Current Synthetic and Systems Biology
Open Access
ISSN: 2332-0737
ISSN: 2332-0737
Review Article - (2023)Volume 11, Issue 2
Cryopreservation is a process that preserves structurally intact live cells and tissues by freezing them at tremendously low temperatures for prolonged periods. Ice recrystallization is the major restraint in the cryopreservation of cells, resulting in lethal cryoinjury. Ice Recrystallization Inhibitors (IRIs), low molecular weight cryo-protectants, restrict the ice growth and alleviate cell injury during the process of freezing. They may possess the ability to enter the cells and alter their cryo-biological response to freezing and influence intracellular ice recrystallization as well. In the presence of abridged quantities of all CPAs including glycerol, ice recrystallization inhibitors can shield the cells against cryoinjury during the recrystallization process and can also be used as effective cryo-additives to freeze cells significantly Red Blood Cells (RBCs). One efficient cryo-additive reticent ice nucleation rather than ice recrystallization indicates the pertinence of blocking both processes along with underlining the relevance of this evolving class of Cryo-Protective Agents (CPAs). Cryopreservation approaches for certain cells i.e. RBCs have been revealed to be extremely successful and extensively applied but for other cells have proven to be challenging. Subsequently, this is due to the multifaceted nature of the freeze-thaw injury, the intricacy of preserving organic matter, and the erraticism of procedures and regulations used across the world. This review will focus on the cryopreservation of RBCs in the presence of Ice Recrystallization Inhibitors (IRIs).
Cryopreservation; Ice recrystallization inhibitors; RBCs; Cryo-protective agents; Cryoinjury
Since the nineteenth-century low temperature cell storage has been studied. Numerous breakthroughs in cryopreservation have occurred since that time, and protocol optimization remains a major research topic. Various types of cells and tissues are preserved in order to perform scientific research, as well as utilization in the medical field such as hepatocyte and pancreatic islet transplantation, blood transfusion, bone marrow transplantation, artificial insemination, and fertilization outside the body, are all possible with cryopreservation [1]. Cryopreservation techniques have made it possible that large amounts of the required cell types to be kept on standby and accessible for use on demand.
The demand for cryopreservation has been progressively high in many fields including biology, and medicine. At low temperatures, the deterioration rate is declined but the lifespan of cells can be protracted via freezing. Long-term cryopreservation of cells is also required to maintain their bioactivity though, the cells and tissues of the organisms are easily damaged during the process of freezing with osmotic and mechanical injury [2]. Osmotic injury refers to the damage of cells by osmotic dehydration and it is caused by the freezing of extracellular solution which leads to enhancing the rate of concentration of the solutes. While mechanical injury is the puncture injury or damage of cells via sharp ice crystals. These two types of injuries i.e., osmotic and mechanical confine the cryopreservation development.
Cooling rates (fast/slow)
There is a variety of cryo-injury mechanisms but usually, cells that possess highly permeable membranes need to be cooled at faster rates while cells with less permeable membranes tend to be cooled at much slower rates. Depending on the type of cell, cryopreservation is achieved with either slower or fast cooling rates. But in most cases, ice will prefer to form the cell’s exterior.
Cryopreservation principles
In order to properly and accurately comprehend the function of cryo-protective agents, the impact of subzero temperatures on normally healthy tissue must be understood. Cells are usually considered fatal without cryo-protectants when exposed to below 0°C temperatures. Because both intracellularly and extracellularly water accounts for about 80% of tissue mass and freezing water, has the greatest impact on unfavourable biochemical as well as structural variations that are predicted to cause unsafe freezing damage. Almost two theories are independent and elucidate the detrimental freezing effects. In the first one, ice crystals automatically shatter cell membranes, rendering structurally whole cells hard to attain and subsequently thawing, and in the second one, during cooling, as ice crystals develop within the cell, devastating increases in the concentration of solutes happen to the prevailing liquid phase. The final consequence is the same whether the automatic freezing effects are dominant; unsafe cellular cooling and thawing are irreconcilable methods with life. Utilization of cryoprotectants, as well as the assortment of an adequate cooling and thawing pace, are the two important precautionary activities that must be taken into consideration to limit these effects [3-5].
Approaches to cryopreservation
All biopreservation methods strive to keep living tissues stabilized and alive via constraining metabolism and significantly slowing down chemical as well as metabolic processes that cause deterioration during ex-vivo storage.
Short-term preservation: Short-term preservation of living cells that cannot be properly cryopreserved because of too much damage at deep-subzero temperatures can be accomplished at temperatures a few degrees above freezing (4°C for a few hours). This has provided ways for preserving living tissues and organs that have so far proven refractory to cryopreservation methods effectively developed for single-cell systems.
Long-term preservation: Long-term preservation necessitates significantly lower temperatures (-196°C or even lower for days or hours) as compared to short-term preservation and in the proximity of cryo-protective agents, it requires the tissue to endure the inflexibilities of transfer of heat and mass in the course of protocols intended to enhance cooling and warming.
Ice recrystallization
Ice growth is a key issue during the cryopreservation of clinically significant biomolecules that is not hindered by traditional solvent-based CPA. The ice crystallization rate is maximum at 0°C and -10°C so its detrimental influence is most noticeable during the thawing phase of cryopreservation.
The process of growth, as well as recrystallization, is increased by the diffusion of unfrozen water from the serum phase to the crystal phase, during storage. The rate of the crystallization process is determined by the unfrozen phase's viscosity. Ice crystal formation of highly viscous solutions is very low due to their lower temperature and lower kinetic energy. Recrystallization is primarily caused by two processes: Accretion and migration [6]. The joining of two or more adjacent ice crystals to form a single, larger crystal is known as accretion. Because ice crystals do not move, this process prompts their close vicinity. The melting of smaller crystals and the movement of the melted liquid to the surface of larger crystals constitute migration. The temperature of the product affects this mechanism.
Increased temperature leads to the liquefaction of the ice crystals either partially or completely, however reduction in temperature again caused the freezing of the liquid on the larger crystals. The rate at which water molecules diffuse to the larger ice crystal surface, identified as diffusion kinetics, has a significant impact on migration. The crystals' movement or diffusion is heavily influenced by the viscosity of the serum phase. Higher viscosity leads to a lower diffusion rate [7].
Cryo-Protective Agents (CPAs)
Cryoinjury caused by freezing and thawing is reduced by the utilization of Cryo-Preservative Agents (CPAs). Maintenance of cell volume and reduction in the osmotic imbalances is occurred by the CPAs such as glycerol and dimethyl sulfoxide during cryopreservation as these CPAs have the ability to cross the cell membrane [8]. Non-permeable CPAs such as Hydroxyethyl Starch (HES), Poly Ethylene Glycol (PEG), Poly Vinyl Pyrrolidone (PVP), and various sugars (trehalose and dextran) do not easily cross the cell membrane so they have the role of stabilizing cell membranes in the process of cryopreservation (Table 1).
| Cryo-protective Agents (CPAs) | Types | Structure | Properties | Citations | |
|---|---|---|---|---|---|
| Permeating cryo-protective agents | Dimethyl Sulfoxide (DSMO) | 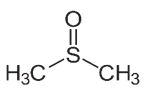 |
The molecules that easily cross the cell membrane. | Squires, et al. | |
| Ethylene glycol | 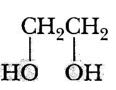 |
||||
| Glycerol | 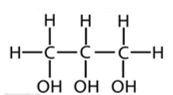 |
||||
| Non-permeating cryo-protective agents | Osmotically active | Sucrose | 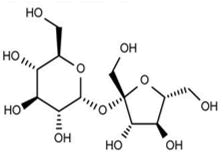 |
The molecules that do not easily cross the cellular membrane. | Oldenhof, et al. |
| Trehalose | 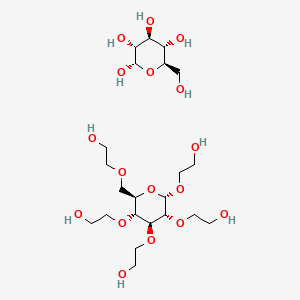 |
||||
| Osmotically inactive | Hydroxyethyl starch | 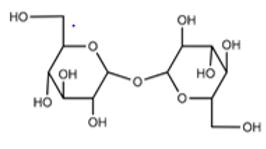 |
|||
Table 1: Classification of cryo-protective agents, their structure and properties.
Significant quantities of glycerol are often used for the cryopreservation of human RBCs in North America and almost all of Europe. Glycerol at 40% (w/v) combined with a slow cooling rate of 1°C/min hinders cell shrinkage and retains the volume of the cell. Nevertheless, in order to prevent intravascular hemolysis, the glycerol CPA should be removed due to transfusion [9-10]. This procedure is time-consuming and necessitates the use of specialized tools, and the washed RBCs have a shelf life of about twenty-four hours if performed in an open system.
Ice Recrystallization Inhibitors (IRIs)
The capability of biological antifreeze to prevent cryoinjury on exposure to cold temperatures caused them to become of greater interest in science and industries. The complexity of their structures made it challenging to recognize their procedure of inhibition of ice crystallization. However, these chemicals are the base of the newly designed ice crystallization inhibitors including along with the more recently reported small molecule ice recrystallization inhibitors.
Anti-Freeze Proteins (AFPs)
Several antifreeze solutions are evolved by the nature to reduce the process of recrystallization in which proteins and glycoproteins are included which are formed from plants, insects as well as fish. Although AF(G)Ps have the intrinsic potential to block ice recrystallization, their successful implementation as cryo-protectants to a wide range of cells ex-vivo has been limited due to their secondary property, Dynamic Ice Shaping (DIS). The morphology of ice crystals is altered by the effect produced by the connection of AFPs and AF(G)Ps with the ice crystals lattice [11]. The interaction of AFPs and AF(G)Ps with the ice crystal lattice results in a (concentration-dependent) effect that changes the ice crystal morphology. These morphological changes result in "needle like" structures that cause extensive mechanical damage, cancelling out the cryo-protective benefits of Ice Recrystallization Inhibition (IRI).
| Classification | Mass | Amino acids | Properties | Source | Diverse structure | Roles | Authors |
|---|---|---|---|---|---|---|---|
| AFGP | 2.6-33 kDa | 3 | Repeating three-residue peptide | Antarctic notothenioides | 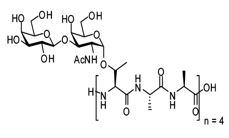 |
Inhibits bacterial ice nucleators. | Parody-Morreale, et al.; Tejo et al. |
| Type I | 3.3-4.5 kDa | 37 | Straight α-helix, high alanine residue content | Flounders, sculpins |  |
Maintain the transmembrane ionic gradient and improve cryopreservation of cells and tissues. | Arav, et al.; Kim, et al. |
| Type II | 11–24 kDa | – | Globular cysteine rich | Sea raven, smelt, herring, long snout poacher |  |
Infrequent in cryopreservation due to association with cytotoxicity in cells, tissues, and organs. | Wang, et al.; Kim, et al. |
| Type III | 6.5–14 kDa | no amino acids | no cysteine residues | Antarctic eelpout (Macrozoarces americanus), wolf fish |  |
Improves antifreeze activity and protects against freezing damage. | Bagis, et al. |
| Type IV | 12 kDa | 108 | Enriched in glutamine and glutamate | Longhorn sculpin |  |
Eskandari, et al.; Bang, et al. |
Table 2: Comparison between the characteristics of Anti-Freeze Glycoproteins (AFGPs) and Anti-Freeze Proteins (AFPs type I-IV).
These side effects have resulted in inconclusive results, with failures to improve rat cardiac explant and mouse spermatozoa cryopreservation. Beneficial effects, on the other hand, have been obtained with rat islets and rat hepatocytes (Table 2).
Cryopreservation of RBCs by using IRIs
For the preservation (as of cells) by subjection to extremely low temperatures of RBCs, HES, a non-permeable CPA is an effective cryo-protectant. It has been established that RBCs cryopreserved with 11.5 percent (w/w) HES can be transfused without the need for post-thaw HES removal [12-14]. In cases of substantial blood loss due to trauma or surgery, HES is routinely used as a blood plasma volume expander to replenish lost volume in the circulatory system. Because α-amylase degrades HES in the bloodstream, it can be given transferred with RBCs, removing the need for post-thaw processing before transfusion. However, ultra-fast cooling rates and extremely low storage temperatures (-165°C or -195°C) are required to enable high RBC recovery [15].
Ice formation and ice recrystallization are two of the most common causes of cryoinjury. Glycerol and DMSO, for example, are CPAs that do not control ice development. As a result, the capacity to prevent ice recrystallization would be a highly desirable characteristic in a CPA. Antifreeze glycoprotein analogs with carbon-linked (c-linked) chains have previously been shown to suppress ice recrystallization in our lab. Cryo-protectants have been found to be successful using these complicated, high molecular-weight analogs with "customtailored" antifreeze action. However, because of the significant production required for the creation of large amounts for cryopreservation applications, their usage as CPAs has been limited.
It has been discovered that several tiny carbohydrate based compounds can act as Ice Recrystallization Inhibitors (IRIs). These small-molecule IRIs have been demonstrated to be useful cryo-additives for freezing human RBCs, boosting the proportion of intact red blood cells after thawing in the presence of lower glycerol (15 percent w/v) concentrations. Prior to this discovery, RBC cryopreservation with 15% glycerol and moderate cooling rates yielded insignificant RBC recovery [16]. While employing IRIs with only 15% glycerol provides for a much faster deglycerolization time, frozen RBC units still need to be deglycerolized and cannot be transfused immediately.
RBC transfusions can help patients with leukemias, hemolytic anemias, and traumas that cause significant loss of blood. RBC transfusions can help patients with leukemias, hemolytic anemias, and traumas that cause significant loss of blood. Cryopreservation is the only technology that allows access to massive amounts of RBC units when large numbers of RBC transfusions are required. However, because current protocols do not allow for the direct transfusion of RBCs after thawing, cryopreservation of RBCs for normal transfusion is not a common practice.
When huge numbers of RBC transfusions are required, cryopreservation is the sole technology that allows access to enormous amounts of RBC units. However, because existing protocols do not allow direct transfusion of RBCs after thawing, cryopreservation of RBCs for normal transfusion is not a frequent practice. Clinical cryopreservation treatments use high concentrations of glycerol (40 percent v/v) as a cryo-protectant; nevertheless, to avoid intravascular hemolysis following thawing, time-consuming deglycerolization processes are required.
Although cryopreservation procedures for Red Blood Cells (RBCs) have shown to be effective and widely used, cryopreservation of other cells is still a challenge. This is due to the complexities of freeze-thaw damage, the difficulties of maintaining biological material, and the wide range of techniques and protocols applied. In many cases, ice recrystallization during thawing causes cellular damage to cryopreserved cells, tissues, and organs. Sugars, alcohols, and a variety of other small molecules are routinely used in combination to minimize these effects [17].
Briard, et al. demonstrated in 2016 that ice recrystallization inhibitors can minimize ice development in-vitro while using less glycerol and that this ability is linked to increased post-thaw survivability using anucleate human RBCs as a model. They further showed that these chemicals can protect cells from ice recrystallization induced damage during Transient Warming Events (TWEs). TWEs have also been linked to decreased postthaw viability in sperm, placental cord blood, peripheral blood mononuclear cells, and tissue allographs. These characteristics make small-molecule ice recrystallization inhibitors extremely desirable as novel cryo-protectants with a distinct mechanism of action.
The importance of small molecule IRIs for the freezing of human RBCs was established by Poisson, et al. in 2017. Human RBCs can be frozen using HES and a slow freezing rate (5°C/min) employing small-molecule IRIs, resulting in quantifiable post-thaw recovery and RBCs that can be immediately transfused without washing [18]. These findings highlight the necessity of developing new cryo-protectants that can prevent ice formation (recrystallization) during freezing, and suggest that these compounds could be used in a variety of cryosolution formulations.
Transplantation of Hematopoietic Stem and Progenitor Cell (HSPC)
Patients with hematological cancers and nonmalignant hematological diseases such as hemoglobinopathies, immunodeficiency syndromes, and hereditary metabolic abnormalities can benefit from the transplantation of Hematopoietic Stem and Progenitor Cells (HSPC) products. Furthermore, HSPCs are being employed in a growing number of unique and emerging regenerative therapies, such as the treatment of neurological illnesses, heart disease, and diabetes. Hematopoietic stem cell transplantation using Umbilical Cord Blood (UCB) can be associated with delayed neutrophil and platelet engraftment when compared to Bone Marrow (BM) or mobilized peripheral blood due to the limited volume that can be collected and the resulting lower dosage of cells. UCB, on the other hand, has various advantages, including quick availability, lower risk of Graft Versus Host Disease (GVHD), and a slight risk to donors as well as fewer HLA-matching requirements.
UCB units are treated and kept at -196°C for future use, even though stem cell grafts from adult donors are collected and injected as rapidly as feasible without cryopreservation. Furthermore, in autologous hematopoietic transplantation, patients' cells are harvested, cryopreserved, and reinfused after conditioning to restore hematopoiesis. Cryopreservation raises a number of concerns that could jeopardize transplantation success. First, HSPCs are cryopreserved with dimethyl sulfoxide, a cryo-protectant (DMSO). The DMSO in the thawed HSPC product has been demonstrated to have negative effects on the immune system. The digestive, renal, hepatic, central nervous, cardiovascular, and respiratory systems are all involved. The removal of DMSO before transfusion has been used in an attempt to decrease toxicity. People with pre-existing illnesses are at more risk. However, Yang, et al. found that eliminating DMSO multiple times after regular interval washing reduces viability and recovery from UCB CD34+ cells. The second concern with cryopreservation is that after cryopreservation, substantial quantities of apoptotic CD34+ cells (up to 30%) are found, which is linked to a lower ability to engraft. Furthermore, Sasnoor, et al., discovered that cryopreservation reduces growth factor responsiveness, which is linked to a lower ability to proliferate and differentiate after thawing (Figure 1).
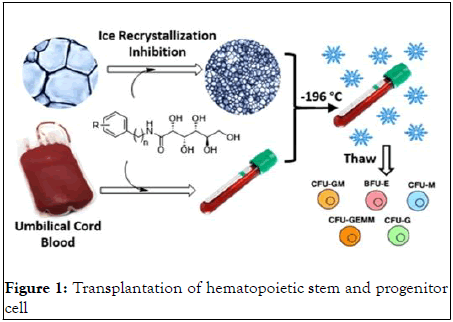
Figure 1: Transplantation of hematopoietic stem and progenitor cell.
The ability of prevention of ice recrystallization and protection from HSPCs from cryoinjury, a new class of cryo-protectants (N-aryl-D-aldonamides) was tested. A number of potent ice recrystallization inhibitors have been identified. When these nontoxic small compounds were added to the usual cryoprotectant solution, they improved the preservation of functionally divergent hematopoietic progenitors in colonyforming units and long-term culture-initiating cell experiments. The post-thaw recovery of myeloid progenitors was not improved by structurally similar chemicals that did not block ice recrystallization. These findings show that supplementing the cryopreservation solution with chemicals that can inhibit ice recrystallization improves the post-thaw function and potency of HSPCs in UCB. The rise of it could mean a lower chance of engraftment failure and more usage of cryopreserved organs [19].
Splat cooling method
The activity of ice recrystallization inhibitors is evaluated by a method termed as splat cooling method or assay. In the splat cooling method, an analyte is diffused in Phosphate-Buffered Saline (PBS) and 10 μL of this analyte droplet is released through a micropipette with the aid of a plastic tube (2 m in height and 10 cm in diameter) on a polished aluminum block. The droplet freezes immediately on the block of polished aluminum and the resultant wafer is gently detached from the block’s surface and then placed for annealing to a cryo-stage at -6.4°C [20]. Following 30 minutes, the wafer is photographed between crossed polarizing filters with a Nikon CoolPix 5000 digital camera attached to the microscope. Each wafer is imaged three times, and the procedure is carried out with two extra wafers. Ice crystals impulsively nucleate during freezing from the frost solution and these early crystals are tiny and reasonably homogenous in size. The phenomenon of recrystallization occurs during the annealing cycle, indicating a significant increase in the size of ice crystals. Domain recognition software was used to analyze the images of ice wafers. The activity of IRIs is expressed as a percentage of MGS (mean grain or ice crystal size) as compared to PBS control.
Ice recrystallization inhibitors possess the ability to permeate the cell membrane and mitigate the response of cells to freezing and thawing. Furthermore, these low molecular weight cryoprotectants can readily modulate the pace and degree of intracellular ice growth at subzero temperatures. These findings indicate the significance and potential of IRIs as novel cryo-protectants that have the ability to preserve the cells and tissues and restrict intracellular ice growth during freezing. The o-aryl-glycosides, a novel IRIs class, are effective cryo-additives useful for RBCs freezing with a decreased amount of glycerol. The production of these molecules lends itself to large-scale preparation for cryopreservation applications, intending to potentially improve cryopreservation techniques for therapeutically essential cells.
The authors declare no conflict of interest.
Declared none.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Sattar A, Ijaz H, Azam I, Idrees M, Azam M (2023) A Review of Cryopreservation of Cells by Ice Recrystallization Inhibitors (Iris). J Curr Synth Syst Bio. 11:031.
Received: 28-Dec-2022, Manuscript No. CSSB-22-21195 ; Editor assigned: 30-Dec-2022, Pre QC No. CSSB-22-21195 (PQ); Reviewed: 13-Jan-2023, QC No. CSSB-22-21195 ; Revised: 15-Mar-2023, Manuscript No. CSSB-22-21195 (R); Published: 22-Mar-2023 , DOI: 10.35248/2332-0737.23.11.031
Copyright: © 2023 Sattar A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.