Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2019)Volume 10, Issue 6
Introduction: Air pollution induces skin damage and accelerating skin aging processes, mainly through oxidative stress. Pollutant substances, such as particulate matter (PM10, PM2.5) and tropospheric ozone (O3), can activate specific intracellular receptors called AhR (Aryl-Hydrocarbon Receptors) and this activation is involved in the skin aging process. Deschampsia antartica aqueous extract (DAE) has shown, in vitro, to reduce the pollutant-induced AhR activation. Ferulic acid (FA) and vitamin C (VC) are well known potent anti-oxidant substances. A dermatological product in serum formulation containing DAE/FA/VC has been recently commercialized. This product has demonstrated in vivo to protect skin from pollution damage during winter season when PM10 levels are very high. No data have been published so far regarding the protective effect of this serum during summer months when the main atmospheric pollutant is O3.
Objective and materials and methods: We carried out a prospective, single-blind, controlled 28-day study during the summer season (characterized by elevated O3 tropospheric levels) in 20, photo type II-III, women (mean age 38 years) with mild-moderate skin aging, living in an urbanized, high pollution area (City of Rome) compelling to spend at least 2 hours daily outdoor. The study’s outcomes were to evaluate the effects of treatment on skin barrier function, assessed by TEWL measurement (Tewameter) and corneometry, skin appearance by high resolution photographs with Visioface Quick (Courage and Khazaka) and the effect on squalene peroxide (SQOOH)/squalene (SQ) skin ratio measured with face swabs.
Results: The trial was conducted between July the first, and August 2, 2019. All the subjects concluded the trial. During the study period, elevated O3 concentration (>120 microg/m3) were registered in 22 out of 31 days (71%) (The ARPA official database, Agenzia regionale per la protezione ambientale). The product induced, in comparison with baseline values, a significant improvement of skin barrier function and hydration (-14% of TEWL and +16% skin hydration), significant improvements in skin appearance and SQOOH/SQ ratio (-19%) were also observed. The 90% of the subjects involved in the trial evaluated the product very well regarding cosmetic acceptability.
Discussion: The results of the present study has demonstrated that a serum containing DAE/FA/VC can improve skin barrier function, skin appearance and counteract skin oxidative stress in women living in high O3 pollution urban area during the summer period.
Deschampsia antartica aqueous extract (EDA); Air pollution; Ozone; Skin aging; Squalene
Air pollutant substances are in direct contact with skin [1]. Pollution induces skin damage therefore favoring skin aging processes, mainly through oxidative stress [2]. In particular, air pollution causes alteration of skin barrier functions, oxidative stress, and inflammation [3]. Anti-oxidative skin potential is reduced after pollutants exposure [4]. Pollutant elements, mainly particulate matter (PM10, PM2.5), speed up skin aging process through specific activation of intracellular receptors called AhRs (Aryl-Hydrocarbon Receptors) [5]. PM10 and PM2.5 are common pollutants during winter months like December and January. On the contrary, during summer season the main pollutant is tropospheric O3 [6]. Increased levels of Ozone can cause direct skin barrier damage [7]. In particular ozone causes a strong oxidative stress to the skin, depleting the physiological antioxidant defensive mechanisms, like skin vitamin C and vitamin E levels [8]. Several publications have proved that AhRs are involved in the pathophysiology of skin, including photocarcinogenesis, skin dark spot formation, and skin inflammation [9]. The main pollutant substances like PM10 and Ozone are agonist of AhR [10]. Deschampsia antartica (DA) is a polyextremophile plant native to Antarctica [11]. This plant lives under high salinity, solar irradiation and oxygen concentration, low temperature and extreme dryness [12]. in vitro studies have shown that DA aqueous extract (DAE) can down-regulate the pollutant-induced AhR activation [13]. Ferulic acid [14] (FA) and vitamin C [15] are potent antioxidant substances. The standardized Crypthomphalus aspersa (a snail of the Helicidae family) secretion (SCA), very rich in proteins, antioxidant and glycosaminoglycans, is commonly used in cosmetic products as an anti-aging and skin regenerating substance [16]. A serum containing DAE/FA, vitamin C and SCA (10%) and has been recently commercialized (EC serum). In a clinical study conducted in 20 women, this product has demonstrated to protect skin from pollution damage during winter season when PM10 levels are very high [17]. No published clinical data are available regarding the clinical protective effects of this serum against the negative effects of tropospheric ozone (commonly elevated during summer months) on the skin.
We carried out a prospective, single-blind, controlled, 28-day study to evaluate the skin protective effects of EC-serum in women living in urbanized area during the summer period, characterized by high tropospheric ozone levels.
Subjects
A total of 20 women aged from 35 to 40 years, with a phototype (Fitzpatrick): II and III, residents in an urban area (City of Rome), compelled to spend at least 2 hours a day outdoors were enrolled in the trial. During the entire study duration, the volunteers were asked not to use any topical facial products (cosmetics/drugs/medical devices) or sunscreen or any products with a SPF. For daily hygiene, subjects were instructed to use a non-aggressive cleansing procedures (i.e., Dove soap) was allowed. Table 1 reports the baseline characteristics of participants.
| Baseline characteristics | |
|---|---|
| Number | 20 |
| Mean age, years, mean ± SD | 38 ± 6 |
| Fitzpatrick photo type, % | II: 40% |
| III: 60% | |
| Subjects with sensitive skin, n (%) | 10 (50%) |
| Trans Epidermal Water Loss, (TEWL) g/m2/h, mean ± SD | 11.75 ± 4 |
Table 1: Baseline characteristics of participants.
Ethical aspects
All the enrolled women gave their written informed consent. The trial was carried out according to: 1) the Declaration of Helsinki (June 1964) and its successive amendments [18] principles, 2) the Good Clinical Practices for conducting clinical trials for drugs ICH E6 (R1) of 10/06/1996 (CPMP/ICH/ 135/95) international recommendations [19]; 3) the Directive of the European Parliament and Council 2001/20/EC concerning the harmonization of legislative, statutory and administrative provisions of the member States relating to the application of good clinical practices when conducting clinical trials for drugs for human use-OJ/EC of 01/05/2001; and 4) the Colipa guidelines recommendation, August 1997 [20]. The Local Ethical Committee approved the final study protocol. The study was performed at the Institute of Eurofins BioPharma Product Testing, certified ISO 9001:2015 and equipped with material and technical means suitable for non-invasive clinical research studies, compatible with the safety requirements for human subjects.
Application of DAE/FC/VC serum
The subjects should apply the tested product at home under the normal conditions of use, for 28 consecutive days. The product was applied on the face twice daily (in the morning and in the evening). Written specific instructions were given to each subject for the correct application of the tested product: 1) do not wash the face after the application of the serum (a gentle cleansing of the face with non-aggressive soaps was allowed); 2) do not apply any other skincare products, 3) do not use any make-up products during the study period.
Trial outcomes
The aim of the study was to evaluate the clinical effects of the facial treatment on skin barrier function, assessed by Trans Epidermal Water Loss (TEWL) measurements and corneometry to assess skin hydration, and the anti-oxidative protective skin effect evaluating the squalene peroxide (SQOOH)/squalene (SQ) skin ratio assessed by face swabs. Tewameter TM 300 (Courage & Khazaka) was used to perform TEWL measurements. TEWL was expressed as g/m2/h. Highresolution photographs of the face were performed under standardized lighting with Visioface Quick (Courage & Khazaka) to evaluate the effect on skin appearance. Standardized digital photographs of the full face were taken at D0 and at D28 using the VISIA-CR photo station (Canfield Imaging System, Fairfield NJ). All measurements were done by the same technician, before any application (D0/T0) then after 28 consecutive days of product use (D28). The samples for sebum analysis were taken from forehead with a swabbing method at D0 and at D28. Swab homogenates were centrifuged at 10.000 g and subsequently analyzed for squalene mono-hydroperoxide (SQOOH) and SQ content with liquid chromatography system according to Jourdain et al. [21]. The subject-based evaluation of the cosmetic efficacy of the tested product was performed using a 11-item questionnaire adapted to the investigational product and completed by each evaluated subject, after 28 consecutive days of use.
Statistical methods
A formal calculation of sample size for this trial was not performed in relation with the consideration that this was a pilot study. Statistical analysis was done using GraphPad statistical software ver. 13.0 (La Jolla, CA, USA). Continuous variables have been expressed as mean ± Standard Deviation (SD). The paired T-test and the Wilcoxon tests were used for the analysis of the study outcomes comparing baseline (D0) and D28 values. We calculated also the 95% Confidence intervals of the difference in all the variables evaluated. A p-value of <0.05 was considered significant.
All the enrolled women concluded the 28-day study period. No adverse events imputable to the investigational product were observed by the investigator during the study. The product was very well tolerated, and no sensation of discomfort was reported by the subjects during the entire study period. The trial was conducted between July the first, and August 3, 2019. During the days when the clinical trial was conducted, elevated O3 tropospheric concentration (>120 microg/m3) urban areas were registered (ARPA official database, Agenzia regionale per la protezione ambientale) in 22 out of 31 days. The O3 average during the study period was 127 ± 30 microg/m3. TEWL values at baseline were 11.7 ± 4 g/m2/h. After treatment TEWL was reduced significantly (P=0.008; Wilcoxon Test) to 10.05 ± 2 g/m2/h, with an absolute difference of 1.7 (95% CI: 1.1-2.0) (Figure 1). This represents a reduction of -14% in the TEWL mean values in comparison with baseline. Skin hydration level significantly increased after treatment with the serum in comparison with baseline (from 42.2 U to 49 U), representing a percentage increase of 16% (Figure 2) with an absolute difference of 6.8 (95% CI: from 4 to 8.8). SQOOH/SQ ratio significantly improved observed at day 30 in comparison with baseline data (-19%).
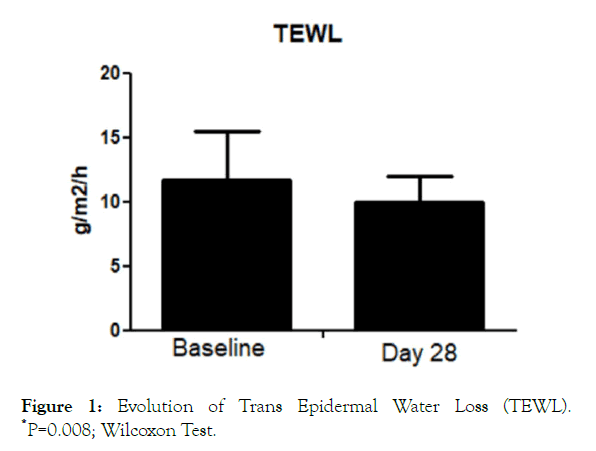
Figure 1. Evolution of Trans Epidermal Water Loss (TEWL). *P=0.008; Wilcoxon Test.
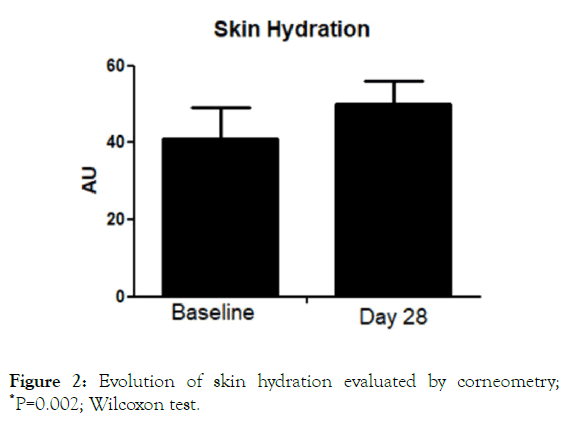
Figure 2. Evolution of skin hydration evaluated by corneometry; *P=0.002; Wilcoxon test.
The SQOOH/SQ ratio at baseline was 21.7 ± 13% and 17 ± 10% after treatment (P=0.05; Wilcoxon test) with an absolute difference of 4.7 (95% CI: 1.6-7.8) (Figure 3). Figure 4 reports four high definition pictures of two subjects at baseline and after treatment demonstrating improvement of skin appearance, showing the skin texture, skin lightening and skin color uniformity. The product was evaluated very well by >90% of the treated subjects regarding cosmetic acceptability. In more details, 75% of the subjects stated that the product has improved skin complexion, 80% affirmed that the product has made the skin brighter. All treated women (100%) stated that the tested product has made the skin smoother. No relevant side effects were reported.
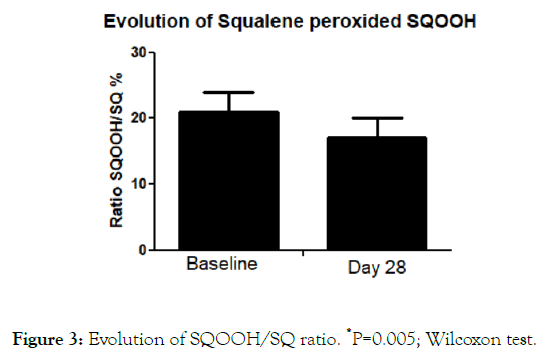
Figure 3. Evolution of SQOOH/SQ ratio. *P=0.005; Wilcoxon test.

Figure 4 High resolution pictures of two subjects evaluating skin appearance (A and A1: Baseline, D0; B and B1 After treatment, D28).
The World Health Organization [22] affirms that 93% of the population worldwide is exposed to high levels of outdoor and/or indoor pollution. Air pollution exposure is associated with premature skin aging, wrinkles formation, dark spots but also urticaria, and eczema [23]. Pollutant substances, such as PM10, PM2.5 and ozone, induce oxidative stress through the production of reactive oxygen species (ROS) and the secretion of pro-inflammatory cytokines such as TNF, IL-1, and IL-8 [24]. Exposure to air pollutants increases the production of dermal matrix metalloproteinases (MMP) including MMP-1, MMP-2, and MMP-9. The increase of MMP favors dermal collagen degradation [25]. Skin aging is speed up by air pollution substances through specific activation of intracellular receptors called AhR (Aryl-Hydrocarbon Receptor) [26]. Substances like PM10, PM2.5 and ozone can affect skin barrier functions, inducing oxidative stress, and inflammation [27]. A clinical trial has demonstrated that the exposure to high traffic air pollution is related to skin aging in light-skinned women [28,29]. A decrease content of squalene in the sebum, an increased lactic acid skin levels and an altered cohesion of stratum corneum have been observed in subjects living in high pollution area [30]. Benzopyrene, a potent pollutant substance, increases the skin amount of squalene peroxides. This modification contributes to skin hyperpigmentation and dark spot formation [31]. Pham et al. have demonstrated that oxidized squalene is a sensible marker of environmental pollution [31]. In our study, in accordance with a previous clinical experience, we demonstrated that the use of DAE/FA/VC serum can decrease the formation of squalene peroxides at skin level, reducing the ratio SQOOX/SQ. SQOOX is believed a secondary mediator of environmental-induced stress on the skin [32,33]. Many commercialized cosmetic products proclaim anti-pollution effects [34]. However, these claims should be supported and proved by specific in vitro and in vivo testing [35]. The serum we have used in this trial contains Deschampsia antartica aqueous extract, vitamin C and ferulic acid. in vitro experiments have shown that Deschampsia antartica extract can reduce the pollutant induced AhR activation [9]. This extract is able also to reduce and attenuate the negative effect of tobacco smoke, heavy metals and oxidant substances on cell viability of cultivated human dermal fibroblasts [36]. Ferulic acid is a potent plant antioxidant and it can improve the chemical stability of vitamins C [37]. Topical vitamin C has a relevant anti-oxidant and lightening effect. In our study we observed that the 28-day use of DAE/FA/VC serum strengths skin barrier function and it reduces the oxidative stress of the skin induced by high tropospheric ozone levels. The main goal of the present trial was to evaluate the capability of the serum to protect the skin of subjects living in a high pollution environmental, during a time frame (summer season) characterized by high outdoor pollution (especially Ozone). During the clinical evaluation, high pollution days (Ozone exceeding 120 microg/m3) represented 71% of the entire study period. This further supports the fact that we tested the skin protective effect of this serum under an appropriate timeframe for assessing its real anti-pollution effect.
Twenty-eight-day use of a serum containing DAE/FA vitamin C and SCA significantly enhances skin barrier function, improving skin appearance and to counteract the skin oxidative stress in women living in high pollution urban area during summer months, characterized by elevated ozone levels.
Citation: Milani M, Piacentini M, Celleno L (2019) A Serum Containing Deschampsia antartica Extract, Ferulic Acid and Vitamin C has AntiPollutant Effects on Skin Exposed to High Tropospheric Ozone Levels: A Controlled Single-Blind, Prospective Clinical Trial in Women Living in Urbanized, High Air Pollution Area during the Summer Season. J Clin Exp Dermatol Res. 10:510. Doi: 10.35248/2155-9554.19.10.510
Received: 04-Oct-2019 Accepted: 18-Oct-2019 Published: 25-Oct-2019
Copyright: © 2019 Milani M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.