Endocrinology & Metabolic Syndrome
Open Access
ISSN: 2161-1017
ISSN: 2161-1017
Research Article - (2019)Volume 8, Issue 1
Background: Primary Hyperparathyroidism (PHPT) is the third most common endocrine disorder, with an estimated prevalence of 1 to 4 per 1,000 in the general population. It is well established that vitamin D deficiency co-exists with PHPT. However, there are very few studies that have compared the prevalence of vitamin D deficiency between symptomatic and asymptomatic PHPT patients.
Aim: This research evaluated the prevalence of vitamin D deficiency in patients with PHPT, and compared the prevalence of vitamin D deficiency in patients with symptomatic and asymptomatic disease.
Methods: This observational cohort study employed a retrospective design where clinical records of 400 new patients referred to the metabolic bone clinics for investigation of hypercalcaemia, between 2010 and 2017, were reviewed. The study population was grouped as ‘asymptomatic’ or ‘symptomatic’ based on the absence or presence of at least one classical hypercalcaemia-related symptom.
Results: PHPT is more prevalent in women with female to male ratio of 4.4:1. Symptomatic patients were significantly younger compared to the asymptomatic group (60.97 year+15.356 vs 65.88 years+13.924, p=0.001). There was a high prevalence of vitamin D deficiency (64.25%) with no difference between the symptomatic and asymptomatic groups. The prevalence of osteoporosis was 53.35% whilst the prevalence of renal stone was 13.54% with no between group differences.
Conclusion: PHPT is more common in women than in men. Symptomatic patients were younger compared with those without symptoms. Vitamin D deficiency is highly prevalent in patients with PHPT regardless of whether the patients were symptomatic or not.
Primary hyperparathyroidism; PHPT; Hypercalcaemia
25(OH)D: 25-hydroxyvitamin D; BMD: Bone Mineral Density; BMI: Body Mass Index; CARMS: Clinical Audit Registration and Management System; CT: computed tomography; DR: Distal Radius; FN: Femoral Neck; g/L: Gram per Litre; HPT- Hyperparathyroidism; IOM: Institute of Medicine; kg: Kilogram; log: Logarithmic Value; LS: Lumbar Spine; m: Meter; mg: Milligrams; ml/min: Millilitre per Minute; mmol: Millimoles; mmol/L: Millimoles per Litre; MRI: Magnetic Resonance Imaging; n: Number of Patients; nmol/L: Nanomoles per Litre; PHPT: Primary Hyperparathyroidism; PTH: Parathyroid hormone; TH: Total Hip; UK: United Kingdom of Great Britain; UoW: University of Wolverhampton; US/USA: United States of America
Primary hyperparathyroidism (PHPT) is an ancient disease, with the earliest documented case discovered in an early Neolithic cemetery in Germany from a woman between 25 to 35 years of age [1]. PHPT is the most common cause of hypercalcaemia in which parathyroid hormone (PTH) is excessively secreted from one or more of the four parathyroid glands [2]. PHPT is diagnosed biochemically by the presence of hypercalcaemia, with increased or inappropriately normal plasma PTH level [3]. It is the third most common endocrine disorder, with an estimated prevalence 1 to 4 per 1,000 in the general population and an incidence of approximately 28 cases per 100,000 individuals [4-6]. PHPT can present at any age, with women three to four times more likely to be affected, particularly those between 50 and 60 years of age [7].
It has long been established that patients with PHPT have coexisting vitamin D deficiency [8]. Patients with PHPT also frequently have non-classical symptoms linked to PHPT that may be at least in part due to vitamin D deficiency. Treating coexisting vitamin D deficiency in patients with PHPT can sometimes improve symptoms prior to definitive parathyroid surgery. We aimed to explore whether symptomatic patients with PHPT were more likely than asymptomatic patients to be more deficient in vitamin D.
Currently there is a ubiquitous, but unsubstantiated, reluctance in providing adequate vitamin D supplementation in patients with PHPT, due to fear of aggravating hypercalcaemia [9]. This is despite convincing evidence of the benefits of vitamin D supplementation, particularly in reducing the degree of hyperparathyroidism and its associated symptoms [10]. Understanding the true prevalence of vitamin D deficiency amongst patients with PHPT has a significant implication to clinical practice as it will further support the recommendations from the ‘Fourth International Workshop on the Management of Primary Hyperparathyroidism’ [11]. In particular, this consensus statement emphasises the importance of a more proactive approach in evaluating end-organ involvement (osteoporosis and kidney stones), assessing vitamin D status, adequate vitamin D supplementation and timely surgical or medical management.
Research design and study samples
This observational cohort study employed a retrospective design in evaluating the prevalence of vitamin D deficiency in patients with PHPT. In this retrospective cohort study, patients with confirmed diagnosis of PHPT, from 2010-2017, were identified and the prevalence of vitamin D deficiency and symptoms of PHPT were reconstructed for analysis.
In total, 400 patients were included in the final analysis. Illustrated in Figure 1 below is the method of sample selection. Patients included in the study were those with confirmed diagnosis of PHPT as defined by one of the biochemical criteria below Bilezikian et al. [12]: high serum adjusted calcium level (>2.60 millimoles per litre [mmol/L]) and an elevated or inappropriately normal serum PTH levels and no evidence of secondary cause or normal serum adjusted calcium level (2.20-2.60 mmol/L) with a high PTH levels and no evidence of secondary cause.
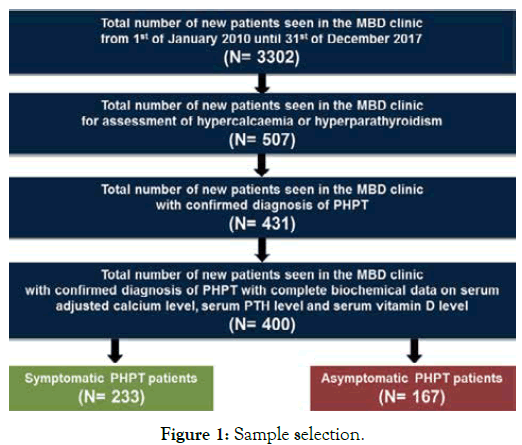
Figure 1. Sample selection.
The study population was grouped as “asymptomatic’ or ‘symptomatic’ based on the absence or presence of at least one of the following:
Generalised and musculoskeletal symptoms including: bone pains, muscle pains, joint pains, muscle weakness, fatigue/tiredness (which were not attributed to other medical conditions)
Gastrointestinal symptoms including: constipation, abdominal pains, gastric reflux, vomiting, and low appetite (which were not attributed to other medical conditions)
Renal symptoms including: osmotic symptoms of polyuria, polydipsia and nocturia (which were not attributed to other medical conditions)
Neurocognitive symptoms including: memory problem, poor concentration and sleep problems (which were not attributed to other medical conditions).
Statistical analyses
Statistical analyses were carried out using SPSS software version 22. Continuous variables were tested for normality using Shapiro- Wilks test. Data are presented as means ± standard deviations (SD) for continuous variables that are normally distributed and as medians with interquartile (25th and 75th percentile) range for continuous variables that are skewed. Between-group differences were analysed using independent-samples T test (e.g. symptomatic versus asymptomatic) and one-way ANOVA (e.g., vitamin D deficient versus insufficient versus sufficient). Categorical variables are presented as numbers of cases and percentages. Fisher’s exact test was used to compare categorical variables. Spearman’s Rho test was used to assess the correlation between the level of serum vitamin D and the level of serum calcium and PTH. The Jonckheere-Terpstra test was performed to assess the correlation between the level of serum vitamin D and the number of symptoms. All statistical tests performed were two-tailed with the minimum level of significant set at p<0.05.
Demographic of the study population
Table 1 summarises the general demographic characteristics of the 400 patients with confirmed diagnosis of PHPT. The majority of 400 patients were women (81.50%). The mean age of patients was 63.02+14.954 years with symptomatic patients significantly younger compared to those who were asymptomatic (60.97+15.356 years versus 65.88+13.924 years, p=0.001).
| Overall | Symptomatic | Asymptomatic | p value | |
|---|---|---|---|---|
| (n= 400) | -58.25% | -41.75% | ||
| (n= 233) | (n= 167) | |||
| Age | 63.02 (± 14.954) | 60.97 (± 15.356) | 65.88 (± 13.924) | 0.001 |
| Sex (Male) | 18.50% (n= 74) | 14.45% (36/233) | 22.75 (38/167) | 0.069 |
| Sex (Female) | 81.50% (n= 326) | 84.55% (197/233) | 77.25% (129/167) | |
| Weight (kg) | 75.26 (± 17.322) | 75.44 (± 18.107) | 75.02 (± 16.229) | 0.816 |
| Height (m) | 1.62 (± 0.093) | 1.61 (± 0.091) | 1.62 (± 0.096) | 0.779 |
| BMI | 28.81 (± 6.042) | 28.92 (± 6.330) | 28.65 (± 5.636) | 0.656 |
Table 1: General demographic characteristics of patients with PHPT.
Biochemical characteristics of patients with PHPT
The overall mean serum adjusted calcium level for the entire study population was 2.70+0.174 mmol/L with no significant difference between the symptomatic and asymptomatic groups (2.71+0.187 mmol/L versus 2.69+0.153 mmol/L, p=0.134) (Table 2).
| Normal value | Symptomatic | Asymptomatic | p value | |
| -58.25% | -41.75% | |||
| (n= 233) | (n= 167) | |||
| Adjusted calcium Level (mmol/L) | 2.20-2.60 | 2.71 (± 0.187) | 2.69 (± 0.153) | 0.134 |
| Serum PTH level | 1.60-6.90 | 1.08 (± 0.203) | 1.08 (±0.202) | 0.897 |
| (pmol/L) | (log value) | (log value) | ||
| 11.50 (2.10–54.80) | 11.60 (3.30–64.70) | |||
| (median) | (median) | |||
| 25 (OH)D level | >50 | 1.56 (± 0.272) | 1.59 (± 0.257) | 0.323 |
| (nmol/L) | (log value) | (log value) | ||
| 38 .00 (10.70-119.00) | 41.00 (13.00–140.00) | |||
| (median) | (median) | |||
| 24-hour urinary calcium excretion (mmol/24h) | 2.50-7.50 | 7.51 (± 3.492) | 6.38 (± 4.168) | 0.134 |
| (n=73) | (n=38) | |||
| Calcium/Creatinine | >0.01 | 0.665 (± 0.459) | 0.73 (± 0.502) | 0.652 |
| Ratio | (n=31) | (n=14) |
Table 2: Biochemical characteristics of patients with PHPT.
Clinical presentation and prevalence of vitamin D deficiency
Table 3 summarises the clinical symptoms reported by the 233 symptomatic patients. The most commonly reported symptoms were polydipsia (35.19% [n=82]), generalise aches and pains (33.48% [n=78]), tiredness/lethargy (29.18% [n=68]), polyuria (26.61% [n=62]) and constipation (25.32% [n=59]).
| Hypercalcaemia related symptoms | Frequency | Prevalence* |
|---|---|---|
| Polydipsia | 82 | 35.19% |
| Generalised aches and pains | 78 | 33.48% |
| Tiredness/Lethargy | 68 | 29.18% |
| Polyuria | 62 | 26.61% |
| Constipation | 59 | 25.32% |
| Nocturia | 40 | 17.17% |
| Bone Pain | 19 | 8.15% |
| Abdominal pain/discomfort | 19 | 8.15% |
| Depression/Anxiety | 12 | 5.15% |
| Memory problems | 6 | 2.58% |
Table 3: Prevalence of hypercalcaemic related symptoms in symptomatic patients PHPT (n=233).
Summarised in Table 4 below are the differences in the prevalence rates of vitamin D deficiency and end-organ complications in patients with symptomatic and asymptomatic PHPT.
| Overall | Symptomatic | Asymptomatic | p value | |
|---|---|---|---|---|
| (n= 400) | -58.25% | -41.75% | ||
| (n= 233) | (n= 167) | |||
| Vitamin D deficiency (%) | 64.25% | 65.24% | 62.87% | 0.673 |
| (25[OH]D of < 50 nmol/L) | (257/400) | (152/233) | (105/167) | |
| Osteoporosis (%) (Any Site) | 43.35% | 42.70% | 44.27% | 0.818 |
| (137/316) | (79/185) | (58/131) | ||
| Renal stone (%) | 13.54% | 14.81% | 11.76% | 0.512 |
| (44/325) | (28/189) | (16/136) | ||
| Nephrocalcinosis (%) | 5.23% | 5.29% | 5.15% | 1.00 |
| (17/325) | (10/189) | (7/136) | ||
| Parathyroid adenoma (%) | 73.15% | 75.73% | 67.39% | 0.001 |
| (109/149) | (78/103) | (31/46) |
Table 4: Prevalence of vitamin D deficiency, end-organ complications in patients with PHPT.
The relationship between vitamin D status and the severity of PHPT
Evaluation of the relationship between the levels of serum vitamin D and the levels of PTH showed a significant inverse correlation, such that patients with high serum vitamin D level had much lower PTH level and vice versa (p=0.000) (Figure 2). In contrast, as shown in Figure 3, there was no correlation seen between the level of serum vitamin D and serum adjusted calcium level (p=0.064).
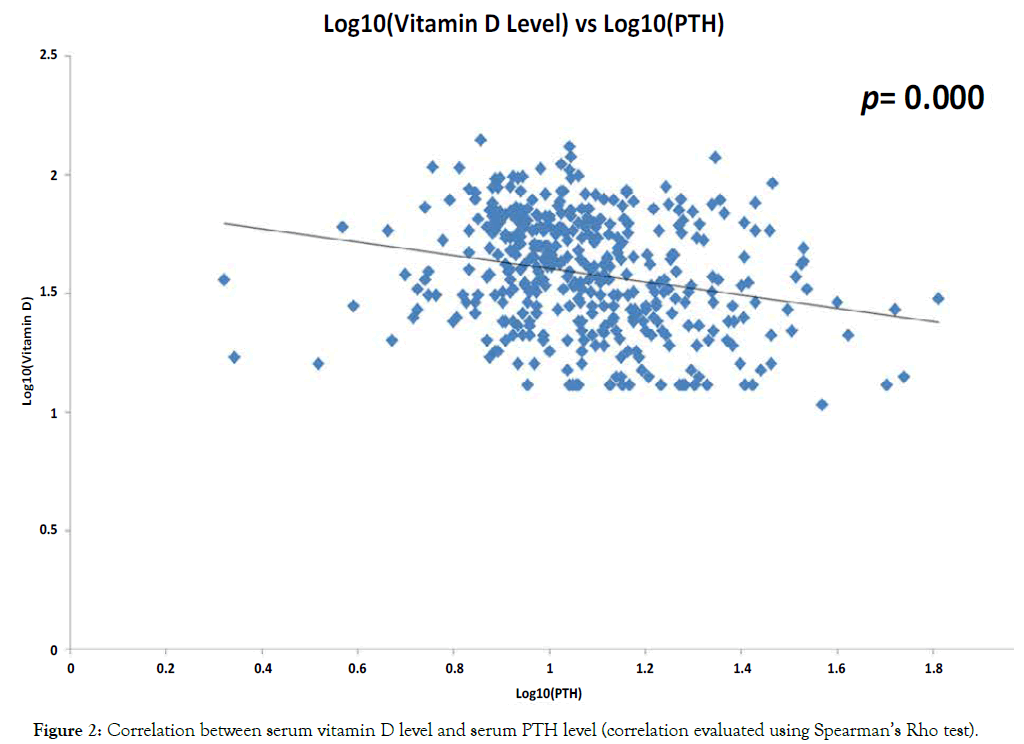
Figure 2. Correlation between serum vitamin D level and serum PTH level (correlation evaluated using Spearman’s Rho test).
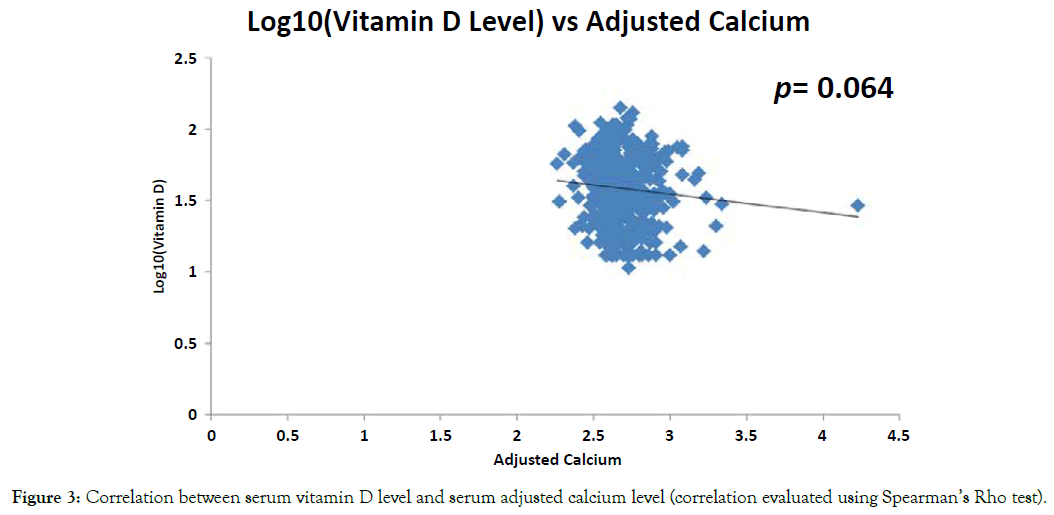
Figure 3. Correlation between serum vitamin D level and serum adjusted calcium level (correlation evaluated using Spearman’s Rho test).
To further evaluate whether there was a relationship between the level of serum vitamin D and the severity of biochemical presentations and complications of PHPT, the study population was further stratified according to their serum concentration of 25(OH)D as deficient, inadequate or sufficient using the NOS (2013) criteria. As show in Table 5, there was no difference in the demographic and general characteristics (age, sex, weight, height and BMI) between the deficient, inadequate and sufficient groups.
| Vitamin D (25[OH]D) Status | ||||
|---|---|---|---|---|
| Deficiency | Inadequate | Sufficient | p value | |
| (<30 nmol/L) | (30-50 nmol/L) | (>50 nmol/L) | ||
| -33.50% | -30.75% | -35.75% | ||
| (n=134) | (n=123) | (n=143) | ||
| Age | 61.50 (± 15.667) | 63.20 (± 15.196) | 64.30 (± 14.008) | 0.294 |
| Sex (Male) | 17.16% (23/134) | 19.51% (24/123) | 18.88 (27/143) | 1 |
| Sex (Female) | 82.84% (111/134) | 80.49% (99/123) | 81.12% (116/143) | |
| Weight (kg) | 76.90 (± 17.369) | 74.67 (± 18.400) | 74.23 (± 16.304) | 0.4 |
| Height (m) | 1.61 (± 0.088) | 1.62 (± 0.095) | 1.62 (± 0.096) | 0.902 |
| BMI | 29.58 (± 6.520) | 28.48 (± 6.076) | 28.36 (± 5.494) | 0.192 |
| Adjusted calcium level (mmol/L) | 2.71 (± 0.194) | 2.70 (± 0.172) | 2.68 (± 0.154) | 0.298 |
| Serum PTH level (pmol/L) | 1.13 (± 0.230) | 1.07 (± 0.198) | 1.03 (± 0.164) | 0 |
| (log value) | (log value) | (log value) | ||
| 13.80 (2.20–54.80) | 11.20 (2.10 – 64.70) | 10.40 (3.70–29.20) | ||
| (median) | (median) | (median) | ||
| 24-hour urinary calcium excretion | 6.52 (± 3.279) | 6.67 (± 3.334) | 7.84 (± 4.237) | 0.219 |
| Ca/Creatinine ratio | 0.59 (± 0.420) | 0.60 (± 0.427) | 0.85 (± 0.525) | 0.209 |
| Renal stone | 14.81% (16/108) | 16.00% (16/100) | 10.26% (12/117) | 0.404 |
| Nephrocalcinosis | 3.70% (4/108) | 6.00% (6/100) | 5.98% (7/117) | 0.705 |
| Parathyroid adenoma | 80.00% (40/50) | 75.00% (30/40) | 66.10% (39/59) | 0.247 |
| BMD (FN T-Score) | -1.27 (± 1.185) | -1.33 (± 1.114) | 1.35 (± 1.103) | 0.868 |
| BMD (LS T-Score) | -0.78 (± 1.548) | 0.89 (± 1.724) | -1.08 (± 1.666) | 0.392 |
| BMD (DR T-Score) | -1.51 (± 1.737) | -1.96 (± 1.727) | -1.96 (± 1.542) | 0.108 |
| BMD (TH T-Score) | -1.10 (± 1.310) | -1.07 (± 1.284) | -1.22 (± 1.170) | 0.655 |
Table 5: Biochemical and clinical presentations of patients with PHPT according to the level of serum 25(OH)D expressed in nmol/L.
It is well known that PHPT is more common in women compared with men with a female to male ratio of 3-4:1 [7-13]. In this study, 81.50% of the study population were women giving a similar female to male ratio of 4.4:1 reflecting the well-known difference in the prevalence of PHPT between genders. The mean body mass index (BMI) for the study population was 28.81 (+6.042) suggesting that patients with PHPT are overweight. This finding is in line with the finding from a meta-analysis conducted by that subjects with PHPT were heavier compared to the controls. The meta-analysis found that on average, the body weight was 3.1 kilograms (kg) (or 1.1 kg/m2 in terms of BMI) (95% confidence interval; p=0.00001) higher in patients with PHPT than in controls.
This study showed a high prevalence of symptomatic disease (58.25%). This could be due to selection bias owed mainly to the nature of the tertiary referral centre for metabolic bone disorders (MBD), which may have attracted a disproportionate cohort of patients with symptomatic disease. The data from this study showed a significant difference in age between the two groups, with patients from the symptomatic group being significantly younger compared with that from the asymptomatic group (60.97+15.356 years versus 65.88+13.924 years, p=0.001). The age difference observed in this study was not seen in a study conducted involving a much smaller study population of 140 patients with PHPT (64 symptomatic and 76 asymptomatic). Several population-based studies demonstrate that PHPT is a disease of middle-aged women; they also showed that it can occur at any age and can affect either gender [14-16].
Prevalence of vitamin D deficiency
Vitamin D inadequacy (25[OH]D level of 30-50 nmol/L) and deficiency (<30 nmol/L) are common in PHPT [8,17,18]. It is well established that the severity of PHPT is heightened when co-existing severe vitamin D deficiency is present [19]. As shown in Table 4, a significant proportion of the study population had serum vitamin D level of <50 nmol/L (64.25%). The median serum vitamin D level was 39.0 nmol/ (range of 10.7–140.0), with subjects from the symptomatic group having slightly lower levels compared with the asymptomatic group, but the difference was not statistically significant (38.0 nmol/L versus 41.0 nmol/L, p=0.323). This finding is consistent with that of Moosgaard et al. [15] who found that 81% of PHPT patients had vitamin D deficiency (25[OH]D levels of <50 nmol/L) compared with 60% of sex-and-age-matched controls (p=<0.001). Using different vitamin D level thresholds, Walker et al. [8] found the prevalence of vitamin D deficiency (25[OH]D levels of <50 nmol/L) and insufficiency (25[OH]D levels of 50-74 nmol/L) amongst PHPT patients as 19% and 35% respectively.
The only study that compared the clinical presentation of symptomatic and asymptomatic patients with PHPT did not specifically evaluate the prevalence of vitamin D deficiency in the study population [20]. However, Cipriani et al. [20] noted that the mean serum vitamin D levels in both groups were low at 26.6 nmol/L and 29.5 nmol/L respectively. The finding from this study regarding the high prevalence of vitamin D deficiency in patients with PHPT, which was corroborated by several other studies, highlights the importance of evaluating the vitamin D status in patients with PHPT as emphasised by the recommendation from the Fourth International Workshop on PHPT [11].
The biochemical and clinical presentations of PHPT patients with co-existing vitamin D deficiency
The result from this study, as shown in Figure 2, regarding the strong negative correlation between serum vitamin D and PTH levels confirmed prior findings that lower vitamin D levels is associated with high PTH levels [17-22]. This well-known phenomenon has a very significant implication in clinical practice. High levels of PTH increases the metabolism of 25(OH)D to 1,25(OH)D (calcitriol) which further exacerbates the state of vitamin D deficiency [23,24]. Furthermore, PTH also stimulates the transformation of pre-osteoclasts into mature osteoclasts and persistently high levels of PTH can lead to osteopenia and osteoporosis which can increase the risk of fractures in patients with PHPT [25-28]. These findings thus reinforce the importance of evaluating the vitamin D status of patients with PHPT and supplementing those with vitamin D deficiency [11].
Vital to the care of patients with PHPT are radiological evaluations to assess for kidney stones and osteoporosis [11]. From this cohort, the overall prevalence of kidney stone was found to be 13.54% with no difference between the symptomatic and asymptomatic groups (14.81% versus 11.76%, p=0.512). Contrastingly, data from Cipriani et al. [20] showed a much higher prevalence of renal stones in PHPT patients of 55%. Furthermore, Cipriani et al. [20] also found a significantly higher prevalence of kidney stones in the symptomatic PHPT patients (78.1%) compared with the asymptomatic patients (35.5%), a trend which was not seen in this study. Data from this study also showed an overall high prevalence of osteoporosis of 43.35% (Table 4). Compared with the prevalence of osteoporosis in the general population, estimated at 2% at 50 years to more than 25% at 80 years (National Institute for Health and Care Excellent, 2012), the prevalence rate of osteoporosis amongst patients with PHPT is much higher as confirmed by the findings from this and other several studies.
The correlation between the level of serum vitamin D and the severity PHPT
We hypothesised that vitamin D deficiency would be associated with more severe symptomatology and complications of PHPT, such as renal stones and osteoporosis. Our data do not support this hypothesis, however (Table 5). Even though a very high prevalence of osteoporosis was found both in the symptomatic and asymptomatic groups, there was no evidence that those with lower vitamin D levels have lower bone mineral density (BMD) or higher incidence of renal stones. This finding is consistent with that of Viccica et al. [29] and Walker et al. [8] who both found no significant difference in the prevalence of renal stones and BMD status in vitamin D insufficient, deficient and sufficient PHPT patients.
The findings from this study on the effects of vitamin D status on the prevalence of osteoporosis and renal stones cannot be generalised to populations with more severe PHPT or more widespread prolonged vitamin D deficiency. Severe or prolonged vitamin D deficiency in PHPT accompanied by higher PTH elevations is likely to have a greater impact upon the skeleton [8]. It is also possible that there is a threshold of 25(OH) D level below which deleterious effects occur (e.g. <22.5 nmol/L) as previously reported [30]. As vitamin D supplementation is now a common practice, the lack of frequent profound and prolonged vitamin D deficiency in PHPT patients from Western countries may have contributed to the effect observed in this study.
This study highlights key areas in practice that need to be reviewed and evaluated. Firstly, the need for a more consensus approach in the evaluation of patients with PHPT is evident. As there was no locally agreed protocol/guideline available within the study institution, the decision regarding the appropriateness of screening tests was decided mainly by individual clinicians based on their professional judgement. As a result, the majority of patients included in this study did not have comprehensive urinary calcium screening. This can be addressed by introducing a locally agreed clinical guideline on the diagnosis and management of patients with PHPT.
This study also underlined several potential areas of research that can be explored further from this large cohort of patients with PHPT including: the correlation between serum calcium levels and the severity of the disease; the correlation between serum PTH levels and the degree of end organ damage, and the effects of vitamin D supplementation on the biochemical and clinical picture of the disease. Additionally, this study can also be extended to look at: the prevalence of fragility fractures amongst patients with PHPT; the management of PHPT (surgical or medical); the outcome of surgical management; the size of parathyroid adenoma and its correlation to the severity of the disease, and long-term outcome of those patients who were treated surgically and conservatively.
It is clear that further systematic studies are needed to better understand and characterise PHPT, particularly with respect to the risk of cardiovascular, neurological, metabolic, kidney and skeletal disease. Nonetheless, the need for a comprehensive biochemical and radiological screenings is well established and should be included in the evaluation of patients in order to guide appropriate management, improve patients’ perception of the disease, and help PHPT patients to understand their condition and its management.
This study has several limitations. Firstly, due to its retrospective nature, it was not possible to access and validate missing or incomplete historic data, including medical and medication histories. Secondly, the patient population was from a tertiary specialised referral centre for metabolic bone disease where patients with more severe disease are being referred to. This is evident by the over-representation of symptomatic patients in the study population.
The main strength of this study focuses on the large population of PHPT patients consecutively seen in the MBD clinic over a period of 8 years. Secondly, the diagnosis of PHPT was validated by retrieving the biochemical data and thereby excluding those subjects who did not meet the diagnostic criteria for PHPT. Furthermore, subjects with hyperparathyroidism due to other condition such as chronic kidney disease and FHH were also excluded.
There is no conflict of interest that could be perceived as prejudicing the impartiality of the findings from this research.
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Sincere gratitude is owed to my academic supervisor, clinical supervisor, employer, family and partner for all the support and encouragement.
Citation: Criseno S, Virk J, Nightingale P, Gittoes N (2019) A Retrospective Cohort Study Evaluating the Prevalence of Vitamin D Deficiency and its Impact on the Biochemical and Clinical Presentations of Patients with Primary Hyperparathyroidism (PHPT). A Case Report. Endocrinol Metab Syndr 8:298.
Received: 11-Jan-2019 Accepted: 21-Feb-2019 Published: 28-Feb-2019
Copyright: © 2019 Criseno S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.