Review Article - (2022)Volume 6, Issue 1
Empagliflozin or Jardiance could be prescribed in forms of immunotherapy or polypharmacy with metformin or insulin for patients with diabetes as an angiotensin converting enzyme inhibitor, angiotensin receptor blocker, Sodium Glucose Contransporter Type 2 (SGLT2) inhibitor and albuminuria lowering agent. The aim of this review was to provide updated information associated with the clinical pharmacokinetics and pharmacotherapy of empagliflozin. This review was conducted according to published PRISMA guidelines. SGLT2 plays a crucial role in glucose transport and intraadrenal osmolality. In fact SGLT2 actively mediated 90% reabsorption of glucose by the kidneys. The over activation of SGLT2 in diabetic patients, leads to an increase in glucose and sodium reabsorption. Empagliflozin inhibits this contransporter that leads to loss of glucose in the urine and a reduction in hyperglycemia. Similar to Glucagon-Like Peptide 1(GLP-1) agonists, empagliflozin could cause reduction in albuminuria. The drug is orally active with a competitive binding to glucose. By oral administration the drug has a rapid absorption and metabolized by glucuronide conjugation. Excretion is through urine and feces. Pharmacotherapy used empagliflozin by reducing kidney disease progression’s could improve conditions in patients with; diabetes (types 1 or 2), overweight and blood pressure. In addition in those with chronic kidney disease empagliflozin could improve serum mineral and bone markers. Further evidence-based pharmacotherapy studies recommended establishing the drug bioavailability, efficacy and side effects’.
From May 2013, a medication that could be used with other drugs, regimented diet and exercise have got marketing approval in type 2 diabetes in which called empagliflozin or Jardiance. In combination with other classes of drugs it has potential for lowering glucose in type 2 diabetes. (Figure 1) shows the mechanism of action of empagliflozin as a potent inhibitor of Sodium Glucose Contransporter 2 (SGLT2) [1]. Peak plasma concentration reported 1.3-3 hours after oral taken drug. Half-life ranged from 10.3-18.8 hours. Trough concentrations remain constant after day 6. Plasma binding protein is about 80%. [2,3] Oxidative stress was mentioned as the main contributor to diabetic kidney disease onset. In fact in the kidney, the proximal tubular cells require a high energy supply to reabsorb proteins, metabolites, ions, and water. Increased glucose level in urine and ROS enhance the activity of sodium/glucose co-transporter type 2 (SGLT2), which in turn exacerbates oxidative stress [4]. The cardiovascular benefits of empagliflozin also were reported based on clinical studies in rats [5]. By glucose lowering effects and stimulating glycosuria excretion, the drug offers cardio protective effects [6]. In animal model and patients there is a report regarding a reduction in HbA1c upon chronic treatment with empagliflozin [7]. In prediabetes metabolic syndrome rats, SGLT2 inhibition reduces visceral adipocytes hypertrophy and ameliorates cardiac injury [8]. Empagliflozin inhibit weight gain by enhancing fat ingestion and decrease obesity induces inflammation and insulin resistance in white adipose tissue and liver [9]. In hypertensive heart failure rat model [10], the drug improved hemodynamics and reduce blood pressure [11]. For diabetic macro vascular disease and cognitive deterioration [12,13], empagliflozin could be considered as a potentially talented beneficial drug. Down-regulation of SGLT1, SGLT2 and GLUT1 expression could be an explanation for its properties [14]. Glomerular or tubular hypertrophy, focal segmental glomerulosclerosis or bulbous sclerosis, are the histological changes of kidney in patients with obesity, that reactive oxygen species inhibitors and SGLT2 inhibitors and melatonin suggested to be used [15]. A recent report confirmed that the drug might slow the loss of kidney function and delay the estimated onset of projected end-stage kidney disease in those with type 2 diabetes and cardiovascular disease complicated nephritic range proteinuria [16]. With respect to the Reno protection for the treatment of Type 2 diabetes in those with a baseline eGFR of more than 60 ml/min/1.73 m2, HbA1C>7%, or young age (<65 years), empagliflozin could be suggested as the preferred choice of management [17]. The aim of this review was to provide updated information associated with the mechanism of action, clinical pharmacokinetics and pharmacotherapy of empagliflozin.
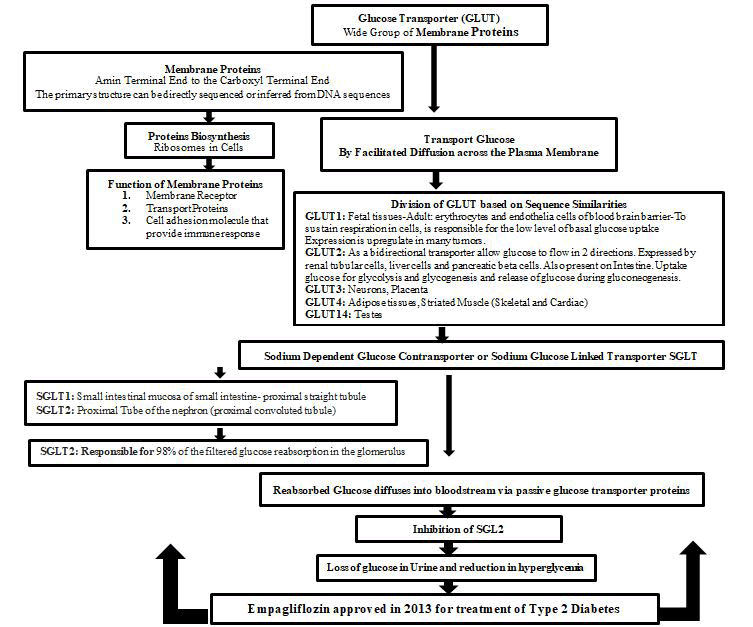
Figure 1: Mechanism of Action of Empagliflozin.
This review was conducted according to published PRISMA guidelines (Figure 2) [13]. The clinical question to guide the review was obviously defined following the structured PICOS framework. The PICOS question is: individuals with empagliflozin and disease (Population), not intervention (Intervention) from the start of the study time period (Comparisons) efficacy and side effects (Outcomes) using existed published articles (Study design)? The electronic databases PubMed, Scopus, and Web of Science were systematically searched. Search strategies used selected subject headings and key words related to empagliflozin and disease combined with (“Pharmacokinetics” and “Efficacy” and “Side effects”). Reference lists of reviews and retrieved articles were checked to identify potentially relevant articles. Studies were eligible if they met the following predetermined selection criteria: 1) the study is population based. 2) Outcome measure was incidence and prevalence. The titles and abstracts of all manuscripts identified by the demands were individually studied for their suitability. Inconsistencies were considered and determined over agreement. The full text of all articles supposed actually appropriate. 40 relevant studies included review articles, clinical trials and case reports from the used references were evaluated.
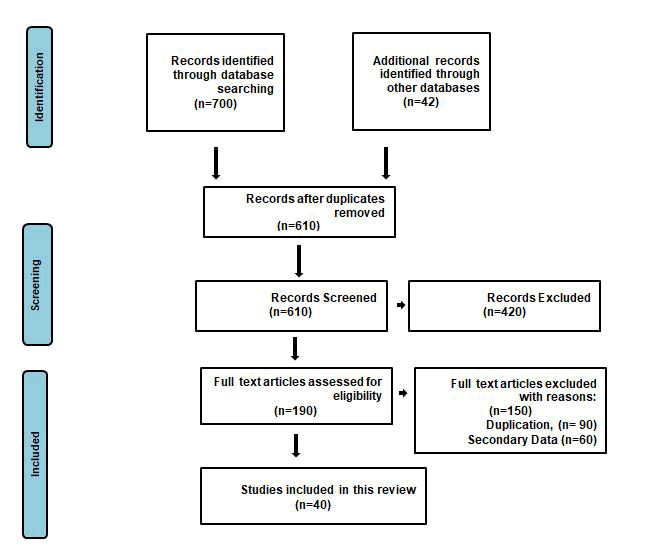
Figure 2: Flow diagram of study selection for the systematic review.
Table 1 shows pharmacokinetics properties, clinical and side effects of empagliflozin. The recommended guideline for patients with type 2 diabetes mellitus is based on initiating pharmacotherapy with empagliflozin an oral antidiabetic drug. Study showed that chronic kidney disease was associated with 303 urinary peptides that the most fragments mentioned as collagen types I, II and III, uromodulin, albumin and beta-2-microglobulin [18-20]. Pharmacotherapy used as empagliflozin could be a promising strategy for prevention and treatment of diabetic cardiomyopathy empagliflozin. The prescription of empagliflozin is robust with regard to efficacy and safety. Poly pharmacy with metformin considers empagliflozin as a first line of pharmacotherapy regarding reduction in risk of cardiovascular disease, absence of weight gain and low risk of hypoglycemia. Associated improvement of overweight linked inflammation and insulin resistance, empagliflozin swelling energy spending and adipose tissue burning [21]. Improvement in indices of left ventricular diastolic cardiac dysfunction by regularizing the size and number of mitochondria is another mechanism of action in diabetic patients with cardiovascular disease as well. [22] Moreover, due to hemodynamic properties that connected with reduction in intraglomerular pressure, empagliflozin could cause long-term protection in function of kidneys [23]. By raising serum uric acid empagliflozin could improve the metabolic disease that called hyperuricemia [24]. Regarding glucose tolerance in type 1 diabetes, animal study described an important advance with empagliflozin [25]. In population with type 2 diabetes, empagliflozin decreases aminotransferases [26].
Table 1: Empagliflozin: Pharmacokinetics properties, clinical use, side effects.
| Pharmacokinetics properties | |
|---|---|
| Tmax: 1-1.3 h, T1/2: 10.3-18.8, PB; 30%Polypharmacy with Valproate Concentration of Valproate | |
| Clinical use | Side effects |
| Type 2 Diabetes | Urinary Tract Infections (UTIs) |
| Weight and Blood Pressure | Fungal Infections of the groin |
| Cardivascular Disease | Joint pains |
| Type 1 Diabetes | Skin infection of the groin |
| Contraindications: Allergi Reaction to Drug, End Stahge Kidney Disease, Diabetic Keto Acidosis | Diabetic ketoacidosis with normal blood sugar |
| Contraindicate in those with significant kidney disease | |
Abbreviations: Tmax: Time to reach Maximum Concentration,T1/2: Half-life, PB: Protein Binding
We describe in this work an updated review of empagliflozin or Jardiance as an albuminuria lowering agent, SGLT2 inhibition, angiotensin receptor blocking and angiotensin converting enzyme inhibition. Research conveyed that in type 2 diabetes patients with; 1) higher baseline HbA1c levels, 2) better renal function, 3) shorter duration of disease, empagliflozin could be considered as a drug of choice associated with glucose-lowering agent [27]. Its’ immunotherapy for one and half year was tolerated well with a sensible reduction in HbA1c and weight when compared to placebo [28]. Polypharmacy based on insulin and empagliflozin for four weeks in Japanese participants with type 1 diabetes increased; 1) urinary glucose excretion, 2) improved glycemic control and 3) reduced total daily insulin needs and body weight. There was no indication of hypoglycemia or diabetic ketoacidosis in that population [29].
Visceral adipose tissue is associated with endogenous glycerol-derived hepatic gluconeogenesis and empagliflozin reduces endogenous glycerol-gluconeogenesis in obese adults without type 2 diabetes mellitus. These findings suggest a mechanism by which SGLT2 inhibitors may prevent type 2 diabetes mellitus in obesity [30]. In older patients, the drug reduced risks of cardiovascular mortality, heart failure and renal outcomes [31]. A recent meta-analysis confirmed that empagliflozin has a good effect on the HbA1c level in patients with type 2 diabetes mellitus [32]. The drug reduces lipid metabolism of diabetic nephropathy through AdipoR1/P-AMPK/phosphorylated acetyl-CoA carboxylase pathway and delay diabetic nephropathy progress [33]. Managing empagliflozin for a period of six months in those with type 2 diabetes and fatty liver disease (non-alcoholic) confirmed an improvement of liver steatosis and fibrosis [34]. Polypharmacy with valproate could cause an increase in blood trough concentrations of valproate [35,36]. At the 6-month follow-up of empagliflozin, urinary acetyl-β-D-glucosaminidase excretion was decreased. Renal tubular damage may be relieved by the prescription of SGLT2 inhibitors through a reduction in the dose of loop diuretics administered and the production of erythropoietin [37-40].
Therefore, this review presents simplicity toward clinical pharmacokinetics aspects’ and pharmacotherapy associated with proteinuria, SGLT2, angiotensin receptor and angiotensin converting enzyme.
In summary we updated review of Empagliflozin for treatment of diabetes, overweight and blood pressure, heart failure with or without diabetes and kidney disease. Its’ pharmacotherapy could provide short and long term benefit regarding to lowering albuminuria irrespective of it’s’ status. In addition the drug reduces the dose of loop diuretics and increases the production of erythropoietin. It is effective in decreasing HbA1c and reducing body weight. Polypharmacy with valproate needs further vigilance due to increase in blood concentrations of valproate. However it could be recommended for patients with chronic kidney disease but publications suggested that it might not be suitable choice of treatment for those with end stage renal disease.
Citation: Ghamari ZT (2022) A Quick Review of SGLT2 Inhibitor Empagliflozin in Clinical Practice. J Pharma Reports. 6:127
Received: 24-Jan-2022, Manuscript No. JPR-22-15953; Editor assigned: 26-Jan-2022, Pre QC No. JPR-22-15953; Reviewed: 09-Feb-2022, QC No. JPR-22-15953; Revised: 24-Mar-2022, Manuscript No. JPR-22-15953; Published: 31-Mar-2022 , DOI: DOI: 10.35248/JPR.22.6.127
Copyright: © 2022 Ghamari ZT. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.