Case Report - (2023)Volume 9, Issue 2
A Pancreatic Neuroendocrine Carcinoma with Super High Procalcitonin Mimicking Sepsis Shock: Case Report and Literature Review
Xuzhen Qin1*, Ling Qin2, Ningning Li3, Xiang Wang3, Chunmei Bai3 and Congwei Jia4Abstract
Besides medullary thyroid cancer, a latent and relatively mild increase of serum Procalcitonin (PCT) has been found in Neuroendocrine Neoplasms (NENs). Here we are aiming to supply more information about NENs related PCT elevation, in order to broaden the clinical experiences about diagnosis and treatment of shock in tumor patients. We reported an advanced Pancreatic Neuroendocrine Carcinoma (pNEC) with liver and lung metastasis, in which a rare pseudo-sepsis shock with extremely high serum PCT level (exceeding 100 ng/ml) had been demonstrated. A series of screening tests to exclude bacterial infections, including blood culture, urine culture and even Metagenomic NGS (mNGS), had been performed. Given negative evidence of bacterial infection and useless broad-spectrum antibiotics treatment, steroid was used to relieve the serious inflammation and its related shock. The patient’s condition was improved and discharged. In summary, despite the ubiquitous use of PCT used in bacterial infection and sepsis shock, pNEC could cause the high level of serum PCT and even result in severe inflammation accompanied by shock. As for diagnosis and treatment strategies, pNEC should be regarded as one of rare differential diagnosis when experimental antibiotics are not working. The potential mechanism of PCT elevation and its role in prognosis of pNEC still needed to be further studied.
Keywords
Pancreatic neuroendocrine carcinoma; procalcitonin; sepsis shock; inflammation
Introduction
During the past three decades, Procalcitonin (PCT) has been considered as a sensitive marker of bacterial infection. Non-infectious conditions inducing PCT production occurs in trauma, post-surgical procedures, heatstroke and tumor. Concerning various tumors, high level of circulating PCT is widely known in patients with medullary thyroid cancer which causes a specific yield of PCT. In non-medullary thyroid cancers, Neuroendocrine Neoplasms (NENs) have been found to secrete PCT to the blood, while the extremely elevated level of PCT (over 100 ng/ml) released by NENs in circulation is still quite rare [1].
In this case report, a 68-year-old man with a pathologically identified Pancreatic Neuroendocrine Carcinoma (pNEC) has experienced lung and multiple liver metastatic lesions, while the PCT is unexpectedly elevated from 32 ng/ml at the onset to over 100 ng/ml. Sequentially, the patient develops high fever and shock, which has been firstly diagnosed as sepsis shock and treated with broad spectrum antibiotics. Because of a refractory shock without response to experimental antibiotics and a lack of bacterial infection evidence, prompt steroid is implemented to relieve the severe inflammation and shock. It turns to be perplexed and inclined to make a misdiagnosis of sepsis shock when a surging level of PCT secreting NENs presents present high fever and decreasing blood pressure. Here, we demonstrate that pseudo-sepsis shock with extremely high serum PCT level can be caused by an advanced Pancreatic Neuroendocrine Carcinoma (pNEC) with multiple liver metastasis, in order to extend the spectrum of differential diagnosis and draw more attention to the role of PCT among non-infectious diseases.
Case Presentation
A 68-year-old man was admitted to our hospital with one year abdominal distension and pain accompanied by watery diarrhea for 4 months, decreased appetite for 5 months and significant weight loss of 12 kg over 6 months. He had experienced Polymyalgia Rheumatica (PMR) for 3 years, and prednisone tapered to 5 mg per day had been introduced to alleviate PMR. He had Type 2 diabetes for 6 years, and coronary artery disease for 3 years. He had been addicted to heavy smoking and moderate drinking for nearly 40 years. He had no chronic hepatitis and family history of liver disease. Due to occupation, he had ever been exposed to radiocobalt 60 and petrol for nearly 30 years. Physical examination showed middle and right upper abdominal tenderness without rebound tenderness. His liver was palpated in the subxiphoid region.
On admission, his body mass index was 23 kg/m2. Blood test showed routine hematological test (White Blood Cells (WBC): 11,400/μl, hemoglobin 10.1 g/dl, platelet 18.9 × 104/μl), inflammatory status (High-sensitivity C-reative Protein (hsCRP) 9.3 mg/l, Erythrocyte Sedimentation Rate (ESR) 43 mm/h, ferritin 151 ng/ml), mild liver dysfunction (gamma-glutamyl transpeptidase, 47 u/l) and normal pancreatic and renal function. Coagulation tests were slightly abnormal for Prothrombin Time (PT) is 12.8 sec and for Activated Partial Thromboplastin Time (APPT) is 24.5 sec, and fibrinogen 3.53 g/l. Moreover, a markedly high Procalcitonin (PCT) level ranging from 32 ng/ml at beginning to >100 ng/ml when disease relapsed (cut-off value: 0.05 ng/ml) was observed (Figure 1). Hepatitis B surface antigen and hepatitis C virus antibody were both negative; the levels of Carbohydrate Antigen 242 (CA242) (24 u/ml [normal range: <20 u/ml]), Neuron-Specific Enolase (NSE) (43 ng/ml [normal range: <16.3 ng/ml]), Progastrin-Releasing Peptide (ProGRP) (>50000 pg/ml [normal range: <69 pg/ml]) and cytokeratin 19 fragments (Cyfra211) (15 ng/ml [normal range: <3.5 ng/ml]) were elevated. Dynamic Computed Tomography (CT) showed multiple tumors (max diameter 52 mm) occupying the entire liver (Figures 2A and 2B) and 31-mm tumor at the head of the pancreas with mild enhancement at the portal venous phase (Figure 2C). An initial chest CT on admission was nearly normal, while a metastasis nodule in the middle of right lung lobe was revealed after 4 weeks hospitalization (Figure 2D). For further definite diagnosis, the patient underwent pancreatic mass and liver nodules biopsy through endoscopic ultrasound guided fine-needle aspiration method. Histologically, the lesion contained poorly differentiated cells with minimal cytoplasm and irregular nucleus. It was positive for chromogranin A, synaptophysin, and Ki67 (labeling index 80%) (Figure 3). Therefore, the patient was diagnosed as pNEC with liver and lung metastasis at the fourth week of admission (Figures 1-3).
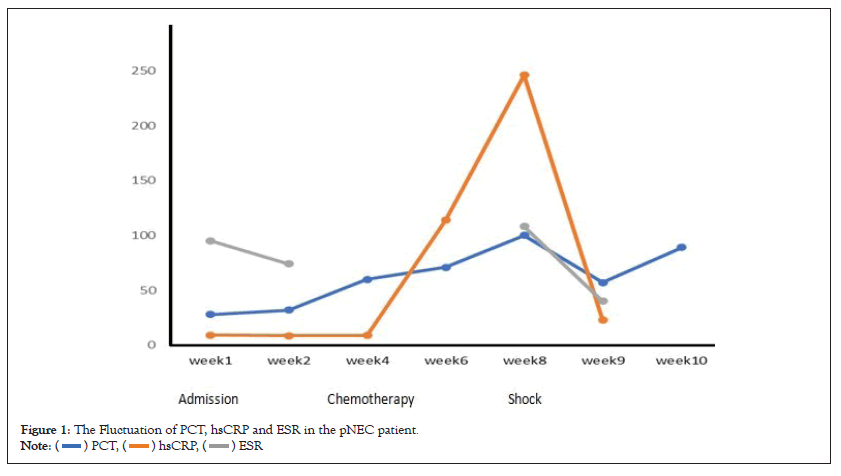
Figure 1: The Fluctuation of PCT, hsCRP and ESR in the pNEC patient.

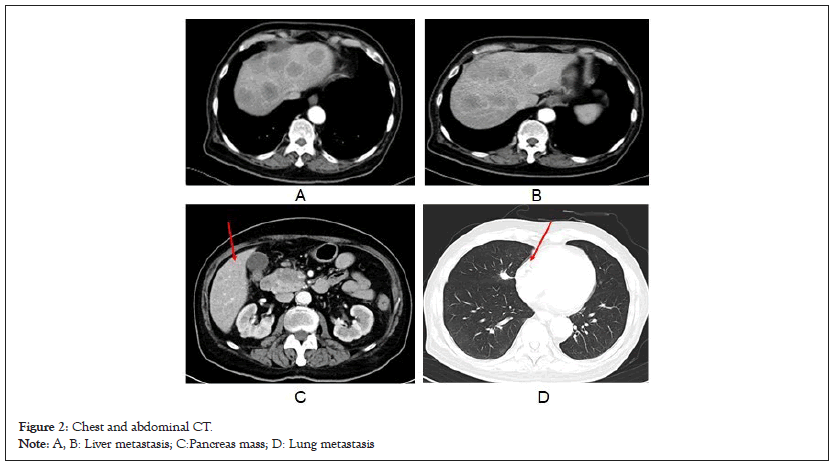
Figure 2: Chest and abdominal CT.
Note: A, B: Liver metastasis; C:Pancreas mass; D: Lung metastasis.
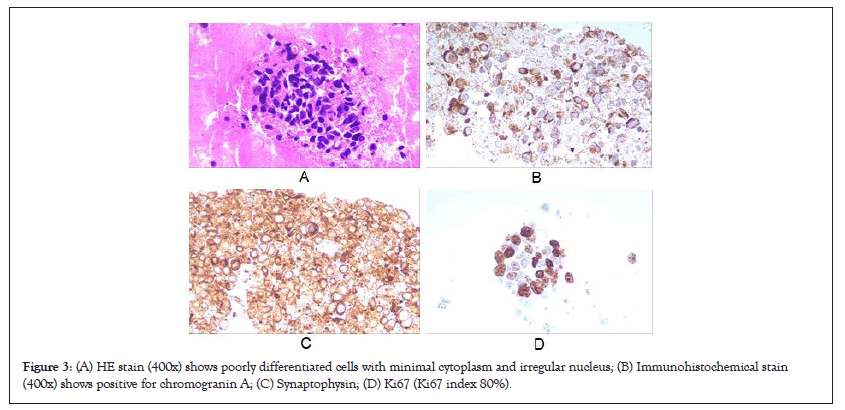
Figure 3: (A) HE stain (400x) shows poorly differentiated cells with minimal cytoplasm and irregular nucleus; (B) Immunohistochemical stain (400x) shows positive for chromogranin A; (C) Synaptophysin; (D) Ki67 (Ki67 index 80%).
On the first week after admission, he had developed fever with 39°C for 3 days. Given the negative blood bacteria culture and improved clinical status by Non-Steroid Anti-Inflammatory Drugs (NSAIDs), no active infection diagnosis could be made. On the fourth week after admission, he acquired a pNEC diagnosis and received chemotherapy (etoposide 160 mg/d, Day 1-3, carboplatin 580 mg, Day 1), then discharged. One month after the first cycle of chemotherapy, he had fever with 40°C, accompanied by severe shock (blood pressure 85/49 mmHg, heart rate 135 bpm) and decreased urine output. Blood routine test showed WBC 33,400/μl, hemoglobin 9.3 g/dl and platelet 6.3 × 104/μl). PCT was more than 100 ng/ml, while inflammatory markers were soaring rapidly (hsCRP 246 ng/ml, ESR 108 mm/h). Blood lactic acid was 2.6 mmol/l. Given highly suspected sepsis shock, experiential antibiotics (meropenem and vancomycin) and vasoactive drugs were used; two sets of blood bacteria and urine culture were repeated simultaneously. Metagenomic Next-Generation Sequencing (mNGS) for peripheral blood sample was adopted to exclude infection as a complement to classic microbe culture. CT in emergency clinic showed the lesions of lung and abdomen remained stable and no abscess was developed. Moreover, microbe screening results were negative and shock was not rectified as common sepsis shock at the first day. Therefore, hydrocortisone (100 mg three times a day) was given against shock at the second day. The temperature came down and the vital sign recovered quickly. After 3 days' resuscitation, we discontinued antibiotics therapy but maintained steroid to relieve the serious inflammation. Inflammatory markers (hsCRP, ESR) were decreased accordingly, while PCT decreased mildly and then rebounded quickly (Figure 1). Although the patient recovered smoothly, he refused further treatment for the malignancy and was transferred to palliative care center.
Results and Discussion
This case report demonstrates a pNEC patient presenting pseudo-sepsis shock with an extremely elevated PCT level. In the present case, the highest point of PCT level appeared in an advanced pNEC patient with liver and lung metastasis. Moreover, the most surprising thing was when pseudo-sepsis shock was relieved by steroid therapy not antibiotics, the unexcepted high PCT level significantly declined. As far as we know, this is the first case present a pNEC with extremely high PCT lever (over 100 ng/ml) which can mimic sepsis shock.
It is well-known that PCT has been widely used as a sensitive and specific biomarker of bacterial infections since 1993 [2]. PCT, a protein making up of 116 amino acids, is recognized as the peptide precursor of calcitonin. Under normal conditions, PCT is mainly produced by thyroid C cells with very low concentration (<0.05 ng/mL) in the circulation. However, during an inflammatory response, in spite of the presence or absence of bacterial infections, diverse tissues and cells can produce and release PCT into the blood [3]. Various organs, consisting of liver, lungs, kidneys, adrenal glands, prostate gland, small intestines and testes as well as peripheral blood mononuclear cells and granulocytes, have been proven to produce PCT [4]. Among them, the liver is still the pivotal source of PCT under different disease conditions [5].
NENs include a broad spectrum of clinical manifestation and can metastasize on diagnosis. Due to substantial clinical features and different response to therapy, research about optimal biomarkers predicting prognosis and metastasis may contribute to understand the physiology of tumor. Currently, Chromogranin A (CgA), serotonin, ProGRP and NSE, are used for NEN’s diagnosis, however the parameters cannot be accurately associated with disease progression [6,7]. In addition, specific biomarkers, such as insulin, Vasoactive Intestine Peptide (VIP) and gastrin, cannot be applied to all types of NENs [6]. Therefore, it is necessary to explore novel biomarkers for NEN diagnosis, prognosis, clinical stage and evaluation of treatment response. In this case, PCT shows its potential role in reflecting advanced stage NEC.
Previously, it had been observed that mildly elevated PCT levels occurred in several non-infectious clinical settings, including uncomplicated live cirrhosis and acute liver failure without infection [3]. Moreover, high elevated PCT levels also have been indicated to be a tumor biomarker for medullary thyroid cancers. However, compared with the thyroidal tumor, extrathyroidal neoplasms were reported to yield lower PCT levels (approximately 1 ng/mL), except the five previous case reports of NENs with an unexpectedly high PCT level (over 100 ng/ml) in the circulation (in Table 1, case 6 is the present case) [8-12]. In Table 1, all cases with extremely elevated PCT (over 100 ng/ml) were all experienced multiple liver metastasis and three cases had also showed pathological evidence of PCT-secreting feature. Once a previous study had revealed that serum PCT could be increased in the NENs of the digestive system, especially in patients with a high tumor burden. The elevated PCT as a promising tumor marker could help evaluate treatment response, predict disease prognosis, and monitor tumor progression [1]. Therefore, we boldly speculated that the abnormal elevation of PCT in these patients may be contributed to the neuroendocrine neoplasms itself and the aggressive liver metastasis. As the main pool of PCT production under disease condition, the Kupffer cells-rich liver occupied by amount of neuroendocrine tumor cells may yield a high level of PCT released to the blood. It is unclear that whether liver involvement would be the leading cause of extremely elevated PCT rather than other organs' metastasis for neuroendocrine neoplasms, and whether high PCT level could be used as a way to predict the occurrence or tumor burden of liver metastasis. Future cautiously planned studies are needed to unveil the underlying relationship between extremely elevated PCT level and neuroendocrine neoplasms (Table 1).
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Reported Year | 2017 | 2017 | 2019 | 2020 | 2020 | 2022 |
| Country | France | Japan | Japan | China | China | China |
| Age (Years) | 54 | 65 | 71 | 78 | 64 | 68 |
| Sex | Male | Female | Male | Male | Female | Male |
| Chronic diseases | N/A | CHB and RA | N/A | N/A | HTN and DM | PMR and CAD |
| Symptom before diagnosis | N/A | Chronic abdominal pain | Refractory diarrhea | Fatigue and fever | Fatigue, decreased appetite and diaphoresis | Abdominal pain and watery diarrhea |
| Tumor type | SCLC with liver metastasis | pNEC with multiple liver metastases | pNEC with multiple liver metastases | primary hepatic NEC | pNET with multiple liver metastases | pNEC with multiple liver and lung metastases |
| Highest PCT elevated time | After 1st etoposide combined with cisplatine | After 1st gemcitabine and nab-paclitaxel 6 weeks | 3 weeks after diagnosis without therapy | At diagnosis | At diagnosis | After 1st etoposide Combined with carboplatine |
| Symptom with highest PCT level | Fever | Fever with epigastralgia and vomiting | Fever | Fever | No fever | Fever with shock |
| Max PCT value (ng/ml) | 137 | 140 | 3783.13 | 100 | >100 | >100 |
| Max calcitonin (pg/ml) | N/A | 6298 | 68670 | N/A | >2000 | N/A |
| hsCRP (mg/dl) | 120 | 13.4 | N/A | N/A | normal | 246 |
| Response to antibiotics | No | No | No | No | N/A | No |
| Further Tumor therapy | Palliative care | Palliative care | N/A | TACE | Systemic chemotherapy and TAE | Palliative care |
| Pathological Evidence proven PCT secreting | No | Yes | Yes | No | Yes | No |
Note: CHB: Chronic Hepatitis B Infection; RA: Rheumatoid Arthritis; PMR: Polymyalgia Rheumatica; HTN: Hypertension; DM: Diabetes; CAD: Coronary Artery Disease; pNEC: Pancreatic Euroendocrine Carcinoma; NET: Neuroendocrine Tumor; SCLC: Small Cell Lung Carcinoma; TACE: Transcatheter Arterial Chemoembolization; TAE: Transcatheter Arterial Embolization; hsCRP: High-Sensitivity C-Reative Protein; PCT: Procalcitonin
Table 1: The neuroendocrine neoplasms with extremely high PCT production.
Fever accompanied with inflammatory response is ubiquitous among the present high PCT-secreting tumor case series. However, shock presentation, especially imitating “sepsis shock”, was quite rare. As far as we know, the present case may be the first case displaying severe fever and shock which had been considered as sepsis shock at the settings of high PCT level (>100 ng/ml). The severe shock showed no response to experimental broad-spectrum antibiotics. The anti-inflammation drugs, such as steroids, can relieve the symptoms (fever and low blood pressure). Unexpectedly, PCT level only decreased mildly for a short time and then bounce back to a high level (89 ng/ml) in spite of stable clinical status. Additionally, the subsequent microbe pathogen screening excluded bacterial infection discreetly. Therefore, it was reasonable to hypothesize that the patients’ shock resulting from high inflammatory status was caused by tumor itself not bacterial infection. Besides that, we also reviewed throughout the course of our patient’s disease. PCT had been obviously elevated (32 ng/ml) at admission and gradually increased to 60 ng/ml at diagnosis, while the patient had not developed severe sepsis or sepsis shock as what we expected in common bacterial infections. According to previous studies, the diagnostic utility of PCT for different infection conditions has been tested widely, with cut-off values ranging from 0.1 ng/ml to 7 ng/ml [13]. Furthermore, the level of PCT may be useful to evaluate the severity of bacterial infection, decreased rapidly after antibiotic therapy and were recognized as a guide to decide the course of therapy for infectious disease [14]. It was quite discordance between the high level of PCT and clinical feature in our case. Although the studies about the value of PCT testing in non-infectious diseases were rare, we should pay attention to some disorders, such as NEN, which could lead to a systemic inflammation resulting in elevated PCT levels but show bad response to antibiotics.
PMR remains a clinical diagnosis without gold standard criteria. A large number of disorders should be excluded, because PMR may be the early manifestation of an underlying cancer which could become clinically evident within a certain timeframe [15]. In the present case, the old man with pNEC experienced abdominal syndrome after two years of PMR. Although some studies found a short-term (less than 6 to 12 months after PMR diagnosis) association between PMR and cancer, in the long term, the definite relationship between PMR and neoplasm was still a puzzle. Furthermore, the relative lack of specific tumor was proven to be secondary to PMR as what pSS had done. It is unclear whether the pNEC of our patients is definitely associated with PMR. Considering PMR per se and its predisposition to elder patients, we should be prudent to exclude tumor at diagnosis and relatively prolong the follow-up period for PMR patients.
Our case report has several limitations. Firstly, immunohistochemical analysis of PCT had not been realized due to the limited sample. Secondly, we ignore the screening of calcitonin level in the circulation. Finally, our patient denied further chemotherapy, which made it impossible to observe the change of PCT level after further anti-tumor treatment.
Conclusion
In conclusion, this presentation showed that the advanced pNEC could cause extremely high level of PCT in circulation, although lack of pathological evidence. It is rare that PCT secreting pNEC imitates sepsis shock, but such complicated clinic condition should be considered when experimental anti-sepsis shock therapy has no effect.
Ethics Statement
Written informed consent to participate in this study was provided by the participants.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
QL was involved in the writing and interpretation of the manuscript.
L-NN was involved in the acquisition of all the clinical data and participated in the revision.
J-CW participated to the analysis of pathological data.
Q-XZ provided the laboratory support and participated in the revision.
WX and B-XM were involved in the revision of the manuscript.
All authors approved the final version of the manuscript.
Funding
This work was supported by grants from National High-Level Hospital Clinical Research Funding (2022-PUMCH-A-059, 2022-PUMCH-A-214) and the Natural Science Foundation of Tibet Autonomous Region (XZ2021ZR-ZY13(Z)).
References
- Chen L, Zhang Y, Lin Y, Deng L, Feng S, Chen M, et al. The role of elevated serum procalcitonin in neuroendocrine neoplasms of digestive system. Clin Biochem. 2017;50(18):982-987.
- Assicot M, Bohuon C, Gendrel D, Raymond J, Carsin H, Guilbaud JJ. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341(8844):515-518.
- Dong R, Wan B, Lin S, Wang M, Huang J, Wu Y, et al. Procalcitonin and liver disease: A literature review. J Clin Transl Hepatol. 2019;7(1):51.
- Meisner M. Update on procalcitonin measurements. Ann Lab Med. 2014;34(4):263.
- Parker GA, Picut CA. Immune functioning in non lymphoid organs: the liver. Toxicol Pathol. 2012;40(2):237-247.
- Oberg K, Modlin IM, De Herder W, Pavel M, Klimstra D, Frilling A, et al. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol. 2015;16(9):435-446.
- Ciobanu OA, Martin S, Fica S. Perspectives on the diagnostic, predictive and prognostic markers of neuroendocrine neoplasms. Exp Ther Med. 2021;22(6):1-4.
- Billy PA, Parmeland L, Brunette S, Lecordier S, Pecquet M. A major procalcitonin elevation without sepsis in a metastatic small cell lung carcinoma. Ann Biol Clin. 2017;75(5):572-575.
- Hagiya H, Matsui T, Kitamura T, Inoue T, Shigekawa M, Yoshida H, et al. Pancreatic neuroendocrine tumor abnormally secreting procalcitonin. Pancreas. 2017;46(1):e7-9.
- Takahashi K, Ozawa E, Nakao K, Aoki S, Takase Y. Hepatobiliary and Pancreatic: A procalcitonin-secreting and calcitonin-secreting pancreatic neuroendocrine carcinoma. J Gastroenterol Hepatol. 2019;34(6):964.
- Han X, Zhong H, Hong D, Li C, Su H, Xu K. Elevated procalcitonin levels in primary hepatic neuroendocrine carcinoma: Case report and literature review. Medicine (Baltimore). 2020;99(31):e21210.
- Wang WZ, Xu LJ, Hu DM, Tang W. Case of an abnormal procalcitonin-producing metastatic pancreatic neuroendocrine tumor. Clin Case Rep. 2020;8(11):2269-2272.
- Hamade B, Huang DT. Procalcitonin: where are we now? Crit Care Clin. 2020;36(1):23-40.
- Gregoriano C, Heilmann E, Molitor A, Schuetz P. Role of procalcitonin use in the management of sepsis. J Thorac Dis. 2020;12:S5-S15.
- Muller S, Hider S, Helliwell T, Partington R, Mallen C. The real evidence for polymyalgia rheumatica as a paraneoplastic syndrome. Reumatismo. 2018;70(1):23-34.
Author Info
Xuzhen Qin1*, Ling Qin2, Ningning Li3, Xiang Wang3, Chunmei Bai3 and Congwei Jia42Department of Infectious Disease, Peking Union Medical College, Beijing, China
3Department of Oncology, Peking Union Medical College, Beijing, China
4Department of Pathology, Peking Union Medical College, Beijing, China
Citation: Qin X, Qin L, Li N, Wang X, Bai C, Jia C (2023) A Pancreatic Neuroendocrine Carcinoma with Super High Procalcitonin Mimicking Sepsis Shock: Case Report and Literature Review. Appli Microbiol Open Access. 9.252.
Received: 28-Mar-2023, Manuscript No. AMOA-23-22614; Editor assigned: 30-Mar-2023, Pre QC No. AMOA-23-22614 (PQ); Reviewed: 14-Apr-2023, QC No. AMOA-23-22614; Revised: 24-Apr-2023, Manuscript No. AMOA-23-22614 (R); Published: 02-May-2023 , DOI: 10.35284/2471-9315.23.9.252
Copyright: © 2023 Qin X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.