Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2019)Volume 10, Issue 4
A clinic based prospective study was carried out during the period of 1st May 2018 to 30 April 2019 in a private clinic located in the Centre of Kowloon district; Hong Kong. The objectives were to study the proportion of clinically diagnosed SSS patients attending the private clinic setting, demographic and clinical characteristics. The study suggested that SSS was a common disease encountered in the private dermatology clinic with a female gender predominance over the face region and at the age group of age 20 to 30. Our study also suggested that SSS patients could be identified by a validated ten item Sensitive Scale–10 questionnaire with a cut-off score of above 20. This is in accordance with that reported in the documented literature. A cut off value of patients scored above 20 in the questionnaire without concomitant skin disease at the same time can be diagnosed to have probable SSS. The score may be effectively used as a monitoring tool of the progress of SSS after management including counseling explanation, education and treatment.
Sensitive skin syndrome; Clinic base setting; Diagnosis; Demographic and clinical characteristics; Sensitive skin-10 questionnaire
Sensitive skin syndrome (SSS) is a common skin condition characterized by subjective sensation of skin irritation, general discomfort, tightening, itching and burning pain under circumstances that are unremarkable to the unaffected. The underlying cause and pathogenesis of the condition is obscure. Epidermal barrier defects due to genetic mutation of adhesion focus macromolecules, intraepidermal nerve fiber expansion resulting from nerve growth factors in the skin, dorsal root ganglion (DRG), central nervous system (CNS), neurogenic inflammation and trans epidermal water loss (TEWL) are all suggested [1-6]. The absence of a confirmatory diagnostic test made clinical management difficult and poses significant challenges to the collection of accurate reliable epidemiological data on this important condition. In view of this, a prospective study was performed locally in Hong Kong in the big bay area region of southern China during 1st May 2018 to 30 April 2019 to study the proportion of newly attended patients diagnosed suffering from SSS by a dermatologist; their demography and clinical characteristic with the aid of the validated ten item questionnaire; Sensitive Scale–10 (SS–10) (Appendix 1a) [7]. The objectives were to estimate the proportion of SSS patients attending a private clinic setting, their background demographic and characteristic clinical features. Based on the collected data, a larger scale community based epidemiological study may be designed to study the prevalence and possible associated risk factors of the condition.
A clinic based prospective study was carried out during the period of 1st May 2018 to 30 April 2019 in a private clinic located in the Centre of Kowloon district; Hong Kong. The objectives were to study the proportion of clinically diagnosed SSS patients in the private clinic setting, demographic characteristics including age, gender, occupation, marital status, site of involvement, medical history and concomitant skin dermatosis especially atopic dermatitis, seborrheic dermatitis, psoriasis, rosacea and acne vulgaris in patients diagnosed clinically with sensitive skin syndrome (SSS) by a dermatologist. By using the validated ten item questionnaire; Sensitive Scale-10 (SS–10) to substantiate the clinical diagnosis, the SS–10 score was recorded and studied with respect to the clinically diagnosed SSS [7]. The score was also used in the subsequent follow up consultation to monitor progress of the disease after management with counseling and emollients only. The study was carried out aiming at collecting local background demographic and clinical data on the common skin condition of SSS and provided basis for further larger scale community epidemiological survey on the prevalence and risk factors associated with SSS using the SS–10.
All newly attended patients during this study period were recorded and consulted by a dermatologist in a private clinic setting. Prior to the consultation, the patients were asked voluntarily to complete the SS-10. The SS-10 was translated into a Chinese version with additional data entry particularly on age, gender, occupation, marital status and family composition; etc. (Appendix 1b). During the consultation, the diagnosis, past medical history, drug history and allergy history of the patients were recorded on the respective case notes by the dermatologist. Patients diagnosed SSS were followed up and managed by counseling, explanation and education of the syndrome plus emollients only. The SS–10 in the follow up visit was recorded. Telephone consultation was carried out by trained nurse for following up the SSS patients.
The clinical diagnosis of SSS in our study is based on the definition suggested by International Forum for the Study of Itch (IFSI): SSS is a syndrome defined by the occurrence of unpleasant sensations (stinging, burning, pain, pruritus, and tingling sensations) in response to stimuli that normally should not provoke such sensations. These unpleasant sensations cannot be explained by lesions attributable to any skin disease [8]. The SS–10 was used as an assisting tool to substantiate, monitor and follow up patients with SSS. The exclusion criteria of the study are those clinic attendees who refused completing or filling in the SS-10; thus; cannot be monitored; patients worked in the cosmeceutical industries who have prior knowledge of the condition and those refused to participate in the study. Patients diagnosed to have SSS would be explained, counseled and educated on the syndrome by both the dermatologist and specialist nurse in the clinic setting and managed with emollients only without topical steroids or other medications. The patients were then followed up by the dermatologist with the aid of the SS-10. The proportion of the patients diagnosed with sensitive skin syndrome with their entered SS-10 score and characteristics of the patients were recorded and analyzed. The patients diagnosed to have SSS would be further telephone followed up one month after the last consultation to exclude the development of dermatitis, rosacea and other skin diseases.
A total of 1111 newly attended patients were recruited in the prospective study. 84 (7.56%) of them were diagnosed by the dermatologist to have SSS. The demographic characteristics of the patients of the study were shown in Table 1. Out of the remaining 1027 were diagnosed as atopic dermatitis, seborrheic dermatitis, allergic contact dermatitis, psoriasis, rosacea, acne vulgaris and other skin diseases like warts, tinea infections and cellulitis (Table 2).
| Demographic characteristics of the patients of the study | |||
|---|---|---|---|
| Diagnosed SSS (N=84) | Not diagnosed SSS (N=1027) | Chi square (p<0.001) | |
| Gender ratio (Female to male) | 61:23=2.65:1 | 493:534=0.92:1 | p<0.001 |
| Age group (%) | |||
| 1 | 1 | 10 | p<0.001 |
| 2 | 29 | 20 | p<0.001 |
| 3 | 37 | 20 | p<0.001 |
| 4 | 18 | 20 | p<0.001 |
| 5 | 14 | 20 | p<0.001 |
| 6 | 1 | 10 | p<0.001 |
| Marital status (%) | |||
| Married | 53.57 | 50.8 | p>0.001 |
| Unmarried | 41.67 | 38.2 | p>0.001 |
| Divorced | 4.8 | 9 | p>0.001 |
| Occupation | |||
| Clerical | 90 | 68 | p<0.001 |
| Manual | 0 | 26 | p<0.001 |
| Unemployed | 2 | 2 | p>0.001 |
| Retired | 8 | 4 | p>0.001 |
Table 1: Demographic characteristics of the patients diagnosed SSS compared with those not diagnosed SSS.
| Number of patients who reported past histories of skin diseases in SSS patients (%) | Number of patients with specific skin diagnosis other than SSS during the consultation (%) | |
|---|---|---|
| Atopic dermatitis | 11 (13) | 253 (24.60) |
| Seborrheic dermatitis | 8 (10) | 71 (6.90) |
| Allergic contact dermatitis | 20 (24) | 263 (25.60) |
| Psoriasis | 0 (0) | 62 (6.00) |
| Rosacea | 0 (0) | 41 (4.00) |
| Acne vulgaris | 3 (4) | 123 (12.00) |
| Other skin diseases like warts, tinea infections, skin nodules, etc | 2 (2) | 214 (20.84) |
| Total | 44 (52.38) | 1027(100) |
Table 2: Proportion of skin diseases associated with SSS diagnosed patients and non SSS diagnosed patients in the study.
The patients diagnosed SSS have a predominant female to male gender ratio of 2.65 to 1 compared to an almost one to one ratio of the non SSS group which is statistically significant. The age distribution of the SSS group is mainly within normal distribution with a peak of 37% at the range of age 20 to 30 with very few below 10 or above 50. The non SSS controlled group has a more evenly distribution among all age groups. The SSS groups have less divorced couples when compared with the control (p<0.001) but the absolute number is relatively small. Most; up to 90% of the SSS diagnosed; are clerical or managerial by occupation while there were more manual workers in the non SSS diagnosed group.
All patients clinically diagnosed SSS scored above 20 in the SS– 10 with an average score of 41.38. They are followed up and managed with counselling, explanation and education plus emollients and avoidance of aggravating factors. Most of them showed improvement of the SS–Score in the subsequent follow up (Figure 1).
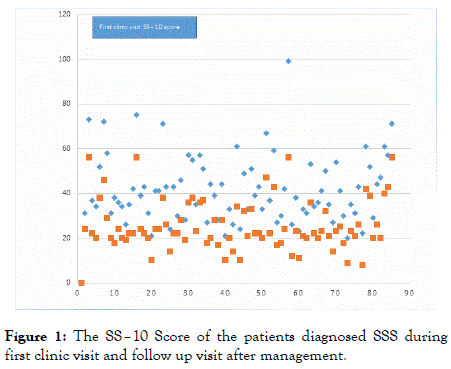
Figure 1. The SS–10 Score of the patients diagnosed SSS during first clinic visit and follow up visit after management.
For the site of occurrence of sensitive skin; face was the commonest (60%) followed by the genital organs (16%), the scalp (13%), the hands (8%) and the body including the trunk and limbs (3%). Most of the facial skin sensitivity occur in the female gender; 92% versus 8% in the male (p<0.01). While for the genitalia; 85% were of the male gender and most frequently affecting the scrotal and perianal region (Table 3) (Figures 2 and 3).
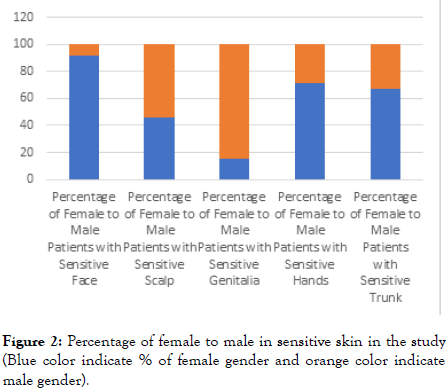
Figure 2. Percentage of female to male in sensitive skin in the study (Blue color indicate % of female gender and orange color indicate male gender).
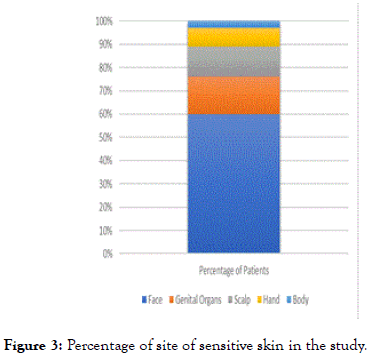
Figure 3. Percentage of site of sensitive skin in the study.
| Site of sensitive skin | Total number of patients (%) | Female (%) | Male (%) | Chi square (p value) |
|---|---|---|---|---|
| Face | 50 (60) | 46 (92) | 4 (8) | <0.001 |
| Scalp | 11 (13) | 5 (46) | 6 (54) | <0.001 |
| Genital organs include scrotum, vulva and | 13 (16) | 2 (15) | 11 (85) | <0.001 |
| Hands | 7 (8) | 5 (71) | 2 (29) | <0.001 |
| Body including the trunk and limbs) | 3 (3) | 2 (67) | 1 (33) | <0.001 |
Table 3: The Proportion of the site of sensitive skin in the study and the gender distribution.
The most frequent presenting complaints of the SSS diagnosed patients were skin irritation (98.81%), followed by general discomfort (97.62%), itching (95.24%), tautness (83.33%), pain (73.81%), sensation of heat (65.48%), hot flushes (54.76%), burning (45.24%) and tingling (26.19%) respectively. The main observed clinical sign is skin erythema (92.86%). The average 10 points score of presenting symptoms and signs in descending order are general discomfort (6.72), itching (6.68), skin irritation (6.72), redness (6.6) tautness (4.25), pain (3.40), hot flushes (2.92), sensation of heat (2.62), burning (1.96), tingling (1.44) (Figure 4).
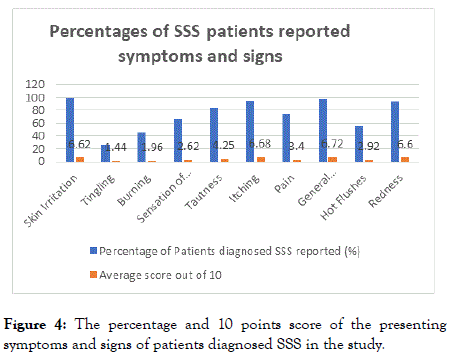
Figure 4. The percentage and 10 points score of the presenting symptoms and signs of patients diagnosed SSS in the study.
SSS is a complex clinical syndrome characterized by subjective unpleasant neurological sensation in non-provoking circumstances [2,3,9]. The known attributes included environmental factors like pollutions, climatic change especially heat, wind, ultraviolet irradiation; modern urban life-style factors like consumption of cosmeceuticals products, hair dyes and endogenous factors like stress, menses, emotional and household burdens [10]. Albeit reported to be a prevalent disease in Europe and United States, there is a paucity of clinical and epidemiological data of SSS locally in Hong Kong and south-east Asia. Our prospective clinic base study is the first survey carried out in collecting background data on SSS in this locality. The data may provide basis for further larger scale community epidemiological survey on the prevalence and risk factors associated with SSS especially utilizing the internationally accepted validated SS–10 Questionnaire which is convenient, non-invasive and quantitative.
Our study suggested that SSS was a common disease encountered in private clinic setting. Our study with a relatively large sample size of 1111 of newly attended patients seeking dermatology consultations in our clinic reported a proportion of 7.56% (N=84) within a one-year period. The proportion of patients diagnosed seborrheic dermatitis, psoriasis and rosacea was 6.90%, 6.00% and 4.00% respectively within the same period. This proportion of SSS in our clinic base study was much lower than those reported in the documented literature globally. Studies on the prevalence of SSS using self-diagnosis and self-report in populations in Belgium, France, Germany, Greece, Italy, Portugal, Spain, Switzerland, United States, Brazil and Japan have yield an estimated prevalence rate of 40% [11-17]. Different methodologies are employed in the collection of these data. Most of these results are based on patient’s selfreported four-point scale on severity of skin sensitivity; very sensitive, moderately sensitive; not very sensitive and not sensitive at all; which may be subject to various expositions. The questionnaire reports usually without a dermatologist clinical diagnosis. The subjective results may be over reported, and the objectivity of survey methodology may be inadequate. A community study carried out in three top metropolitan cities in China; namely Beijing, Shanghai and Guangzhou; report a significantly lower prevalence of SSS; estimated 16% in female and 9% in male on average [18]. Our prospective clinic study carried the advantages of a face to face clinical diagnoses of SSS of clinic attendees by a dermatologist based on the IFSI definition and using the objective validated assessment tool of SS–10 Questionnaire to sustain, monitor and follow up the SSS patients. The reliability of the later has been established and documented [7].
While a confirmatory test is still unavailable, SSS remained a clinical diagnosis by a dermatologist. Our study suggested that patients clinically diagnosed SSS may report a score of above 20 with an average score of 41.38 in the SS-10. This is in line with that reported in the documented literature, SSS patients may be identified with a SS–10 total score of above 20. Other authors also suggested a cut off value of above 20 in supporting a diagnosis of SSS [7]. Hence, it may be argued that a cut off value of patients scored above 20 in the SS-10 without concomitant skin disease at the same time can be diagnosed to have probable SSS. The score may be effectively used as a monitoring tool of the progress of SSS after management including counseling explanation, education and treatment. This is also clear in our study that after topical emollients therapy and counseling, an evident improvement of the score was seen in patients with SSS possibly due to temporary restoration of the defective disturbed epidermal barrier, reduction of TWEL and supportive psychotherapy. The validity of this cutoff value may be of pivotal significance in utilizing the SS–10 Questionnaire in studying the prevalence and risk factors of SSS in the community.
Our study further demonstrated SSS as a skin condition more commonly occur in the female gender. In fact, in our study, female was 2.7 times more likely to suffer from SSS than their male counterpart. This finding had been reported in most epidemiological studies of SSS. While the pathogenesis is still not fully understood, study performed in normal subjects through visualizing the morphology of small nerve fibers in human skin biopsy suggested that women had higher density of intraepidermal nerve fiber than men regardless of the age group [19]. Women may use toiletries and cosmetics to wash their face more frequently. An innate aberrant epidermis and/or a skin barrier damaged by irritants in cosmetics and soaps may stimulate the densely populated nerve fibers triggering the subjective noxious sensory perception of SSS to the brain.
For age distribution, 84% of SSS patients in our study was reported within the group aged of age 19 to 49. Other studies also suggested that SSS is more common in this young to middle age individuals and become less common in age group greater than 50 [20]. Published studies have shown that elderly may be less susceptible to irritant like sodium lauryl sulphate and an elevated sensory threshold with a decrease in structural and functional performance of sensory skin properties [21-23]. Urban restless life-style, application of trendy cosmetic products, hair dyes, fast food diets, stress and emotional unsettlements are potential confounders in influencing the age predilection [24,25]. In vivo studies suggested high stress perception results in an intense cross talk between the skin and skin innervating dorsal root ganglion (DRG) increases the likelihood of nerve growth factor-dependent neurogenic skin inflammation by enhancing sensory skin innervation through intraepidermal nerve fiber [26]. Nerve growth factor stimulate adrenocorticotrophic hormone (ACTH) in the hypothalamicpituitary- adrenal axis which in turn act on the adrenal cortex to secret the stress hormone cortisol. ACTH positively feedback the release of NGF in the cerebral cortex and hypothalamus [26]. Nerve growth factor modulates neurogenesis, neural plasticity and axonal outgrowth but the contrary may be possible if excessive, uncontrolled and prolonged secretions of cortisol inhibit nerve growth factor expression in the brain. Evidences suggested that nerve growth factor may act as a signal protein that critically involved in SSS especially in promoting intraepidermal nerve fiber elongation and branching in the epidermis and dermis; inflammation; nociceptive and pruritogenic neuro sensory transmission from the skin via the DRG to the central nervous system (CNS), ultimately reached the cerebral cortex with the resultant perceptive symptoms; stress, emotional and psychological consequences of SSS secondary to the disharmonious secretions of cortisol via the hypothalamic-pituitary-adrenal axis [26].
Face is the commonest site of presentation of SSS; followed by the genital organs; the scalp, hands and body in descending frequency. The findings are consistent with other documented literatures [27,28]. A significant finding in our clinic setting study is that 42% (10/24) of the total male diagnosed SSS presented with neurological discomfort over scrotum seek dermatology consultation. No specific genital skin or venereal disease is diagnosed. This should raise the attending doctor the possibility of SSS when consulting male patients of sensitive skin irritation over scrotum. The anatomical difference at the site of presentation of SSS in different parts of the body may be due to the variations of epidermal skin thickness and innervation of epidermal and dermal nerve fiber densities and vascular distributions of these sites. Abnormal skin thickness and dry skin type are common SSS clinical presentations [29-31]. Male with frequent scrubbing of the genitalia with soap and detergent apparently to improve skin hygiene may adversely increase the epidermal thickness of the scrotum and subsequently intraepidermal nerve fiber innervation, TWEL and inflammation. Similarly, face of the female gender with a reduced stratum corneum of the epidermis and a higher intraepidermal nerve fiber density and frequent use of skin care products may easily be damaged by irritants in the cosmetics, pollutants and ultra-violet radiations in the environment causing SSS.
The most frequent reported symptoms were skin irritation, followed by general discomfort, itching, tautness, pain, sensation of heat, hot flushes, burning and tingling. More than halves of the patients diagnosed SSS complained skin irritation, general discomfort, itching, tightening, pain, heat sensitivity and hot flushes. These symptoms may reflect the systemic involvement of this syndrome which pathogenetically mediated by hypersensitivity of the sensory nervous system, transient receptor potential vanilloid (TRPV) channels, neuro mediators, transmitters and increased vascular responses resulting inflammation [32,33]. Skin redness was reported in most of the cases but unlike eczema; oozing, swelling, excoriations and signs of chronic eczema like lichenification was not observed in SSS diagnosed patients.
Eczematous and non-eczematous skin condition had been reported to be associated with SSS. In our study, 13% of the SSS diagnosed patients had reported past medical history of atopic dermatitis. Study in the literature suggested that sensitive skin may have an association with atopic diathesis or atopy [17]. Atopic dermatitis patients like SSS share pathogenetic signs of epidermal barrier aberrancy and abnormal distribution of intraepidermal nerve growths both in the epidermis, dermis, DRG and CNS. However, SSS is characterized by no or minimal inflammatory histological signs without specific immunological dysfunction. Hence, despite a possible relation, SSS with a predominant evoked neurological symptomatology apparently more complex and not all SSS pathologies are attributed to an underlying atopic diathesis [13]. SSS is also correlated with noneczematous condition like seborrheic dermatitis, rosacea, psoriasis and acne vulgaris. Our study reported only 10% and 4% of the SSS patients having a history of seborrheic dermatitis and acne vulgaris. It is of interested to note that up to 24% of the SSS patients reported a history of allergic contact dermatitis (Table 2) in our study.
Our study is a clinic setting and physician biased prospective study limited by the fact that patients who complained of SSS symptoms in the community voluntarily choose to attend our private clinic for consultation. The actual prevalence may be much higher in the community who may not have sought consultation. Hence, one of the limitations of our study is under reporting of the true prevalence of SSS. There is no attempt in our original study design to address the prevalence of SSS in the local community nor to generalize the findings epidemiologically. However, the relative consistency of the collected data to the documented literature reflect the validity and significance of the collected data of SSS in our area. Better designed, larger scale study with a bigger sample size in a community setting should be advocated in the future for better improvement.
We report a prospective study using physician face to face diagnosis and validated SS-10 questionnaire to investigate the proportion of SSS Chinese patients attending a private clinic setting. The results show that SSS is a common presentation to the private dermatology clinic with female gender predominance particularly over the face in the age group of age 20 to 30. Clinical associations are history of atopic diathesis, allergic contact dermatitis and a clerical managerial occupational background. SSS may be managed by counseling, education, explanation, emollients and careful follow up but its exact pathogenesis and treatment is still not explained.
Citation: Chan KTM (2019) A Prospective Study on the Characteristics of Sensitive Skin Syndrome in Chinese Patients Attending a Private Clinic- Based Setting in Hong Kong. J Clin Exp Dermatol Res. 10:498. doi: 10.35248/2155-9554.19.10.498
Received: 22-May-2019 Accepted: 31-May-2019 Published: 07-Jun-2019 , DOI: 10.35248/2155-9554.19.10.498
Copyright: © 2019 Chan KTM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.