then each individual was re-measured for the aggressiveness.
Experiment 1: Examining the effect of each amine to females and males.
We administrated 10 mM of each the amine to each 9-14 females or males. Before administration of an amine, each individual was scored for the aggressiveness by the above method. Then, each amine was given to the experimental individuals by rubbing 100 μl solution on the surface of insect jelly. After 24 hours, we recoded the aggressiveness of each individual again.
Experiment 2 and 3: Examining concentration-dependent reaction strength for both an amine and its antagonist (if exist). We used Blonanserin (BS) as an antagonist of DA in this study.
From the results of the experiment 1, we determined the kind of amine that affects aggressiveness of females or males. For the effective amine and its antagonist (if exist), we administrated 0.001, 0.1, 1, 5 or 10 mM of the antagonists' solutions to the individuals, and scored the aggressiveness of them before and after the administration. After scoring of the aggressiveness, we administrated the dilution line of the antagonists to the individuals. After 3 hours from the administration of the antagonist, we administrated 1 mM of the focused amine to the individuals. After 3 hours from the administration of the amine, we re-scored the aggressiveness of individuals.
Analysis
All the statistical tests were conducted by R (version 3.5.2).
For the experiment 1:
For each amine, we compared the average aggressive score between before and after administration of amines. We tested difference in the average aggressiveness by a Mann-Whitney's U test with correction for p values for ties by using the package "ExactRankTests" for R.
For the experiment 2:
We calculated score difference between before and after administration of the amine for all the used individuals. The score-difference data and concentration of the agents were logtransformed then, we calculated a linear regression of the log score-difference on log concentrations. Then, we tested statistical significance of the slopes.
For the experiment 3:
We regressed the log-transformed changes in aggressive scores on the concentrations of the antagonist or the amine. Then, the slopes were tested for its statistical significance.
Effects of amines: Insect-jelly has no effect on the aggressive scores after 24 hours (Figure 2). For females, octopamine rose up significantly the aggressive score (mean score 2.273 to 3.364, V=2, p=0.0313) and thyramine lowered significantly the score (mean average score 3.364 to 2.091, V=45, p=0.004). For males, dopamine rose up significantly the aggressive score (mean score 2.818 to 3.364, V=2, p=0.031) but other amines showed no significant effect (5HT: score 3.300 to 3.200, V=6, p=1.000; OA: score 3.600 to 3.700, V=0, p=1.000; TA: score 3.700 to 3.700, V=4, p=1.000). The results are summarized in Figure 2. Figure 3 shows the regression of change in the male aggressiveness on the DA concentrations. The changes in the aggressive scores increased significantly with the concentration of DA.
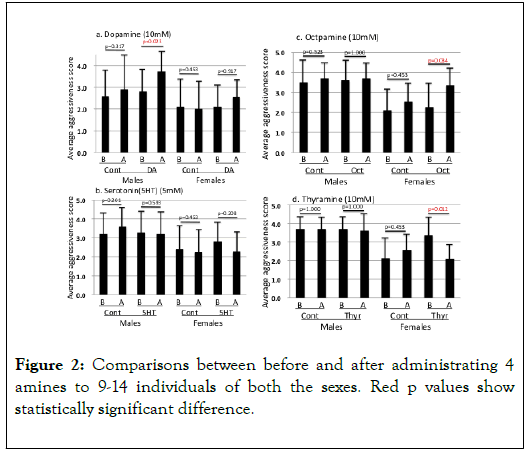
Figure 2. Comparisons between before and after administrating 4 amines to 9-14 individuals of both the sexes. Red p values show statistically significant difference.
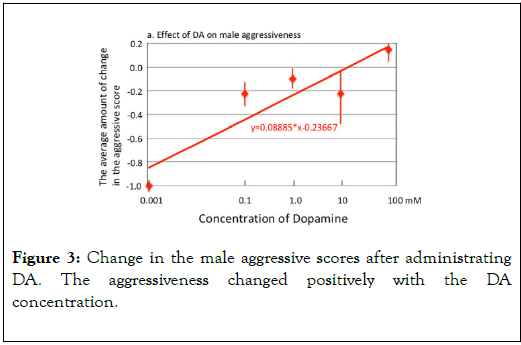
Figure 3. Change in the male aggressive scores after administrating DA. The aggressiveness changed positively with the DA concentration.
DA concentration, but may affect differently to the small (S) or large (L) morphs. To confirm this, first, the change in the average aggressive score was compared between two treatments, 1) 10 mM DA was administrated with the jelly and 2) the Jelly only was given as a control. The aggressive scores are higher significantly in the group 1 comparing with the later (the mean ± S.D. was 0917 ± 1.084 (n=12) in the DA group, and 0.182 ± 0.603 (n=11) in the control), and this difference is statistically significant (exact Wilcox test, W=104, p=0.011). Then, a generalized liner model used a poisson distribution with log link was conducted by setting the difference in aggressive score as dependent variable and the morphs as an independent variable. The model showed that difference in the morphs has no significant effect on the changes in the aggressive scores (estimate=-0.1646, z=-0.485, p=0.628).
Figure 4 shows the regression of change in the male aggressiveness on a DA antagonist (10 mM Blonanserin (BS); Blue line), and that on the DA concentration that has been given after the administration of BS. BS lowered significantly the male aggressiveness (slope=-0.147, t=-3.246, p=0.0476), but irrespective of the administration of BS, DA significantly rose up the male aggressiveness (slope=0.755, t=4.933, p=0.016). In addition, the positive slope of DA in Figure 3 (0.089 without BS) is significantly steeper than that in Figure 4 (0.755 with 10mM BS: ANCOVA, F=6.827, p=0.040).
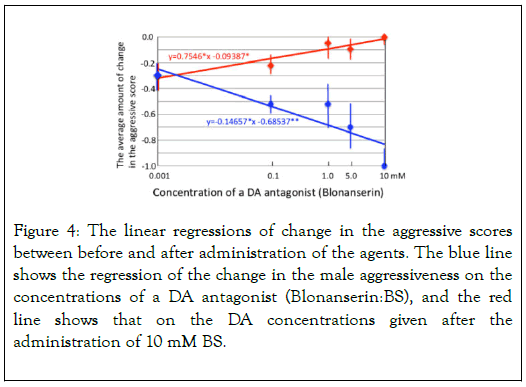
Figure 4. The linear regressions of change in the aggressive scores between before and after administration of the agents. The blue line shows the regression of the change in the male aggressiveness on the concentrations of a DA antagonist (Blonanserin:BS), and the red line shows that on the DA concentrations given after the administration of 10 mM BS.
These results indicated that BS lowers the male aggressiveness that mediating by DA but BS cannot suppress the DA's effect completely. As BS should be ineffective to block neural receptors for the other chemicals e.g. NacDA (a metabolic product of DA), the results in Figure 4 is able to interpret the existence of other chemicals that rise up the male aggressiveness. Figure 5a and 5b shows the regressions of changes in the female aggressiveness scores on the concentrations of OA (a) and of TA (b).
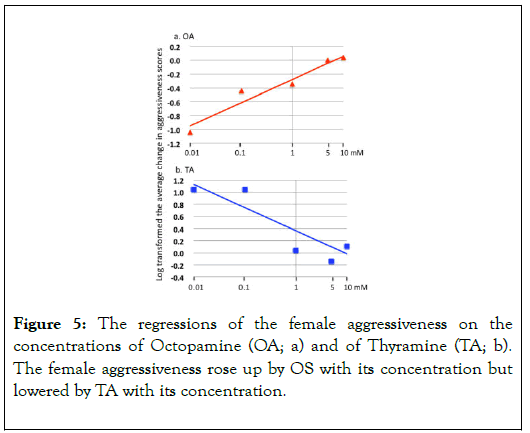
Figure 5. The regressions of the female aggressiveness on the concentrations of Octopamine (OA; a) and of Thyramine (TA; b). The female aggressiveness rose up by OS with its concentration but lowered by TA with its concentration.
These results indicate that the biogenic amines that mediating the aggressiveness are different between females and males in P.inclinatus although the same aggressive posture in both the sex (Figure 1).
Our experiments indicated that although their aggressive posture is similar, the mediating amine is different between females and males in P. inclinatus. It has been known that males of P. inclinatus combat with other males to keep mates at supexude site on tree surfaces [13,14] but females do not combat each other. Why do females take the aggressive posture is unknown, the results of this study suggest that the origin and the neuro-mediation mechanisms are different between females and males.
Another new finding is that DA rises up the aggressiveness of males but no amine lowers their angry. Usually, there are the amines for both the responses as in females (OS rises up and TA lowers). Stag beetle is famous for males’ long mandibles (see Figure 1) by which they combat each other. Our preliminary analysis showed that when the sizes of combatting males are similar, an individual shown higher aggressiveness tends to win (in prep.). Thus, getting high aggressiveness before a combat should be important to get mates. In addition, the morph type did not affect the response to DA (see results). This matches with a previous study in which the small males had not hesitated a combat with the large morph, and have gotten ca. 28% of win in the combats with the large males [14]. A high aggressiveness may overcome size disadvantage of the small morph. A previous study has reported that P. inclinatus males can overcome size disadvantage in a combat with sympatric Lucanus maculofemoratus males. Interestingly, P. inclinatus males close mandibles when something touched from both the upper and lower side of the mandibles, but L. maculofemoratus males close mandibles only when something touched from the lower side [15]. In P. inclinatus, the large males showed a residual correlation between mandible length and eye diameter in an allometry analysis on morphology [16], differently from a previous theory [17]. Whereas, the small males shown a residual correlation between mandible length and length of fore-legs [16]. These facts suggest that tactics for a combat should be different between the two morphs, and the difference may relate with the above mentioned sensitive-difference in the mandible closing mechanism. Manipulation for aggressiveness will elucidate the question that, in P. inclinatus, why small males does not hesitate to combat with large males irrespective of their low winning rate.
As males of P. inclinatus have a much shorter active period than females. The males appear from late June until mid-July, but females are found from a bit later than the male appearance until late August in our study field. This difference is probably due to a fact that males allow own death after mating, but females must survive, at least, until her oviposition after mating [18]. In addition, this stag beetle emerged to adults until the previous winter and wait more than several months for adequate season with good temperature for their activity (usually late June in Hokkaido). The males must get mates during this short period to gain fitness. Although the small males show alternative tactic to get mates [19] mating is difficult without win in combats. Although the small males do not hesitate a combat with the large males, they can win ca. 28% when combatted with the large morph [14]. Therefore, an advantage of keeping an irascible condition may be a cause of the observed asymmetry for aggressiveness mediating pathways between males and females in this stag beetle.
When there is a male dimorphism in Coleopterans, small morphs usually avoid combats with a large morph [4,20], but P. inclinatus males of the small morph do not hesitate from combats, and can get win in ca. 28% [14]. In addition, an allometry analysis showed that, in the large males, the longer the relative mandible length the larger the relative eye's diameter [16], suggesting eye-size is important for the large males. This feature is different from other coleopterans with male-male combats [3,17]. Whereas, the small P. inclinatus males has relatively long forelegs when the mandibles are long [16]. This morphometric difference between the morphs is likely to relate with their combat tactics. If a small male is very aggressive, he may be the winner in combats with not so much aggressive large males. P. inclinatus males have been reported that they can win in combats with larger males of sympatric Lucanus maculifemoratus [15]. Therefore, even in intraspecific combats, more aggressive males may tend to win with a larger male. Artificial administration of DA to males of P. inclinatus will elucidate the role of "motivation for a combat" in the Coleopterans with male-male combats in future.
In P. inclinatus, aggressiveness of females and males are mediated by different biogenic amines. Unusually, the males rise up the aggressiveness by dopamine but there is no amine that lowered aggressiveness. The results suggest that the independent origin and adaptation to take a similar aggressive posture in both the sexes. As the males combat with each other to get mates, the one-direction effect of the mediation shown in the males may relate with this unusual mediation mechanism of aggressiveness.
This study was partly supported by Grants-in-Aid from the Ministry of Education, Culture Sports, Science and Technology of Japan (nos. 15H04420 and 18H02502).
Citation: Hasegawa E, Kudo T (2020) Aggressiveness of Males and Females of the Stag Beetle, Prosopocoilus inclinatus (Coleoptera: Lucanidae), are Mediated by Different Biogenic-amines. Entomol Ornithol Herpetol. 9:223. DOI: 10.35248/2161-0983.20.9.223.
Received: 01-Feb-2020 Accepted: 12-Feb-2020 Published: 19-Feb-2020 , DOI: 10.35248/2161-0983.20.9.223
Copyright: © 2020 Hasegawa E. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.