Journal of Applied Pharmacy
Open Access
ISSN: 1920-4159
ISSN: 1920-4159
Review Article - (2021)Volume 13, Issue 6
The novel and development of nanotechnology have brought a term targeted drug delivery system. Targeting a molecule to a specific site has brought a certain pharmacological action. Nanosponge is a part of nanotechnology which is both hydrophilic and lipophilic in nature. They are colloidal carriers which improve the aqueous solubility of the poorly soluble drug by enhancing and improving the physical and chemical stability and improving the permeability and bioavailability in the body tissues. Nanosponge are tiny sponges which are in the size of colloidal sizes and nanosized cavity. There general application can be seen in oral administration, parenteral administration, topical administration and available in the form of hydrogel. The current review discussion various methods of synthesis and application of nanosponges.
Nanosponge; Hydrophilic; Lipophilic; Aqueous solubility; Route of administration
A nanosponge synthesis and application can determine the helpful outcome of treatment of several Cancer therapies, Breast cancer, inflammation, Brain tumor, antifungal therapy, and treating poison in blood as adsorbent. Generally, it is difficult to understand the chemistry behind the targeted drug delivery system. Whether how much drug dose to be given and to prevent overdose [1,2]. So a new term "Nanosponge" is introduced where it is the resulting combination of the Polymer and crosslinker. It has a size of about 50 nm or less than (Figure 1).
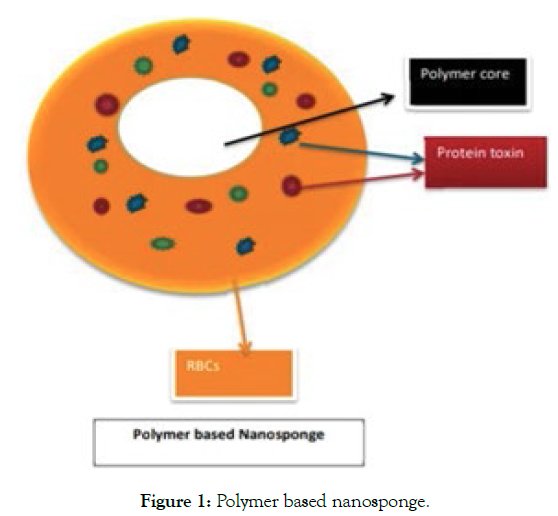
Figure 1: Polymer based nanosponge.
They can withstand a temperature of about 300 degrees high temperature. They encapsulate the molecular drug which is lipophilic in nature by engulfing it within its core when it covers the molecular drug it forms a hydrophilic covering open structure like a membrane [3,4]. For example; Nanosponge likes Sodium alginate Poly-L-Lysine where it encapsulates the antisense oligonucleotides drug molecule within its core and releases the drug for cancer therapy viral infection pathological-disorders. This type of study is used for Pharmacokinetic satisfaction on an in-vitro/in-vivo model called mice. Nanosponges are nano solid in form and can be formulated in the form of oral, parenteral, or inhalational dosage forms (Figure 2 and Table 1).
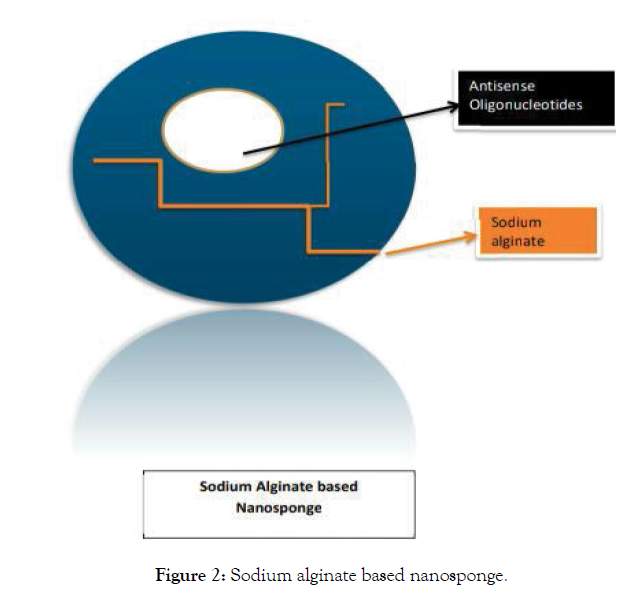
Figure 2: Sodium alginate based nanosponge.
| Name of the Drug | Route of administration | Brand Name | Dosage form | References | |
|---|---|---|---|---|---|
| Iodine | Topical | Mena - Gargel | Solution | [35] | |
| Alprostadil | Intravenous | Prostavastin | Injection | [35] | |
| Dexamethasone | Dermal | Glymesason | Tablet | [35] | |
| Piroxicam | Oral | Brevin | Capsule | [35] | |
Table 1: Some of the marketed nanosponge formulation.
For oral administration, these are prepared in a mixture of excipients which is compressed on either tablet. For Parenteral administration, these are easily mixed with saline and other aqueous solutions. For topical dosage form, nanosponge can be effectively incorporated into the topical hydrogel.
Mechanism of drug release from nanosponge
When the nanosponge-based formulation (Polymer +Crosslinker ) in the encapsulated-based form. These can be either in the form of injection or Cream or lotions applied on the skin. It gets penetrated through the minute skin pore cavities leading to reach the carrier the Blood flowing RBCs which tends to bind it to RBCs cellular component thereby reaching the desired targeting cell by giving almost pharmacological action [5-9] (Figure 3).
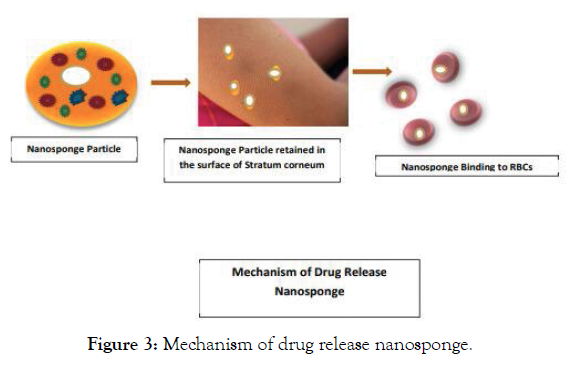
Figure 3: Mechanism of drug release nanosponge.
Advantages
1. The drug encapsulated nanosponge provides a pharmacological action to the targeted site delivery system.
2. It is non-toxic and non-mutagenic.
3. It can reduce the irritation without reducing the efficacy.
4. It can mask the unpleasant flavor of the drug.
5. It tends to improve the stability, flexibility, and elegance of the formulation.
Disadvantages
1. During loading of nanosponge it depends mainly on the degree of crystalline. P-crystalline nanosponge shows different loading capacities [10-12].
2. Since it is tiny molecule chances of dose dumping is possible.
Cyclodextrin-based nanosponges are classified into four different stages depending on their evolution of cyclodextrin nanosponges, chemical composition, and properties (Figure 4).
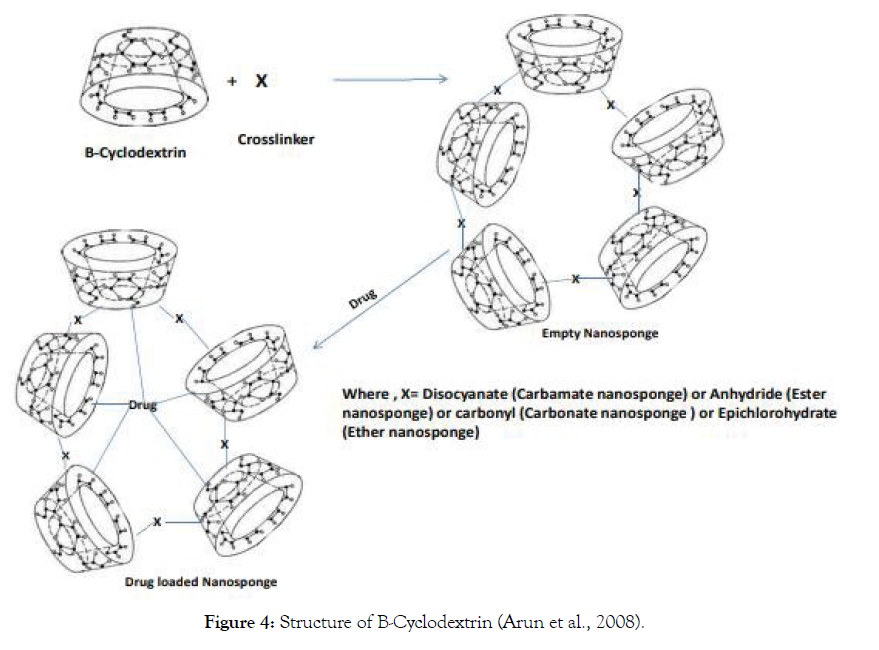
Figure 4: Structure of B-Cyclodextrin (Arun et al., 2008).
Stage 1: Which is also known as the "Plain Nanosponges" includes functional group-based cyclodextrin nanosponges which are connected to crosslinker [13].
(A) Cyclodextrin-based carbamate nanosponges E.g.-: Crosslinkers like Disocyanate encapsulate the drug generally steroids. It is prepared with the help of solventbased synthesis [14].
(B) Cyclodextrin-based Ester nanosponges E.g.: Crosslinkers like Anhydride encapsulates the drug Ibuprofen. It is also prepared with the help of Solvent-based synthesis [15].
(C) Cyclodextrin-based Carbonate nanosponges E.g.: Crosslinkers like Carbonyl encapsulates the drug itraconazole which is prepared with help of either the Solvent extraction method or thermal desorption method [16].
(D) Cyclodextrin-based Ether nanosponges E.g.: Crosslinkers like Epichlorohydrate encapsulate the drug captopril which is prepared by the method Solvent-based synthesis [17].
Stage 2: Which is also known as the "Modified nanosponges" are categorized on the basis of specific properties. Such as fluorescence and electric charge Eg: Crosslinker like carboxylated nanosponges encapsulates the drug Antiviral agent Acyclovir [18].
Stage 3: Which is also known as the "stimuli-responsive nanosponges". They are categorized by external changes in the environment such as temperature, Redox condition, and pH Eg: Glutathione bioresponsive cyclodextrin nanosponges [19,20].
Stage 4: This is also known as the "Molecularly imprinted nanosponges" which is categorized on the basis of the high selectivity of specific molecules. Eg: Biomimetic estimation of glucose using molecular and molecular imprinted polymer nanosponges [21].
Techniques involved in the synthesis process for nanosponges
(I) Melt-based synthesis: This type of technique involves melting up of a combination. Crosslinking agents with cyclodextrin where it's mixed at a maximum speed with the help of a magnetic stirrer at a very high temperature where the fusion of both cyclodextrin and the crosslinking agent takes place. This technique is also called as fusion method. The mixture is left for cooling and washing is done to remove the unreacted substrates [22].
(II) Solvent-based synthesis: This technique involves only solvents, where a polar solvent like Dimethylsulfoxide or dimethylformamide (DMSO/DMF). Finally, crosslinker like carbonyl-based nanosponges is added in the ratio of 1:4. The reaction was maintained at a temperature of 10 degrees celsius for refluxing time for about 1-48hr. Once the solution temperature is settled down to 30-degree celsius and the demineralized water is added to remove the unreacted product and thereby product is filtered under vacuum. The final product is purified with the help of the soxhlet apparatus by the addition of ethanol to it. Later after a certain longer period of time of purification. The final product is subjected to drying [23-25].
(III) Microwave-assisted synthesis: It is the simplest method compared to melt-based synthesis. where this technique exhibits the four-time reduction in the reaction time. It makes the nanosponges property a higher degree of crystallization and shows homogenous distribution with the polymer.
(IV) Ultrasound-assisted synthesis: This technique involves Esterbased cyclodextrin and Diphenyl carbonate are taken together and put in a vial. Then the vial is kept in an ultrasound bath consisting of water in it and heated at a temperature of 90 degrees celsius and sonicated for about 5 hr. Further purification and crystallization are done by washing it with Demineralized water to remove the unreacted product. The final product is filtered with the help of a vacuum and it is further purified and dried with the help of soxhlet apparatus consisting of ethanol [24].
Applications of nanosponges:
(i) Molecular drug targeting: In one of the articles it is claimed that the GSH-responsive cyclodextrin-based nanosponge encapsulated the drug doxorubicin showed therapeutic action and efficacy in human Hep G2 cells (in-vitro) and organotypic cultures of rat precision-cut liver slices (PCLs, ex-vivo) [26].
(ii) As toxin therapy: Polymeric nanosponge tends to absorb the membrane damaging toxins which leads to a diversion away from cellular targets (In-vivo study) in mouse, the nanosponge tends to heal the toxicity of staphylococcal alpha-hemolysin (α-toxin) which can increase the survival capacity of the toxin of mice [27].
(iii) As dermal penetration: In the case of skin absorption cyclodextrin is crosslinked with the pyromellitic dianhydride which tends to give a semisolid formulation that is applied on the skin. Diclofenac is an anti-inflammatory drug which is typically formulated as an ointment form because of its composition. It is able to penetrate the dermal skin. This cyclodextrin nanosponge doesn’t affect during penetration of diclofenac into the skin. But diclofenac dose can be controlled by the addition of pyromellitic dianhydride [28].
(iv) As an anticancer therapy: A major challenging nowadays drug. which are used in the treatment of cancer because of their low solubility and low permeability one such example is paclitaxel anticancer drug which belongs to BCS IV(Biopharmaceutical classification system) where carbonyl diimidazole based cyclodextrin nanosponge encapsulate the drug paclitaxel and take it to the desired targeted cell to show its pharmacological action [29].
(v) For Raman spectroscopy: In recent study which were introduced by Occon optics. The newly equipped RAMSERS- SP surface enhanced Raman spectroscopy. Subtrates tend to use a proprietary gold-silver nanosponge alloy to produce highly sensitive tracing capacity of Raman spectroscopy which in turn helps in detection of explosives, food safety, narcotics and biological research. Here, silver only SERS substrates work with best with 532 nm gold. Substrates are better suited to 785 nm. Both silver gold only SERS substrates combined work with 638 nm Raman excitation [30].
(vi) Oxygen delivery system: Three types of Cyclodextrin nanosponges were prepared by crosslinking. α, β or γ cyclodextrin with carbonyl diimidazole. In this type of study cyclodextrin based nanosponge formulation were developed as oxygen delivery system. For this purpose three type of cyclodextrin based nanosponge were suspended in water and were saturated with oxygen and in-vitro characterized. The efficacy was tested on vero cells. This is to check the capacity or ability to release oxygen in presence or in the absence of ultrasound was determined overtime. Cyclodextrin nanosponge or hydrogel combination systems were used for oxygen permeation through silicone membrane [31].
(vii) Inhibits SARS-CoV-2 infectivity: In one of the research article, it was found that the two types of cellular nanosponges which are derived from plasma membrane from human lung epithelial type II cells or human macrophages. These nanosponges show some protein receptors, both are identified and unidentified, required by SARS-CoV-2 for cellular entry. Then there is the incubation of nanosponges with SARS-CoV-2 takes place which gets neutralized and was unable to infect cells. The nanosponge platform is agonistic to viral mutations. The information on the target of the virus remains the identified host cell; the nanosponge will be able to neutralize the virus [32].
(viii) Removal of neonicotinoid dinotefuran from waste water: The β-cyclodextrin-based carbonate nansponges are compiled with the superparamagnetic ferrosoferric oxide nanoparticle. In one of the article it was claimed that the potential removal of Dinotefuran from waste water. The nanosponges – Dinotefuran inclusion were evaluated by Transmission electron microscopy, Energy dispersive spectroscopy, UV-visible spectroscopy, scanning electron - microscopy, Thermogravimetric analysis, X-ray Powder diffraction and Proton nuclear magnetic resonance [33].
(ix) Property of having a protective agent from light: In one of the article it was encapsulation of Gamma-oryzanol showed a good protection from photodegradation. Gammaoryzanol. This is a ferulic acid ester mixture. Which is an antioxidant are used for stabilizing food and Pharmaceutical raw material [34-36].
In this type of review study, it was found that a nanotechnology type of cyclodextrin-based nanosponge had Pros and cons in the formulation. Accordingly, it was classified as plain nanosponge, modified nanosponge, stimuli-responsive nanosponge, and molecular imprinted nanosponges. Based on it nanosponges technique were used for the synthesis process of nanosponges such as melt- based synthesis, solvent based synthesis, Microwave-assisted synthesis, and ultrasound - assisted synthesis, and useful application of nanosponge in targeted drug delivery system was described in molecular drug targeting, toxin therapy, dermal penetration and as an anticancer therapy.
Citation: Behura PR, Vamshi Krishna T (2021) A Novel Revolutionary Approach of a Synthesis and Application of Targeted Nanosponge Drug Delivery. J App Pharm. 6:297.
Received: 05-May-2021 Accepted: 20-May-2021 Published: 27-May-2021 , DOI: 10.35248/1920-4159.21.13.297
Copyright: © 2021 Behura PR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.