International Journal of Physical Medicine & Rehabilitation
Open Access
ISSN: 2329-9096
ISSN: 2329-9096
Research Article - (2023)Volume 11, Issue 1
This systematic review of the literature presents the important biological and clinical effects of Anabolic Agents (AA) used by athletes to dope themselves, identified in the scientific literature with keywords based on the name of both anabolic androgenic steroids identified by the World Anti-Doping Agency (WADA), in PubMed and Web of Sciences between 2010 and 2021. The selected publications recall the consequences on the cardiovascular and muscular systems, behavioral disorders but also at the hepatic, hematological and hormonal levels. It is useful to recall that the use of anabolic agents by athletes is prohibited and sanctioned by the WADA to preserve the fairness of competitions, but also because of the harmful effects of their use.
Doping in sports; Anabolic agents; Adverse effects
Sports doping is a problematic topic despite World Anti-Doping Agency (WADA) regulations. According WADA's 2019 Anti-Doping Rule Violations Report (ADRV), there were 2,701 adverse analytical findings (1%) out of 278,047 samples collected in doping controls, 57% of the adverse analytical findings were considered an anti-doping rule violation and resulted in a sanction [1]. However, doping prevalence rates in competitive sport had been estimated in a systematic review showing a higher prevalence ranged between 0% and 73%, but the most frequently under 5% in the publication assessed [2]. The most represented sports are bodybuilding (22%), athletics (18%), cycling (14%), weightlifting (13%) and powerlifting (9%) [1]. Among adverse analytical findings, the most represented substance groups were anabolic agents (47%), and thereafter, stimulants (15%) and diuretics and other masking agents (14%) [3].
Anabolic agents used as sports doping can be Androgenic Anabolic Steroids (AAS), which are synthetic derivatives of testosterone, a sex hormone that develops male sexual characteristics and incresases skeletal muscle growth through an anabolic action [4]. The most commonly AAS used by athletes are stanozolol, nandrolone and metandienone according to the National Institute on Drug Abuse [5], and stanozolol (14% of AAS identified in adverse analytical findings), dehydrochloromethyl-testosterone (13%), and drostanolone (10%) according to the WADA [3]. Other anabolic agents are also consumed such as beta-2 adrenergic agonists that have anabolic effects on muscles like clenbuterol [5]. Anabolic agents are used by professional and amateur athletes to improve performance and muscle mass [6]. AA represents a large part of sports doping, and WADA prohibits their use and updates a list of banned substances [7]. Today, it is known that the use of anabolic agents is responsible for adverse effects, especially because athletes have used very high doses compared to therapeutic ones [4].
Based on the importance of AA in sport doping, we performed a systematic review focused on the biological and clinical effects of these agents in sports doping presented in the scientific literature between 2010 and 2021.
This systematic review of the scientific literature was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [8].
Eligibility criteria
Inclusion criteria were publications referring to AA according to the World Anti-Doping Code International Standard for the 2021 Prohibited List (1-androstenediol, 1-androstenedione, 1-androsterone, 1-epiandrosterone, 1-testosterone, 4-androstenediol, 4-hydroxytestosteron, 5-androstenedione, 7α-hydroxy-DHEA, 7β-hydroxy-DHEA, 7-keto-DHEA, 19-norandrostenediol, 19-norandrostenedione, androstanolone, androstenediol, androstenedione, bolasterone, boldenone, boldione, calusterone, clostebol, danazol, dehydrochlormethyltestosterone, desoxymethyltestosterone, drostanolone, epiandrosterone, epi-dihydrotestosterone, epitestosterone, ethylestrenol, fluoxymesterone, formebolone, furazabol, gestrinone, mestanolone, mesterolone, metandienone, metenolone, methandriol, methasterone, methyl-1-testosterone, methylclostebol, methyldienolone, methylnortestosterone, methyltestosterone, metribolone, mibolerone, nandrolone, norboletone, norclostebol, norethandrolone, oxabolone, oxandrolone, oxymesterone, oxymetholone, prasterone, prostanozol, quinbolone, stanozolol, stenbolone, testosterone, tetrahydrogestrinone, trenbolone, clenbuterol, andarine, LGD-4033, ligandrol, Enobosarm, ostarine, RAD140, tibolone, zeranol, zilpaterol) [9], abstract available, sport doping, human species, original article and in English language. Exclusion criteria were defined as follows languages different from English one, book, review, meta-analysis, letter to the editor, case report, animal studies, in vitro studies, analytical studies, epidemiological studies, studies assessing prevention strategies, agents different from anabolic ones (e.g. erythropoietin, plants...)
Information sources
The bibliographic search was performed in PubMed and Web of Sciences databases from January 1st 2010 and June 2nd 2021.
Search strategy
The search strategy in PubMed was done on June 2nd, 2021, with the following keywords sequence: “(1-androstenediol or 1-androstenedione or 1-androsterone or 1-epiandrosterone or 1-testosterone or 4-androstenediol or 4-hydroxytestosteron or 5-androstenedione or 7α-hydroxy-DHEA or 7β-hydroxy-DHEA or 7-keto-DHEA or 19-norandrostenediol or 19-norandrostenedione or androstanolone or androstenediol or androstenedione or bolasterone or boldenone or boldione or calusterone or clostebol or danazol or dehydrochlormethyltestosterone or desoxymethyltestosterone or drostanolone or epiandrosterone or epi-dihydrotestosterone or epitestosterone or ethylestrenol or fluoxymesterone or formebolone or furazabol or gestrinone or mestanolone or mesterolone or metandienone or metenolone or methandriol or methasterone or methyl-1-testosterone or methylclostebol or methyldienolone or methylnortestosterone or methyltestosterone or metribolone or mibolerone or nandrolone or norboletone or norclostebol or norethandrolone or oxabolone or oxandrolone or oxymesterone or oxymetholone or prasterone or prostanozol or quinbolone or stanozolol or stenbolone or testosterone or tetrahydrogestrinone or trenbolone or clenbuterol or andarine or LGD-4033 OR ligandrol or Enobosarm or ostarine or RAD140 OR tibolone or zeranol or zilpaterol and sport and doping and English language not review (Publication Type)), and between 2010-2021.
The search strategy in Web of Science was done on June 2nd, 2021, with the following associations of keywords sequences: 1. “Ts=sport and doping and language: English and document types: Article” (Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S,BKCI-SSH, ESCI Timespan=2010-2021). “Ts=1-androstenediol or 1-androstenedione or 1-androsterone or 1-epiandrosterone or 1-testosterone or 4-androstenediol or 4-hydroxytestosteron or 5-androstenedione or 7α-hydroxy 8DHEA or 7β-hydroxy-DHEA or 7-keto-DHEA or 19-norandrostenediol or 19-norandrostenedione or androstanolone or androstenediol or androstenedione or bolasterone or boldenone or boldione or calusterone or clostebol or danazol or dehydrochlormethyltestosterone or desoxymethyltestosterone or drostanolone or epiandrosterone or epi-dihydrotestosterone or epitestosterone or ethylestrenol or fluoxymesterone or formebolone or furazabol or gestrinone or mestanolone or mesterolone or metandienone or metenolone or methandriol or methasterone or methyl-1-testosterone or methylclostebol or methyldienolone or methylnortestosterone or methyltestosterone or metribolone or mibolerone or nandrolone or norboletone or norclostebol or norethandrolone or oxabolone or oxandrolone or oxymesterone or oxymetholone or prasterone or prostanozol or quinbolone or stanozolol or stenbolone or testosterone or tetrahydrogestrinone or trenbolone or clenbuterol or andarine or LGD-4033 or ligandrol or Enobosarm or ostarine or RAD140 or tibolone or zeranol or zilpaterol and language: English and document types: Article” (Indexes=SCIEXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI Timespan=2010-2021).
Selection process
Two readers (JB and AT) performed a first selection of manuscripts according to inclusion and exclusion criteria based on title and abstract of each manuscript. In the event of disagreement between the two readers, a third reader (DB) took part in the decision with a reading of the full-text of the manuscript, until a consensus was reached. Full-text of each selected manuscript was finally obtained for analysis. Manuscripts could be excluded during analysis of full-texts.
Data collection process
Manuscript data from PubMed and Web of Sciences were collected and imported into Zotero software (Roy Rosenzweig Center for History and New Media at George Mason University). Thereafter, data from Zotero was exported to Excel (Microsoft) software format for data analysis. These two Excel files (PubMed and Web of Science data) were merged in a single Excel one, and keeping only the following information for analysis: publication years, authors, journal name, publication title, abstract, and the unique identifier number used in PubMed (PMID) or the Web of Science identifier.
Data items
From full-text manuscript, each publication was analyzed to retrieve the following information: Name of anabolic agents, doses, durations of use (if available), total number of subjects, number of doped and clean subjects, type of athletes, and biological and clinical effects.
Selection of publications
The bibliographic search identified 689 publications: 415 publications on PubMed and 274 publications on Web of Sciences.
One hundred and sixty-five duplicates between PubMed and Web of Sciences were removed from database. Finally, 17 publications met inclusion criteria and 507 publications were removed from analysis because of exclusion criteria (Figure 1) [10].
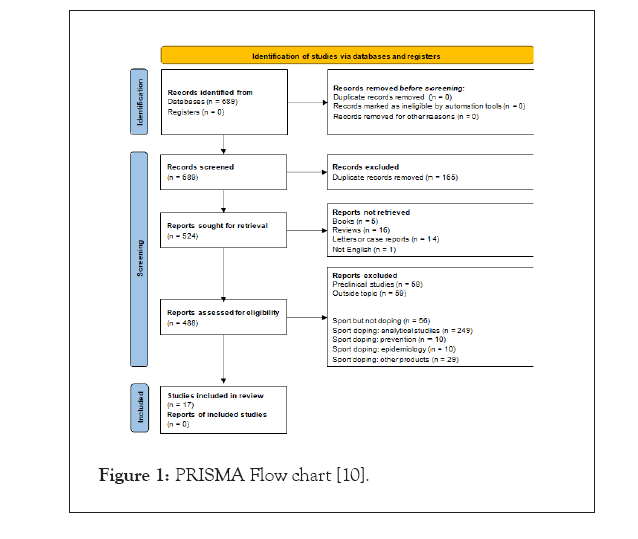
Figure 1: PRISMA Flow chart [10].
The analysis of the selected publications allowed identifying the following system and classes of adverse effects related to the use of anabolic agents in sport doping: Cardiovascular, anthropomorphic, muscular, behavioral, endocrine, biochemical, hematological and hepatic effects.
Cardiovascular effects
Six publications were identified and analyzed as related to cardiovascular effects [4,11-15], with a total of 323 subjects of which 155 were doped and 168 were clean subjects. The clean subjects are represented by 108 athletes and 60 sedentary subjects who do not use anabolic agents.
A significant blood pressure increase was observed in athletes consuming AA [4,12,13,15]. However, in one publication, there was no significant change in blood pressure after a clenbuterol consumption [14].
Heart rate of trained athletes was lower than that of sedentary persons (55.8 ± 7.8 vs. 75.3 ± 10.8, p<0.001) [13]. The use of anabolic agents increased heart rate in doped athletes [13,14], but two studies did not highlight this increase [4,12]. In addition, 4 cases of tachycardia were r13eported among 155 doped subjects (2%) across all publications [11,14].
+myocardial anatomy, left ventricular wall thickness was comparable between doped and non-doped individuals [4,12,13]. In doped athletes, a higher left ventricular mass was observed in doped athletes than in non-doped, this may reflect the higher risk of cardiac hypertrophy for these athletes [4]. About the left atrium of the myocardium, a significant alteration in the emptying fraction and lateral deformation of the left atrium in doped subjects was observed. A correlation between the decrease in left atrium deformation and the decrease in capacity during exercise was reflected by variation of peak VO2 (r=0.68; p<0.001) [12].
The QT interval is shorter in doped subjects compared to non-doped subjects, but no significant difference was demonstrated between doped and sedentary subjects. It is necessary to mention that the mean QT interval was normalized by corrective formulas and only two corrected QT (QTc) were significant with a variation of up to 59 milliseconds (350 ± 33.2 vs. 409 ± 47.8 milliseconds, so these QT interval data should be treated with caution [12]. The risk of atherosclerosis was also increased, as evidenced by the significant increase in coronary plaques in doped individuals. Furthermore, in the same study, 3% of long-term users had an history of heart attack, but none of the non-users [4]. This risk is also marked by decreased endothelial functions such as blood flow-dependent vasodilation [15]. Noteworthy, changes in blood pressure related to platelet concentration and HDL cholesterol are both markers of atherothrombosis risk [15] (Table 1).
| Djordjevic et al. [13] | Severo et al. [15] | Baggish et al. [4] | D'Andrea et al. [12] | Jessen et al. [14] | Börjesson et al. [11] | ||
|---|---|---|---|---|---|---|---|
| Number of subjects | Doped | 10 strength athletes | 10 | 86 | 35 | 6 | 8 |
| Non-Doped | 12 endurance athletes | 12 | 54 | 30 | 0 | 0 | |
| Controls | AAS-free sedentary controls=20 | 0 | 0 | Age and sex matched sedentary=40 | 0 | 0 | |
| Doping detection | Non users: All negative during and out of competition | NC | Test | LH and FSH levels | NC | Self-reported and confirmed by analysis | |
| Dosage/duration | combination of oral and injectable for at least 2 years (up to 5 years) | At least one year | Cumulative lifetime AAS dose (g): 366 (166-608) | Number of weeks per year: 31.3 ± 6.4. Weekly AAS dosage 525.4 ± 90.7 mg. | 80 µg oral clenbuterol | 7 weeks-2 years (mean: 58 weeks) | |
Blood pres051 36+ sure (mmHg) (mean ± SD) |
Doped | Systolic: 133 ± 23 mmHg; P=0.085 Diastolic: 88 ± 14.2 mmHg P< 0.001 | Systolic: 155 ± 27 mmHg; P=0.001 Diastolic: 93 ± 16 mmHg. P=0.005 | Systolic: 118 ± 11 mmHg Diastolic: 76 ± 9 mmHg | Systolic: 138.8 ± 8.5 mmHg; p<0.01 (vs. controls) Diastolic: 85.3 ± 5.5 mmHg | Systolic : before ingestion: 115 ± 10; after 140min: 120 ± 9; p=0.068 Diastolic : before: 63 ± 5; after 140 min: 61 ± 8; p=0.472 | |
| Non-Doped | Systolic: 118 ± 12 mmHg Diastolic: 71 ± 8.4 mmHg | Systolic: 119 ± 12 mmHg Diastolic: 77 ± 10 mmHg | Systolic: 115 ± 10 mmHg Diastolic: 72 ± 9 mmHg | Systolic: 128.4 ± 7.3 mmHg Diastolic: 81.3 ± 4.7 mmHg | |||
| Contrôles | Systolic:124 ± 11 mmHg Diastolic: 74 ± 5.6 mmHg | Systolic: 124.8 ± 5.5 mmHg Diastolic: 84.5 ± 7.2 mmHg | |||||
| Heart rate (bpm) | Doped | 77.5 ± 15.9; p<0.001 | 65 ± 5; p=0.008 | 68.4 ± 8.8; p<0.01: users versus controls | Systolic: before ingestion: 51 ± 9; after 140 min: 71 ± 17 Diastolic : before: 63 ± 5; after 140 min: 61 ± 8; p=0.472 | ||
| Non-Doped | 55.8 ± 7.8 | 62 ± 6 | 67.4 ± 7.7 | ||||
| Controls | 75.3 ± 10.8 | 77.3 ± 4.4 | |||||
| Tachycardia | Doped | 2 cases | 2 cases | ||||
| QT interval | Doped | 348 ± 42.3 ms; p<0.05 | |||||
| Non-Doped | 400 ± 34.2 ms | ||||||
| Controls | 358 ± 18.8 ms | ||||||
| LV Ejection Fraction (%) | Doped | All subjects had preserved left ventricular systolic function (LV ejection fraction>0.55) | 52 ± 11 p<0.001; 71% of users showed LVEFs falling | 56.8 ± 5.4 | |||
| Non-Doped | 63 ± 8 | 61.6 ± 5.1 | |||||
| Controls | 60.8 ± 4.1 | ||||||
| LV mass | Doped | 217.4 ± 47.3 g; p<0.001 | 245 ± 62 g; p<0.001 | ||||
| Non-Doped | 244.8 ± 58.9 g; p<0.001 | 192 ± 40 g | |||||
| Controls | 156.4 ± 19.6 g; p <0.001 | ||||||
| LV mass index | Doped | 97.3 ± 20 g/m2; p<0.001 | 111 ± 61 g/m2; p<0.001 | 69.9 ± 8.4 g/m2; p<0.001: users vs. controls | |||
| Non-Doped | 113.5 ± 28.2 g/m2 | 89 ± 18 g/m2 | 63.4 ± 8.9g/m2 non-users vs. controls | ||||
| Controls | 80.1 ± 11.9 g/m2 | 48.4 ± 5.4g/m2 | |||||
| LV stroke volume | Doped | 41.5 ± 2.2 ml/m2; p<0.001: users vs. controls | |||||
| Non-Doped | 41.5 ± 2.7 ml/m2 | ||||||
| Controls | 35.2 ± 1.7 ml/m2 | ||||||
| Interventricular septum thickness | Doped | 10.7 ± 2.1 mm; p=0.151 | 1.2 ± 0.2 cm; p<0.001 | 6.4 ± 1.7 mm/m2 ; p<0.001: users vs. controls | |||
| Non-Doped | 10.7 ± 1.7 mm | 1.1 ± 0.1 cm ; p<0.001 | 5.7 ± 1.2 mm/m2; p<0.01: non-users vs. controls | ||||
| Controls | 9.8 ± 0.7 mm | 4.7 ± 0.7 mm/m2 | |||||
| Posterior wall thickness | Doped | 10.3 ± 2.1 mm; p = 0.006 | 1.2 ± 0.2 cm ; p=0.003 | 5.9 ± 1.6 mm/m2 ; <0.001: users vs. controls | |||
| Non-Doped | 9.9 ± 1.4 mm | 1.1 ± 0.2 cm | 5.15 ± 1.0 mm/m2 | ||||
| Controls | 8.6 ± 1.1 mm | 4.2 ± 1.2 mm/m2 | |||||
| Flow-mediated dilatation | Doped | 2.4 ± 1.5 %; p=0.032 | |||||
| Non-Doped | 6.7 ± 1.5 % | ||||||
| Endothelium-independant dilatation | Doped | 11.9 ± 1.0 %; p=0.29 | |||||
| Non-Doped | 9.2 ± 1.5 % | ||||||
| Left Atrial (LA) diameter | Doped | 3.6 ± 0.5 cm; p=0.42 | 35.9 ± 3.5mm ; p<0.01: users vs. controls and non-users | ||||
| Non-Doped | 3.5 ± 0.5 cm | 32.5 ± 3.4 mm | |||||
| Controls | 31.4 ± 3.4 mm | ||||||
| LA Volume index | Doped | 31.9 ± 5.3 ml/m2; p<0.001: users vs. controls and non-users | |||||
| Non-Doped | 27.3 ± 2.1 ml/m2 | ||||||
| Controls | 25.4 ± 1.8 ml/m2 | ||||||
| LA active empting fraction | Doped | 37.9 ± 3.2 % ; p<0.01: users vs. controls and non-users | |||||
| Non-Doped | 41.3 ± 3.7 % | ||||||
| Controls | 40.2 ± 4.2% | ||||||
| LA lateral strain | Doped | 33.9 ± 6.4 %. p<0.001: users vs. controls and non-users | |||||
| Non-Doped | 50.4 ± 4.9 % | ||||||
| Controls | 52.3 ± 5.8 % | ||||||
| Longitudinal 4-chamber strain | Doped | -16 ± 4; p <0.001 | |||||
| Non-Doped | -20 ± 3 | ||||||
| Coronary plaque volume | Doped | 3 (0-174) mm3; p =0.012 | |||||
| Non-Doped | 0 (0-69) mm3 | ||||||
| Degree of stenosis for most severe stenosis* | Doped | 0.5 (0-1); p= 0.052 | |||||
| Non-Doped | 0.5 (0-1) | ||||||
| Number of diseased coronary artery segments** | Doped | 0.5 (0-2); p=0.059 | |||||
| Non-Doped | 1 (0-1) | ||||||
| Maximal HR during physical effort | Doped | 172.9 ± 8.4 bpm | |||||
| Non-Doped | 173.4 ± 9.8 bpm | ||||||
| Controls | 178.5 ± 10.4 bpm | ||||||
| Maximal Blood pressure during physical effort | Doped | 180.4 ± 14.8 mmHg; p<0.01: users vs. controls and non-users | |||||
| Non-Doped | 166.8 ± 10.8 mmHg | ||||||
| Controls | 165.8 ± 15.4 mmHg | ||||||
| Rate pressure product during physical effort | Doped | 0.59 ± 0.6 bpm × mmHg × 10 ^ 3 | |||||
| Non-Doped | 0.54 ± 3.4 bpm × mmHg × 10 ^ 3 | ||||||
| Controls | 0.53 ± 0.2 bpm × mmHg × 10 ^ 3 | ||||||
| Maximal workload achieved | Doped | 160.2 ± 24.7 Watts | |||||
| Non-Doped | 200.7 ± 33.5 Watts; p<0.001: users vs. controls and non-users | ||||||
| Controls | 150.5 ± 32.4 Watts | ||||||
| Peak VO2 during physical effort | Doped | 49.3 ± 8.7 ml/kg/min | |||||
| Non-Doped | 55.9 ± 7.4 ml/kg/min | ||||||
| Controls | 44.9 ± 6.4 ml/kg/min |
Note: *p<0.05; **significant difference between Pla and DHEA/(p<0.01, significant difference between Pla and DHEA)
Table 1: Cardiovascular effects.
Anthropomorphic effects
Four publications addressed anthropomorphic effects, with a total of 274 subjects, 122 doped and 130 non-doped [4,13,15,16]. The non-doped subjects were represented by a group of 110 non-doped athletes and 20 sedentary subjects not using anabolic agents.
One of the primary goals of consumers of anabolic agents is to increase their muscle mass leading to anthropomorphic changes. Among these modifications, there is a significant difference between body surface area and Body Mass Index (BMI) of doped and non-doped subjects. Indeed, these two parameters were increased in athletes using anabolic agents [4,13]. This would be related to the body mass of doped subjects higher than that of non-doped subjects, observed in the majority of studies [13,15].
In addition, one study showed a significant decrease in body fat, which can be compared with the increase in lean mass index in doped subjects. There was also an increase in the average lean mass and the average body cell mass in this same category of subjects [16] (Table 2)
| Parameters (mean ± SD) | Publications | |||||
|---|---|---|---|---|---|---|
| Djordjevic et al. [13] | Severo et al. [15] | Baggish et al. [4] | Meinhardt et al. [16] | |||
| Number of subjects | Doped | 10 strength athletes | 10 | 86 | 16 | |
| Non-doped | 12 endurance athletes | 12 | 54 | 32 | ||
| Controls | AAS-free sedentary controls=20 | 0 | 0 | 0 | ||
| Doping detection | Non-users: all negative during and out of competition | NC | Test | - | ||
| Dose/duration | combination of oral and injectable for at least 2 years (up to 5 years) | At least one year | Cumulative lifetime AAS dose (g): 366 (166-608) | 250 mg/wk for 5 weeks | ||
| Height (cm) | Doped | 181 ± 4.6; p<0.001 | (175 ± 7); p= 0.26 | 180 (170-180); p=0.16 | 180 ± 8 | |
| Non-doped | 190 ± 10.8 | 178 ± 6 | 180 (170-180) | 185 ± 5 | ||
| Controls | 182 ± 9.1 | |||||
| Body weight (kg) | Doped | 100.4 ± 18.6; p<0.001 | 93 ± 14; p=0.015 | 83.3 ± 18.5 | ||
| Non-doped | 89.6 ± 14.9 | 77 ± 8 | 90.5 ± 12.2 | |||
| Controls | 78.6 ± 8.3 | |||||
| Body surface area (m2) | Doped | 2.2 ± 0.23; p=0.001 | 2.2 (2.1-2.3); p=19 | |||
| Non-doped | 2.2 ± 0.24 | 2.2 (2.0-2.3) | ||||
| Controls | 1.9 ± 0.12 | |||||
| Body mass index (kg/m2) | Doped | 31 (29-33); p<0.001 | 25.4 ± 3.7 | |||
| Non-doped | 29 (27-31) | 26.1 ± 3.1 | ||||
| Fat-free mass index | Doped | 26 (24–28); p <0.001 | ||||
| Non-doped | 23 (21-25) | |||||
| Mean fat mass (kg) | Doped | 16.2 ± 9.1 | ||||
| Non-doped | 18.8 ± 7.99 | |||||
| Mean lean body mass (kg) | Doped | 63.1 ± 10.2 | ||||
| Non-doped | 67.6 ± 7.1 | |||||
| Mean body cell mass (kg) | Doped | 41.8 ± 7.0 | ||||
| Non-doped | 44.4 ± 5.3 | |||||
Table 2: Anthropomorphic effects.
| Publications | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Meinhardt et al. [16] | Severo et al. [15] | Razavi et al. [21] | Hengevoss et al. [20] | Börjesson et al. [11] | Bordin et al. [17] | Collomp et al. [18] | D'andrea et al. [19] | Jessen et al. [14] | Solheim et al. [22] | ||
| Number of subjects | Doped | 16 | 10 | 72 | 5 | 8 | 20 | 10 men+11 women | 35 | 6 | 9 |
| Non-doped | 32 | 12 | 178 | 5 | 0 | 20 | 30 | 0 | 10 | ||
| Controls | 0 | 0 | 0 | 6 | 0 | 0 | 0 | age and sex matched sedentary=0 | 0 | 0 | |
| Doping detection | - | NC | Self-declaration | NC | Self-reporting and confirmed by analysis | Self-declaration | - | LH and FSH levels | NC | - | |
| Doses/Duration | 250 mg/week for 5 weeks | At least one year | 34.7%<six months, 16.7% to 12 months, 11.1% to 24 months and 37.5% to 12 yrs. | NC | 7 weeks-2 yrs. (average: 58 weeks) | 1g/week for one year | DHEA 100 mg/day | 31.3 ± 6.4 wk per yr. 525.4 ± 90.7 mg per wk. | 80 µg oral clenbuterol | Testosterone esters:250 mg | |
| Testosterone | Doped | Change in testosterone group-change in placebo group (95% CI) : 397.69 (155.62 to 737.75) ng/dL; p<0.005 | 80%>54.69 pg/ml (median (interquartile range)); p=0.003 | Increase | 649.45 (340.9) ng/dl; p=0.25 | Men: basal=6.61 ± 0.40 ng/ml, at mid-treatment : 7.48 ± 0.37 ng/ml*, at end of treatment=7.26 ± 0.29 ng/ml*/ Women: basal=0.38 ± 0.02 ng/ml ++, at mid-treatment=3.41 ± 0.51 ng/ml**++, at end of treatment=3.33 ± 0.46 ng/ml**++ | Serum testosterone increased (p<0.001) from pre-administration (19.8 ± 7.6 nmol/L) to post-administration (81.4 ± 21.9 nmol/L) in the treated group and that the post- administration level was higher (p<0.001) in the treated group (81.4 ± 21.9 nmol/L) compared with in the control group (30.0 ± 5.4 nmol/L) | ||||
| Non-doped | 0%>54.69 pg/ml | No change | 545.2 (156.0) ng/dl | Men: basal=5.76 ± 0.17 ng/ml, at mid ttt=5.92 ± 0.29 ng/ml, at end ttt=6.04 ± 0.33 ng/ml/Women: basal=0.40 ± 0.02ng/ml ++, at mid ttt = 0.42 ± 0.03 ng/ml ++, at end ttt= 0.40 ± 0.02 ng/ml ++ | |||||||
| Estradiol | Doped | 70%>42.6 pg/ml (median (interquartile range)); p=0.04 | Increase in the TE group (99.0 ± 54.2 pmol/L) compared with pre-administration (63.5 ± 24.2 pmol/L, p=0.04) and compared with the control group (58.0 ± 23.2 pmol/L, p=0.05) | ||||||||
| Non-doped | 8.3%>42.6 pg/ml | ||||||||||
| FSH | Doped | 100%<0.7 mIU/ml (median (interquartile range)); p=0.0001 | Decrease | No effect of time | |||||||
| Non-doped | 0%<0.7 mIU/ml | No change | |||||||||
| LH | Doped | Decrease | Decrease | Decrease (p=0.02) from pre-(3.8 ± 1.7 IU/L) to post-administration (1.6 ± 0.7 IU/L) in doped group, Decrease (p<0.001) after administration in doped group (1.6 ± 0.7 IU/L) compared to undoped group | |||||||
| Non-doped | No change | No changes | |||||||||
| Estrone (pg/ml) | Doped | Men: basal=77.1 ± 7.1 ng/ml, mid=160.6 ± 20.8 ng/ml**, late =174.1 ± 9.3ng/ml**/Women: basal = 45.7 ± 2.4 ng/ml, mid =197.9 ± 15.4 ng/ml**, late=253.3 ± 26.6ng/ml **+ | |||||||||
| Non-doped | Men: basal=62.6 ± 5.8 ng/ml, mid=66.7 ± 4.0 ng/ml, end=71.6 ± 5.8 ng/ml Women: basal=53.4 ± 2.8 ng/ml, mid =61.9 ± 8.8 ng/ml, end=62.8 ± 9.0 ng/ml | ||||||||||
| Insulin | Doped | After ingestion: +105%; p=0.009 | |||||||||
| Non-doped | |||||||||||
| Inhibin B | Doped | Decrease | |||||||||
| Non-doped | No changes | ||||||||||
| Clitoral hypertrophy | Doped | 6 cases | |||||||||
| Change of voice | Doped | 5 cases | |||||||||
| Menstrual disorders | Doped | 5 cases | |||||||||
| Impaired libido | Doped | 9.7% | 35% | ||||||||
| Gynecosmastia | Doped | 15% | |||||||||
| Testicular atrophy | Doped | 27.5 % | |||||||||
Note: *p<0.05; **significant difference between Pla and DHEA/(p<0.01, significant difference between Pla and DHEA); +p<0.05, significant difference between genders; ++p<0.01, significant difference.
Table 3: Endocrine effects.
Muscular effects
Muscular effects were described in 3 publications [14,16], with 71 subjects including 32 doped subjects and 39 clean subjects. Muscle effects reported by doped subjects included muscle pain [16,17]. One study reported a case of muscle tremor in six clenbuterol-doped subjects [14].
According Yu, et al. ratios of protein muscle concentration levels in doped athletes, between 5 and 15 years of consumption, versus non-doped ones for CMTK2 (metabolism) and ACTAC1 (mobility and contractility) were increased, but decreased for HSPB1 (cell protection), PGM-1 (metabolism) and myoglobin (transport and storage), suggesting alteration of muscle metabolism and contractility injury. A study in 6 doped subjects using oral clenbuterol (80 μg) showed increases of phosphorylation of mTORSer 2448 (+121%, p=0.004), of PKA-dependent substrates (+35%, p=0.006) and of the resting energy expenditure (+21%, p<0.01) [14].
Endocrine effects
Ten publications addressed the effects of anabolic agents on blood concentrations of hormones [11,14-21]. These studies included a total of 535 subjects including 181 doped subjects, 287 non-doped subjects, 46 sedentary subjects and 21 subjects participating in a crossover study with a doped and a non-doped period.
The administration of testosterone and of synthetic derivatives of testosterone leaded to significant changes in the blood concentrations of sexual hormones. Among these changes, there was significant increases in the concentrations of testosterone, estradiol and estrone [15,18,19,22], but also luteinizing hormone, follicle stimulating hormone, and inhibin B [15,18,19,21]. The use of anabolic agents impacted the hypothalamic-pituitary-gonadal axis through changes in luteinizing hormone, follicle stimulating hormone, and inhibin B variations [19]. Libido alteration, gynecomastia, testicular atrophy [17,20], clitoral hypertrophy, voice changes and menstrual disorders [11] were described (Table 3).
One study also reported an increase in insulin (+105%; p=0.009) after administration of anabolic agents [14].
Biochemical effects
Six publications addressed the biochemical effects, with a total of 249 subjects including 122 doped subjects, 86 non-doped subjects and 41 subjects participating in a crossover study with a doped and a non-doped period [4,14,15,17,18,23].
A decrease in HDL-cholesterol concentration was observed in the doped subjects. In a study comparing 86 doped and 54 non-doped subjects with a consumption of about 366 g AA (166-608 g) during approximately 7.4 years (4.0-11.6 years), higher prevalence of dyslipidemia was reported in the groups using anabolic agents in comparison to non-users [4]. Finally, a significant correlation between decreased endothelial function, reflected by the flow-mediated dilation, and HDL-cholesterol concentration was found (r=0.49, p=0.03) [15].
Clenbuterol use by 6 subjects (80 μg per os) increased the blood concentrations of glucose by 25% (p<0.001), lactate by 87% (p=0.004) and fatty acids by 129% (p=0.001) [14].
The use of 100 mg DHEA during 28 days did not significantly alter the concentrations of adipokines (leptin, adiponectin and resistin) [23] or of cholesterol, glucose, and triglycerides [18,23].
Also, uric acid, urea and creatinine concentrations of a group of 20 subjects doped with 1g per week for one year were significantly increased from the clean group. In addition, there was a correlation between the increase of these markers and the hematocrit concentrations [17]. Urea and creatinine retention could show renal dysfunction caused by the intake of these anabolic agents [24] (Table 4).
| Publications | |||||||
|---|---|---|---|---|---|---|---|
| Severo et al. [15] | Baggish et al. [4] | Bordin et al. [17] | Collomp et al. [18] | Gravisse et al. [24] | Jessen et al. [14] | ||
| Number of subjects | Doped | 10 | 86 | 20 | 10 men+11 women | 20 | 6 |
| Non-doped | 12 | 54 | 20 | 0 | |||
| Controls | 0 | 0 | 0 | 0 | 0 | 0 | |
| Doping detection | NC | test | Self-declaration | - | - | NC | |
| Doses/ duration |
At least one year | Cumulative lifetime AAS dose (g):366 (166-608) | 1g/week for one year | DHEA 100 mg/day | 100 mg DHEA for 28 days | 80 µg oral clenbuterol | |
| Total cholesterol | Doped | 151 (35) mg/dl; p=0.78 | Men: basal=1.54 ± 0.11 g/L; mid-treatment=1.43 ± 0.10; end of treatment=1.47 ± 0.10/Women: (p<0.05, significant difference between Men and Women) basal=1.70 ± 0.09; mid-treatment=1.79 ± 0.11; end of treatment=1.70 ± 0.08 | ||||
| Non-doped | 154 (25) mg/dl | Men: basal=1.47 ± 0.1 g/L; mid-treatment=1.45 ± 0.13; end of treatment=1.46 ± 0.11/Women: basal=1.76 ± 0.13; mid-treatment=1.79 ± 0.11; end of treatment=1.74 ± 0.10 | |||||
| Triglycerides | Doped | 100 (62) mg/dl; p=0.83 | Men: basal=0.65 ± 0.08 g/L; mid-treatment=0.77 ± 0.12; end of treatment=0.79 ± 0.14/Women: basal=0.78 ± 0.11; mid-treatment=0.66 ± 0.04; end of treatment=0.65 ± 0.11 | ||||
| Non-doped | 96(37) mg/dl | Men: basal=0.59 ± 0.07 g/L; mid-treatment=0.76 ± 0.13; end of treatment=0.69 ± 0.10/Women: basal=0.89 ± 0.12; mid-treatment=0.83 ± 0.11; end of treatment=0.79 ± 0.10 | |||||
| LDL Cholesterol | Doped | 109(28) mg/dl; p=0.17 | Men: basal=0.87 ± 0.09 g/L; mid-treatment=0.81 ± 0.08; end of treatment=0.82 ± 0.10/Women: basal=1.02 ± 0.10; mid-treatment=1.15 ± 0.12; end of treatment=1.05 ± 0.08 | ||||
| Non-doped | 93 (24) mg/dl | Men: basal=0.85 ± 0.10 g/L; mid-treatment=0.84 ± 0.10; end of treatment=0.83 ± 0.09/Women: basal=1.06 ± 0.12; mid-treatment=1.11 ± 0.11; end of treatment=1.08 ± 0.09 | |||||
| HDL Cholesterol | Doped | 21 (6) mg/dl; p=0.0001 | Men: basal=0.53 ± 0.03 g/L; mid-treatment=0.48 ± 0.03; end of treatment=0.49 ± 0.03/Women: basal=0.52 ± 0.02; mid-treatment=0.51 ± 0.02; end of treatment=0.50 ± 0.02 | ||||
| Non-doped | 42 (37) mg/dl | Men: basal=0.50 ± 0.03 g/L; mid-treatment=0.46 ± 0.04; end of treatment=0.49 ± 0.03/Women: basal=0.52 ± 0.03; mid-treatment=0.52 ± 0.03; end of treatment=0.50 ± 0.02 | |||||
| Dyslipidemia | Doped | Higher prevalence of dyslipidemia | |||||
| Non-doped | |||||||
| Glucose | Doped | Men: basal=0.83 ± 0.03 g/L; mid-treatment=0.86 ± 0.03; end of treatment=0.83 ± 0.03/Women: basal=0.81 ± 0.02; mid-treatment=0.76 ± 0.02; end of treatment=0.85 ± 0.02 | After ingestion: +25%; p<0.001 | ||||
| Non-doped | Men: basal=0.88 ± 0.03 g/L; mid-treatment=0.82 ± 0.03; end of treatment=0.82 ± 0.02/Women: basal=0.79 ± 0.03; mid-treatment=0.81 ± 0.03; end of treatment=0.82 ± 0.03 | ||||||
| Lactate | Doped | After ingestion: +87%; p=0.004 | |||||
| Fatty acids | Doped | After ingestion: +129%; P=0.001 | |||||
| Adipokines | Doped | No changes | |||||
| Uric acid | Doped | 5.9 (0.8) mg/dl; p<0.007 | |||||
| Non-doped | 5.1 (0.8) mg/dl; ref values: 4.5-8.1 | ||||||
| Urea | Doped | 42.8 (6.4) mg/dl; p<0.0001 | |||||
| Non-doped | 34.6 (4.7) mg/dl; ref values: 17-42 | ||||||
| Creatinine | Doped | 1.7 (0.2) mg/dl; p<0.0001 | |||||
| Non-doped | 1.1 (0.1) mg/dl; ref values: 0.7-1.3 | ||||||
Table 4: Biochemical effects.
Hematological effects
Three publications described hematological effects, with a total of 61 subjects including 39 doped and 22 non-doped subjects [15,17,22].
A greater number of abnormal blood profiles in doped individuals was shown by Soldheim, et al. [22] with a significant increase in platelet count, but low variations in hematocrit, hemoglobin concentration and red cells indices (mean corpuscular hemoglobin and mean corpuscular hemoglobin concentration) [15,17]. No significant alteration was demonstrated for white blood cell counts and fibrinogen concentrations [15,25,26].
Hepatic effects
Two publications addressed the effects of anabolic agents on hepatic functions [18,27]. These studies included a total of 166 subjects, including 145 doped subjects and 21 subjects participating in a crossover study with a doped and a non-doped period.
A study carried out with DHEA did not show any significant modification of transaminase (aspartate transaminase and alanine transaminase) concentrations following a 100 mg/day DHEA use [18]. A second study was conducted with doped subjects consuming cocaine. It only showed a significant increase in gamma-glutamyl transferase in cocaine users (39.4 ± 44.6 UI/L vs. 27.2 ± 13.5 UI/L, p=0.02) [27].
Behavioral effects
Three publications addressed cognitive and behavioral effects, with a total of 298 subjects including 100 doped, 198 non-doped [11,17,20]. One study (n=20 doped and n=20 non-doped) showed that 12.5% of subjects reports anxiety and 15% reports depression after one year of consumption of 1 gram anabolic agent per week [17]. Another study showed increase of depression cases in 6.9% of 72 doped subjects with consumption duration between less than 6 months to 12 years.
Anabolic agents act on the behavior of consumers and it was mainly observed an increase in the aggressiveness [11,20], as well as an increase in cases of depression [11,17]. Borjesson, et al. noted memory impairment (1/8 cases), mood changes (3/8 cases) and depression (4/8 cases) when using anabolic agents [11].
The strategy used in this bibliographic research allowed us to have an up-to-date vision of the different biological and clinical effect notified in the scientific literature, caused by the use of anabolic agents, which clearly shows that athletes expose themselves to several risks.
The effects of anabolic agents on the muscles are important to remember, effects that some athletes want for aesthetic purposes, but alter the muscle protein profile and can lead to muscle damage [28]. It should be remembered that training increase the effects of androgens on muscle, do not improve muscular endurance or the contractile quality of the muscle. The increase of muscle mass is associated with hypertrophy of muscle fibers. Endocrine effects, renal dysfunction, heart hypertrophy, increase of BMI, blood pressure increase and atherosclerosis are mainly reported. The endocrine adverse effects primarily relate to gonadal dysfunction and dose-dependent suppression of hypothalamic and pituitary gonadotropins [29]. Indeed, the consumers have more risks to have adverse effects as dystrophy of sexual organs, gynecomastia by peripheral aromatization of androgens to estradiol, but also to liver as hepatocellular adenoma. A hypothesis has been put forward concerning the risk of liver disorders following the consumption of anabolic agents, which introduces the role of the polymorphism of the gene coding for UGT2B17 linked to a deletion type mutation [30]. Indeed, UGT2B17 is an enzyme that catalyzes glucurono-conjugation, involved in the main pathway of elimination of anabolic agents, so individuals of UGT2B17 may increase the risk to develop renal disorders [30].
Furthermore, the use of anabolic agents has important consequences on the behavior of the consumers. It is mainly reported that it increases aggressiveness, but also behavioral disorders such as depression, anxiety or addiction. Maybe it is due to their easy crossing of the blood-brain barrier and binding to brain androgen receptors when using anabolic androgenic steroids [31].
Nevertheless, not all the known effects were found in this bibliographic search, because they were not all detailed in the selected publications. Indeed, it is reported in a review not listed here, that anabolic agents are also responsible for fertility problems with a significant decrease in the fertility index [32]. In addition, the concomitant use of substances such as alcohol, tobacco or illicit drugs may increase the effects of anabolic agents and may lead to changes in the blood lipid profiles of these users, although the mechanisms of action remain to be determined [27].
This study reminds us that non-therapeutic use of anabolic agents made by professional and amateur athletes is not without important risk. According to the different chapters detailed in this study, the use of anabolic agents involves many health risks for the athlete, despite the sanctions incurred in case of control by WADA. Indeed, their use leads to many pathophysiological changes, some of these changes are searched by the consumer, but it is also exposed to the appearance of adverse effects, including the effects on the cardiac system, behavior, or changes in biochemical and hematological profiles. The consumer may be subject to other dysfunctions such as liver and kidney dysfunctions. It is for these reasons that the WADA prohibits and controls the use of these substances, particularly through the athlete's biological passport.
It is therefore important to enhance the scientific literature on the effects of these anabolic agents to better adapt the prohibition list maintained by WADA, but also to implement prevention among athletes who are not all aware of the impacts of using sports doping agents such as anabolic agents.
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
Citation: Tutakhail A, Brionne J, Balayssac D, Coudore F (2022) Adverse Events of Anabolic Agents in Doped Athletes: A Systematic Review of the Literature from 2010 to 2021. Int J Phys Med Rehabil.11:654.
Received: 05-Oct-2022, Manuscript No. JPMR-22-19470; Editor assigned: 10-Oct-2022, Pre QC No. JPMR-22-19470 (PQ); Reviewed: 28-Oct-2022, QC No. JPMR-22-19470; Revised: 04-Nov-2022, Manuscript No. JPMR-22-19470 (R); Published: 15-Nov-2022 , DOI: 10.35248/2329-9096.22.11.654
Copyright: © 2022 Tutakhail A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.