Advances in Automobile Engineering
Open Access
ISSN: 2167-7670
ISSN: 2167-7670
Research Article - (2022)Volume 11, Issue 2
Magnesium is a transition metal, which is an abundant and non-toxic element. Hence magnesium is suggested for biomedical applications. But the problem is due to its poor mechanical and corrosion property, magnesium is not suggested for heavy load applications like automobile and aerospace industries. Hence, researchers should alleviate the poor Magnesium’s mechanical and corrosion properties through alloying. In the automobile industry, Magnesium was used in chassis, engine cradle, seats, and etc. The application of magnesium in the aerospace industry is like some of the Mg alloys like HK31, HM21, HM31, HZ32, ZH42, and ZH62. These Mg-thorium alloys are being utilized for various military applications especially in missiles. Since Mg forms soluble non-poisonous corrosion products that can be harmlessly excreted, implants made of Mg do not require reoperation for the removal of implants like other metal implants. Further, it is also useful in brittle bone disease that the release of magnesium ions above degradation can revive the spreading of brittle bone cells. Similarly, the elastic modulus of Mg (~45 Gpa) compared to various metal material implants like titanium (~100 Gpa) and stainless steel (~205 Gpa) and closer to that of natural bone (~20 Gpa) and it helps reduce the stress shielding phenomenon in implants. In this regard, an attempt is made to summarize the applications that function with the help of Magnesium alloys. Also, Fu-Sheng Pan, Chongqing University, China is in first place with 642 publications in Magnesium alloys and its applications.
Magnesium is a shiny silver-white metal from group 2A alkaline earth metals of the periodic table. So that it is harder metal and less reactive. Magnesium has a high melting point of 650°C. It has an HCP crystal structure. So, in general, having low ductility which does not deform easily, and is extremely brittle. It is the lightest metal of having a low density of 1.74 g/Fcc which is 78% and 35% lighter than iron and aluminum per unit volume [1]. Magnesium and aluminum have similar properties as shown in Table 1. Magnesium is richly available in the earth’s crust of 2.3% [2].
| Properties | Magnesium | Aluminium |
|---|---|---|
| Atomic number | 12 | 12 |
| Atomic weight | 24.32 | 26.98 |
| Crystal structure | HCP | FCC |
| Density | 1.74 | 2.7 |
| Melting point | 650 | 660 |
| Boiling point | 1105 | 2520 |
| Thermal expansion coefficient (µm/mk) | 25.5 | 23.6 |
| Elastic modulus (GPa) | 45 | 69 |
| Tensile strength (MPa) | 240 | 320 |
| Specific strength (kNm/kg) | 35-260 | 7-200 |
| Specific stiffness (MNm/kg) | 21-29 | 25-38 |
Table 1: Prevalence of excessive vaginal bleeding before and after delivery by different socioeconomic and demographic characteristics.
Magnesium was first discovered as an element by Joseph Black in the year 1755 at Edinburgh. A pure and small quantity of magnesium was first isolated by an English chemist sir Humphry Davy in 1808 through the electrolysis of magnesium oxide and mercuric oxide. Later in the year 1831, a French scientist Antoine-Alexandre-Brutus Bussy made a hefty quantity of metal through magnesium chloride with potassium and discovered its properties [3].
Magnesium is an eco-friendly and biodegradable material that is environmentally safe and is used in multi-medical applications [4]. In the human body several cases like dental issues, bone fractures, censorious wounds, and ischemia heart diseases suffered by the patients need help to recover. Ischemia heart disease or coronary heart disease is treated with a coronary stent device which is used to open the arteries to supply wealthy oxygen blood to the heart [5] and restenosis will take time within 6 months. And these stents are also made of magnesium alloy AE21. Magnesium stents were first implanted to the coronary arteries in the year 2003, containing all-alloy AE21 (2% Aluminium and 1% rare earth) [6]. Mg is fixed at 8 most abundant element by mass and placed at 7 position with by molarity in the earth’s crust as shown in the pie chart below.
Magnesium is largely available in minerals (Figure 1) like brine, carnallite, magnesite, and dolomite which are concentrated by conventional methods. It can produce in two methods; one is electrolysis of magnesium chloride through that liquid state magnesium is found. Another one is magnesium ores are reduced through a reactant. Some of the countries of countries like China, the USA, Turkey, Russia, Kazakhstan, Israel, and Brazil produce a large amount of magnesium. Magnesium chloride gets from the sea which contains trillion tones of mg. Only by this source per annum 8,50,000 tons of magnesium is produced.
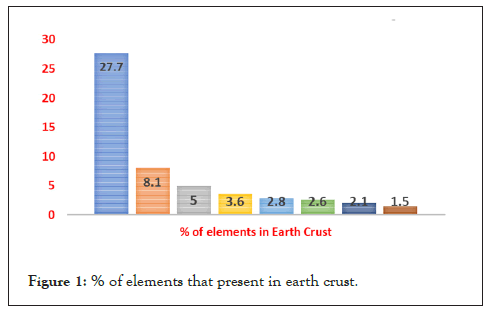
Figure 1: % of elements that present in earth crust.
Scheme of this investigation
The following pattern was followed in this investigation to review the applications of Magnesium in various industries as follows; they are,
• Mg alloys in Automobiles
• Mg alloys in aerospace
• Mg alloys in biomedical implants
Magnesium alloys in automobiles: Nowadays reduction of weight in automobiles is crucial to improve fuel efficiency and corrosion resistance better performance in our surrounding environment. We have been using high-strength steels like 304 and 316 stainless steels one is best for heat resistance and another for corrosion resistance. Some of the metals like aluminum, stainless steel, and other polymers are being used for the reduction of mass.
By additional use of this low-density magnesium alloys will receive higher benefits in the automotive industry [7]. Some of the automotive applications of magnesium alloys are AZ91D (Mg-9Al-1Zn) which is widely used in the automotive industry because of its better corrosion resistance, superb die-cast ability, and fit for room temperature. So, this alloy is used as crankcase, steering box, steering column, brake pedal, manual transmission case, drive brackets and sub frames, etc. as shown in Figure 2. Magnesium alloys like AM60, AM50, and AM20 are having high ductility strength, better corrosion resistance and are good for die cast ability. AM50 and AM20 are used as seat frames in Daimler Benz 500/600 and these alloys are mostly used in internal parts of a car. AM60 alloy is used as wheels in Porche. Some of the magnesium alloys like AE42 AS21 are having high creep resistance with perfect strength [8]. Several mechanical and corrosion properties of magnesium alloys are shown in Table 2.
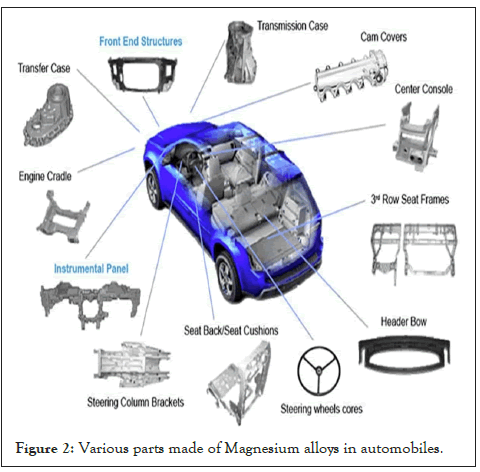
Figure 2: Various parts made of Magnesium alloys in automobiles.
| PROPERTIES OF MAGNESIUM ALLOYS | |||||
|---|---|---|---|---|---|
| MECHANICAL PROPERTIES AND CORROSION PERFORMANCE | |||||
| ALLOY | T.S (MPa) | Y.S. (MPa) | E (%) | CREEP (%) (150C, 35MPa, 200hr) | CORROSION (mg/cm2/day) |
| A380 | 325 | 160 | 3 | 0.19 | |
| AZ91D | 240 | 165 | 3 | 2.54 | 0.11 |
| AM60 | 220 | 130 | 6 | 2.15 | 0.13 |
| AM50 | 200 | 125 | 06 to 10 | 2.15 | 0.22 |
| AM20 | 135 | 105 | 10 | ||
| AS41 | 225 | 135 | 4.5 | 0.25 | |
| AS21 | 170 | 110 | 4 | ||
| AE42 | 225 | 140 | 8 to 10 | 0.33 | 0.21 |
| ZC63* | 240 | 145 | 5 | ||
| ZE41* | 180 | 135 | 2 | ||
Table 2: Convair XC-99 used in between 1940 to 1950.
In 1936, mg alloys were first used in the powertrain to Volkswagen beetle model and between 1950 to 1979 the sales were huge. Magnesium alloys were used as engine blocks to Porsche in the year 1968. Depending upon the process of operations magnesium alloys are divided into two groups. Those are Cast alloys and Wrought alloys.
We need certain alloys for particular operations and selection of these alloys is important for good strength, better resistance of heat, and surrounding environment. A few wrought and cast mg alloys are divided as shown in Table 3.
| Wrought Magnesium alloys | Cast Magnesium alloys |
|---|---|
| AZ91, AM50, AM60, ZK51, ZK61, ZE41, ZC63, HK31, HZ32, QE22, QH21, WE54, | ZK60, M1A, HK31, HM21, ZE41, ZC71, Elektron675, AZ31, AZ61, AZ80. |
| WE43, Electron 21, AZ63, AZ81. |
Table 3: Some of wrought and cast alloys.
Magnesium alloys in aerospace industry: Demand for Mg in the aerospace industry is ultimately increasing worldwide due to the lightest structural metal which is needed in certain areas in aerospace. The metal of lightweight with good strength has widespread use in the different products.
It is one of the largest service industries which serve many functions like military warplanes and helicopters, missiles, spaceships and rockets, commercial airlines and aviation’s, etc. In 1947 a flight named Convair XC-99 as shown in Figure 3 which was the largest airplane made by Convair to the US air force. It serves its great performance between 1940 to 1950 and this aircraft higher amount of Mg alloy used in its airframe as a coating [9]. In the year 1946, the Convair aircraft manufacturing industry built The Convair B-36 ‘’Peacemaker’’ which serves its best between 1949 to 1959 and used magnesium alloys of about 8600 Kg as shown in Figure 4.

Figure 3: Convair XC-99 used in between 1940 to 1950.
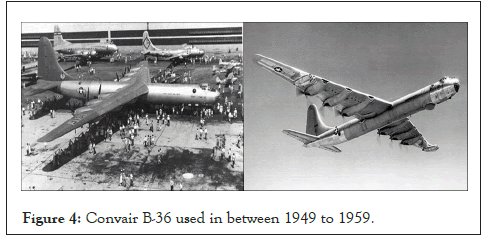
Figure 4: Convair B-36 used in between 1949 to 1959.
A strategic bomber named Tupolev TU-95 is shown in Figure 5. Which is under the missile platform used an average of 1550 Kg of magnesium alloys and serves to the Russian Air force, Soviet Air force and Navy. Also Figure 6 CIM-10A Bomarc missile, which major components were made of Mg composites.

Figure 5: Tupolev TU-95.
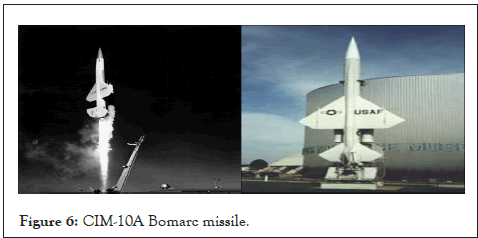
Figure 6: CIM-10A Bomarc missile.
As a result of good damping capacity and having low density (will result from y low weight). Some of the parts like outer shells, control surfaces, and wings are made of Mg alloys [1]. Some Mg alloys like HK31, HM21, HM31, HZ32, ZH42, and ZH62. These Mg-thorium alloys are being utilized for various military applications especially in missiles.
CIM-10A Bomarc missile was the ramjet components is a supersonic surface to area missile (SAM) as shown in Figure 5. It is the US Air force missile used in the cold war [10]. In this missile, Mg thorium is used in engine construction because of its properties of having high strength and less density and better creep resistance of 350°c. Due to this creep resistance, these alloys cannot stay for a longer period and also because of thorium's radioactivity. Also, in Boeing 727 aircraft, magnesium alloys are used and made approximately 1200 parts [2].
Magnesium alloys in biomedical implants: During recent researches and due to its decomposable properties Mg and human bones are having similar density Table 3. This is used in different biomedical implants [11]. Biomedical implants are the instruments used in medical applications to provide support or altering the damaged parts of the human body. As regards the use of Mg wire bonds as human medical implants to reduce bleeding was first proved scientifically by Edward Chuse Payr in 1878.
Since Mg forms soluble non-poisonous corrosion products that can be harmlessly excreted, implants made of Mg do not require reoperation for the removal of implants like other metal implants [12]. Further, it is also useful in brittle bone disease that the release of magnesium ions above degradation can revive the spreading of brittle bone cells. Similarly, the elastic modulus of Mg (~45 Gpa) compared to various metal material implants like titanium(~100 Gpa) and stainless steel(~205 Gpa) and closer to that of natural bone (~20 Gpa) and it helps reduce the stress shielding phenomenon in implants (Table 4) [2].
| Materials | Density (g/cc) | Elastic modulus (GPa) | Compressive yield strength (MPa) |
|---|---|---|---|
| Natural Bone | 1.8-2.1 | March-20 | 130-198 |
| Ti alloys | 4.4-4.5 | 100-117 | 758-1117 |
| Co-Cr alloy | 8.3-9.2 | 230 | 450-1000 |
| Stainless steel | 7.9-8.1 | 189-205 | 170-310 |
| Magnesium | 1.74-2.0 | 41-45 | 20-120 |
| Hydroxyapatite | 3.1 | 73-117 | 600 |
Table 4: Properties of bio medical implants.
Corrosion and bio degradability of mg based materials
The corrosion functioning in both Vivo and Vitro atmosphere is a difficult phenomenon for bio decomposable implants in clinical uses. The main purpose is to promote bio decomposable implants which melt in the body after the work is done. We have observed that pure Mg and popularly available alloys need some more research in it [9].
Mg-based metal and alloys have been used in biomedical applications since 1878 [4]. Mg metals and alloys are used to develop circulatory stents and can reach required angiographic results after completing 4 months and safe desorption [1]. The reasonable period for coronary stents to complete the renovation process of arterial vessels and demean with ideal mechanical integrity is from 6 to 12 months [3]. There are different bio-absorbable stents used in clinical applications. To recover the function of diseased arteries are shown in Figure 7.
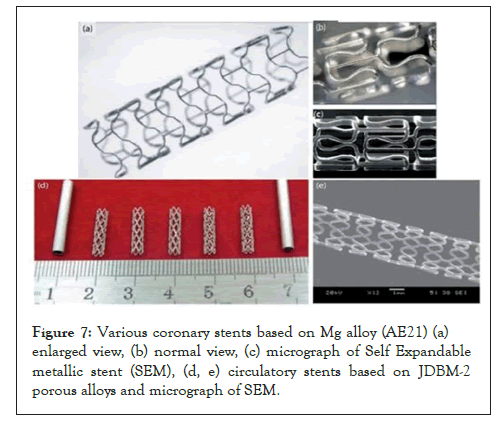
Figure 7: Various coronary stents based on Mg alloy (AE21) (a) enlarged view, (b) normal view, (c) micrograph of Self Expandable metallic stent (SEM), (d, e) circulatory stents based on JDBM-2 porous alloys and micrograph of SEM.
Various Mg based biodegradable orthopedic implants [5], implants for orthopedic applications such as
• Screws (Mgca 0.8 alloy)
• Plates (ZEK 100 alloy)
• Nails (LAE 442 alloy)
• Micro clips for laryngeal surgery
• Wound closing instruments
Dental implants for replacing teeth are shown in Figure 8.

Figure 8: (a) different types of Mg based orthopaedic implants for fixation of bone, (b, c, d) bio-degradable orthopaedic implant, (e) micro clips for laryngeal microsurgery, (f) wound VAC device, (g) dental implants.
In absence of nonorganic elements, buffer system and natural ingredients in the stimulated body [12]. The difficulty of Mg degradation under physiological terms is shown in Figure 9. Figure 9 shows the schematic representation of degradation nature in the physiological atmosphere by producing different corrosion products [12].
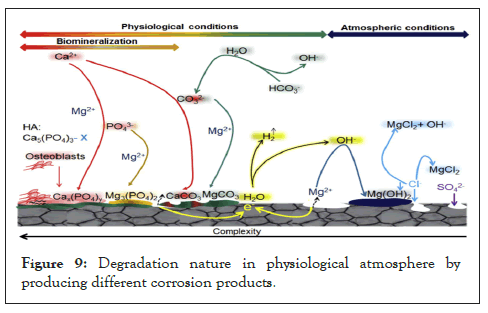
Figure 9: Degradation nature in physiological atmosphere by producing different corrosion products.
Impact of magnesium alloys
Figure 10 shows the schematic representation of articles published in Magnesium alloys and its applications from the year 2012 to 2020. The data is obtained from the diemensions.ai. From Figure 10 it was clearly understood that the articles published in Magnesium alloys were kept on increasing by year by year. This shows the importance Magnesium alloys for industrial scale-up. Also, Table 5 shows the top 10 researchers who published articles in Magnesium alloys for the past decade. Table 5 is composed of the name of the researcher and their respective affiliation, the number of publications, citations, and citation mean. From Table 5 it was found that Fu-Sheng Pan, Chongqing University, China is in first place with 642 publications in Magnesium alloys and its applications.
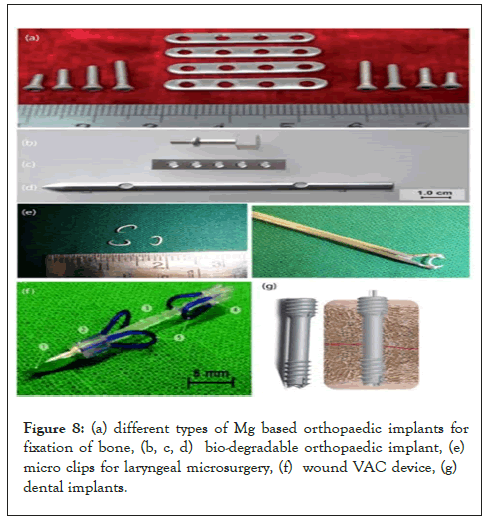
Figure 10: Articles published in Magnesium alloys and its applications from the year 2012-2020.
| S. No | Name Organization, Country | Publications | Citations | Citations mean |
|---|---|---|---|---|
| 1. | Fu-Sheng Pan (Chongqing University, China) | 642 | 10,811 | 16.84 |
| 2. | Manoj Kumar Gupta (National University of Singapore, Singapore) | 459 | 11,092 | 24.17 |
| 3. | Wen-Jiang Ding (Shanghai Jiao Tong University, China) | 455 | 10,477 | 23.03 |
| 4. | Karl Ulrich Kainer (Helmholtz-Zentrum Hereon, Germany) | 330 | 10,249 | 31.06 |
| 5. | Norbert Hort (Helmholtz-Zentrum Hereon, Germany) | 294 | 7,987 | 27.17 |
| 6. | Terence George Langdon (University of Southampton, United Kingdom) | 290 | 18,695 | 64.47 |
| 7. | Yu-Feng Zheng (Peking University, China) | 248 | 13,968 | 56.32 |
| 8. | En-Hou Han (Institute of Metals Research, China) | 244 | 8,724 | 35.75 |
| 9. | Bin Jiang (Chongqing University, China) | 221 | 3,057 | 13.83 |
| 10. | Xiao-Qin Zeng (Shanghai Jiao Tong University, China) | 215 | 4,980 | 23.16 |
Table 5: Researchers published paper in Magnesium alloys and its applications from 2012 to 2021.
This review paper summarizes the applications of Magnesium and its alloys in automobiles, aerospace, and biomedical. Research and development of magnesium-based composites and alloys led to extend the novel magnesium materials in engineering and biomedical applications. From the literature review it is clearly understood the importance of Mg based alloys in various applications. Further, it was found that Fu-Sheng Pan, Chongqing University, China is in first place with 642 publications in Magnesium alloys and its applications followed by Manoj Kumar Gupta, Wen-Jiang Ding. Moreover, magnesium-based alloys and composites replace aluminum-based alloys and composites and steels in many engineering applications which building the path for a multimillion dollar market.
Declaration of conflicting interests
There are no relevant financial or non-financial competing interests to report.
Funding
No funding was utilized for this investigation.
Citation: Sivamaran V (2022) A Review on Magnesium-based Materials in Automobile, Aerospace, and Bio-Medical Applications. Adv Automob Eng. 11:185.
Received: 02-Feb-2022, Manuscript No. AAE-22-48701; Editor assigned: 04-Feb-2022, Pre QC No. AAE-22-48701 (PQ); Reviewed: 18-Feb-2022, QC No. AAE-22-48701; Revised: 22-Feb-2022, Manuscript No. AAE-22-48701 (R); Published: 01-Mar-2022 , DOI: 10.35248/2167-7670.1000185
Copyright: © 2022 Sivamaran V. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.