Journal of Fertilization: In Vitro - IVF-Worldwide, Reproductive Medicine, Genetics & Stem Cell Biol
Open Access
ISSN: 2375-4508
ISSN: 2375-4508
Research Article - (2022)Volume 10, Issue 3
Objective: We assessed the effectiveness of a clinical decision support tool to reduce follicle-stimulating hormone dosage and eliminate the need for an ultrasound after day 5 of an individual superovulation cycle.
Design: Test participants undergoing superovulation were compared with retrospective control subjects.
Subjects: 22 Test and 22 control participants included normal and poor responders and patients with the polycystic ovarian syndrome.
Intervention: A clinical decision support tool was used to forecast stimulatory hormone dosing for an individual cycle based on follicle size distribution on day 1 and day 5.
Main outcome measures: Cumulative stimulatory hormone doses, number of oocytes, numbers of MII oocytes, and high-quality embryos obtained during the cycle.
Results: Test participants required significantly lower cumulative FSH doses during superovulation cycles (average 1856 IU test, 2760 IU control, p<0.001), with significantly higher numbers of high-quality embryos (average 5.7 test, 2.1 control, p=0.0003). Test participants had higher numbers of MII follicles, although the difference was not statistically significant (average 9.7 test, 7.9 control, p=0.271).
Conclusion: The use of the clinical decision support tool eliminated the need for ultrasound exams after day 5, reduced the doses of stimulatory hormone required, and yielded significantly higher numbers of high-quality embryos.
Clinical decision support tool; Superovulation; Optimal control; Opt-IVF
Infertility is a disease affecting more than 48.5 million couples and 150 million individuals globally [1]. Primary infertility is the inability to conceive a first live birth, and secondary infertility is the inability to conceive after a prior live birth. In vitro Fertilization (IVF) is one of the most frequently adopted fertility treatments worldwide. IVF involves four basic steps: Superovulation, oocyte retrieval, insemination/fertilization, and embryo transfer. In both rich and poor countries, IVF remains expensive, with out-of-pocket estimates per cycle of around $10,000-$30,000. The high cost of IVF is a result of expensive drugs, fixed costs for infrastructure, extensive testing required, and labor costs for physicians and other healthcare personnel. Superovulation accounts for the major share of these costs as compared to the other three steps.
Current approaches to superovulation involve almost daily monitoring of follicle development using ultrasound and/or blood tests. The daily dosage of stimulatory hormones is then prescribed by physicians based on empirical data and clinical experience. Factors impacting this decision include age, BMI, anamnesis, day 3 serum FSH, Antral Follicle Count (AFC), and Anti-Mullerian Hormone (AMH) levels [2-7]. Initial doses typically start at 150 or 225 IU of Follicle-Stimulating Hormone (FSH) daily, increasing later to 150 to 300 IU daily for younger patients and up to 450 IU daily for older or less responsive patients. Devroey, et al. have shown that even low initial doses of FSH (100 IU) allowed recovery of high numbers of oocytes in relatively young patients [3]. Higher hormone doses are associated with a higher risk of Ovarian Hyper Stimulation Syndrome (OHSS), which occurs in 1%-2% of women undergoing IVF [8]. OHSS is of particular concern in women with the polycystic ovarian syndrome (PCOS).
We have previously described a mathematical modeling and computerized algorithmic approach to generating personalized hormone dosing for augmented superovulation for both agonist protocols and antagonist protocols [9-11] and have created a decision support tool (Opt-IVF) based on this framework. This approach was validated using data from 150 patients retrospectively. Details of the mathematical modeling, algorithms, and validation have been previously published [9-11]. Briefly, follicular size and distribution on days one and five of the superovulation cycle and the FSH doses administered on days one to four are the input data used by Opt-IVF to create a real-time follicle development model for that individual superovulation cycle, and optimal control theory then applied to forecast the hormone doses from day 5 to the trigger day to maximize the number of mature follicles. The Opt-IVF algorithm can also forecast the best time to begin antagonists, each day's estrogen levels, and the optimal trigger day to maximize the recovery of mature oocytes. We report here on a clinical trial using Opt-IVF to guide superovulation.
All clinical work was conducted at the Akanksha Hospital and Research Institute in Gujarat, India. The institutional review board at Sat Kaival Hospital Pvt. Ltd. Ethics Committee, Gujarat, India, approved the protocol and consent forms. All participants provided written informed consent. Patient safety was reviewed throughout the study by the clinical investigators (Nayana Patel, Niket Patel, M. Patel, H. Bhadarka, P. Ghoghari, H. Thakkar, R. Ainani), who were responsible for clinical work and data collection. U. Diwekar and S. Joag are responsible for study design, Opt-IVF runs, data analysis, and interpretation and writing of this manuscript. All authors contributed to the review and editing of the manuscript and approved the final version for submission. All authors vouch for the accuracy and completeness of the data and the fidelity of the trial to the protocol.
Opt-IVF is a decision support tool based on the earlier work of modeling and optimization of Dr. Diwekar’s group, as stated earlier. Opt-IVF uses follicular distribution data from day 1 and day 5 to obtain personalized parameters for the patient (modeling loop) and then calculates the rest of the dosage profile based on this personalized model using optimal control (optimal control (red) loop) as shown in Figure 1. Opt-IVF can provide an initial dose based on the nomogram proposed by La Marca and Sunkara (2013) [2], which predicts the initial dose based on age, day 3 serum FSH, and AMH or based on heuristics using age, AMH, and AFC values. For the rest of the cycle, it uses follicular distribution data (Figure 2). Figure 3 shows the output obtained from Opt-IVF, as you can see that the optimal FSH (this is a combined dose of FSH+hMG) dosage predicted by Opt-IVF tapers off as the cycle proceeds. This allows for reducing variance in follicular size on the day of retrieval.
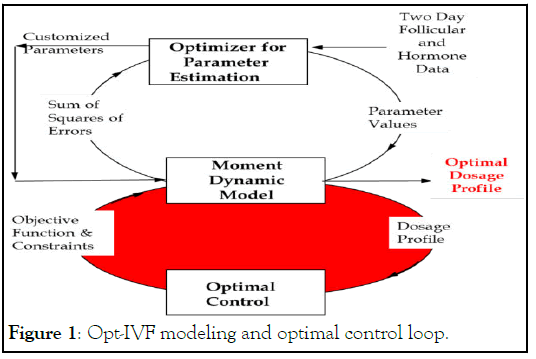
Figure 1: Opt-IVF modeling and optimal control loop.
Figure 2: Dosage profile calculation for the rest of cycle (Inputs).
Figure 3: Opt-IVF output flow.
The trial was registered on ClinicalTrials.gov (ID NCT05377879). The test participants (for whom Opt-IVF dosages are used) were enrolled prospectively, with very recent retrospective controls matched for age and previous IVF history, and with equivalent proportions of patients with PCOS. An antagonist protocol was used in all cases. Antagonist protocol started for all patients with Recombinant FSH (Gonal-F, Merck), R-Hu-LH (Merck), and Cetrorelix 0.25 mg (Bharat Serum and Vaccines Limited). Participants were categorized as expected poor responders, expected normal responders, and PCOS based on age, AMH, and clinical presentation. Initial FSH doses were based on clinical investigators' choice (Initial dose recommendations by Opt-IVF were made available to the clinical investigator). Test participants had an ultrasound (US) to quantitate the number and size of follicles present on day 1 and day 5 of the cycle, and this information was entered into the Opt-IVF decision support tool, which suggested hormone doses for day 5 and all subsequent days of the cycle. All recommendations of the decision support tool were made available to the clinical investigators on day 5. Control participants underwent superovulation cycles in the first half of 2022. Control participants had US on days 1, 5, 7, and 9. The participating clinical investigators retained the ability to override Opt-IVF recommendations based on their clinical judgment. Antagonist initiation day, antagonist dosage, and trigger day were based on the participating clinical investigators' choice. The timing of antagonist initiation and antagonist doses were similar in both groups. There were no differences in the dosing of trigger hormones. MII oocytes were matured with the presence of one polar body.
We measured the number of mature oocytes, MII oocytes retrieved, and the number of high-quality embryos obtained after in vitro fertilization in each cycle. Good quality embryos match with the Recent Gardner Grading. Morphologically Normal with No Fragmentation is known to be good quality. Statistical analyses were performed using Microsoft Excel.
Test and control participant groups were similar in age and the proportions of expected normal responders, poor responders, and participants with PCOS (Table 1).
| Test group | Control group | |
|---|---|---|
| Number of Participants | 22 | 22 |
| Age, Years ± SD | 30.6 ± 4.4 | 32.7 ± 3.5 |
| Expected Normal responders | 8 | 10 |
| Expected Poor responders | 4 | 3 |
| Polycystic Ovarian Syndrome | 10 | 9 |
Table 1: Participant characteristics.
The cumulative total FSH doses administered to test participants were lower than the doses administered to control participants, as shown in Table 2. The differences in dosage were statistically significant (p<0.001) for normal responders, poor responders, and PCOS participants. On average, the reduction in dosage with Opt-IVF was 32%. Although clinical investigators had the discretion to override Opt-IVF recommended doses, they did not do so on any day for any participant. None of the test participants needed ultrasounds after day 5, while all controls required ultrasounds on days 7 and 9.
| Responder category | Participants | Total FSH dose (IU) | Oocytes retrieved | MII oocytes retrieved | High-quality embryos |
|---|---|---|---|---|---|
| Normal test Normal control Probability |
8 | 1856 | 9.4 | 7.6 | 4.5 |
| 10 | 2760 | 8.1 | 6.6 | 1.7 | |
| <0.001 | 0.557 | 0.592 | 0.01 | ||
| Poor test Poor control Probability |
4 | 1931 | 5.3 | 4 | 3.25 |
| 3 | 3000 | 6.3 | 3.3 | 1.67 | |
| <0.001 | 0.727 | 0.779 | 0.284 | ||
| PCOS test PCOS control Probability |
10 | 1901 | 18.9 | 13.7 | 7 |
| 9 | 2667 | 12.8 | 10.9 | 3 | |
| <0.001 | 0.069 | 0.251 | 0.034 | ||
| Total test Total control Probability |
22 | 1890 | 13 | 9.7 | 5.7 |
| 22 | 2755 | 9.8 | 7.9 | 2.1 | |
| <0.001 | 0.141 | 0.271 | 0.0003 |
Table 2: Stimulation cycle FSH doses and outcomes in test and control participants.
The number of mature oocytes retrieved and the number of MII oocytes were similar among test participants and controls overall and in each subgroup (Table 2). The number of high-quality embryos was significantly higher in test participants than in controls overall (p<0.01). A similar difference was also found in PCOS and normal participants on subgroup analysis (p<0.05). Most of the embryos obtained in the test cases were Grade A embryos.
This trial was designed as a non-inferiority trial to test the proposition that using Opt-IVF, a clinical decision support tool, to guide superovulation would allow for a reduction in US testing and a reduction in hormone dosage, with at least comparable outcomes in terms of total oocyte retrieval, MII oocyte retrieval, and high-quality embryos obtained. Using a personalized model and optimal control theory to guide hormone dosing during an individual stimulation cycle represents a new approach in the field of in vitro fertility.
Our data show that utilizing the Opt-IVF clinical decision support tool eliminated the need for transvaginal US exams after day 5. Control participants required, on average, at least 3 ultrasound exams after day 5 of a superovulation cycle. Following the standard practice in resource-poor settings, blood testing of estradiol levels to help guide FSH dosing was not used in either test or control participants. Similarly, superovulation cycles in test participants required significantly lower FSH doses in normal responders, poor responders, and PCOS participants. Reduction in US testing and doses of hormones needed for superovulation is likely to significantly reduce the overall costs, which is particularly valuable in resource-poor settings. None of the participants in either arm of the study developed OHSS or any other significant side effects of hormone therapy.
An unexpected finding was that the test participants had significantly higher numbers of high-quality embryos (p<0.01). Notably, PCOS and normal participants had higher numbers of high-quality embryos (p<0.05), even though the test and control categories had only 10 and 9 participants in PCOS and 8 and 10 participants in normal responder subgroups, respectively. Although higher numbers of high-quality embryos were also noted in test participants categorized as poor responders, the differences from the control group were not statistically significant. The numbers of mature oocytes and MII oocytes were higher in test participants as compared with controls, although the differences were not statistically significant. Additional trials will be required to clarify whether using Opt- IVF can result in better outcomes on this measure. However, our results demonstrate that using a clinical decision support tool results in at least equivalent outcomes compared to standard protocols on all outcome measures. Furthermore, outcomes obtained in the control group are consistent with outcomes in the published literature [12].
We did not measure estrogen levels in either test or control participants. The Opt-IVF optimization algorithm allows the incorporation of estrogen levels in addition to follicle numbers on days 1 and 5 to forecast optimal doses. Future clinical trials will be needed to test whether incorporating information on estrogen levels will be superior to using only follicle number data, as in this study.
We have shown that using a clinical decision support tool, Opt- IVF, to guide stimulatory hormone dosing during superovulation allows using lower overall hormone doses and eliminates the need for ultrasound testing after day 5 of the superovulation cycle. This approach yielded similar numbers of mature oocytes and MII oocytes and higher numbers of highquality embryos in test participants compared with retrospective controls.
ClinicalTrials.gov (ID NCT05377879).
Urmila Diwekar and Sanjay Joag have ownership interests in Stochastic Research Technologies LLC, which owns and markets Opt-IVF. Nayana Patel, Niket Patel, Molina Patel, Harsha Bhadarka, Paresh Ghoghari, Harmi Thakkar, and Richa Ainani have no disclosures.
Attestation statement
• The subjects in this trial have not concomitantly been involved in other randomized trials.
• Data regarding any of the subjects in the study has not been previously published unless specified.
• Data will be made available to the editors of the journal for review or query upon request.
Data sharing statement
Data on individual patients will be shared in spreadsheet format upon request to the corresponding author.
Citation: Diwekar U, Patel N, Patel N, Patel M, Bhadarka H, Ghoghari P, et al.(2022). A Non-Randomized Clinical Trial of a Decision Support Tool to Optimize Superovulation Cycles in Individual Patients. J Fertil In vitro IVF World w Reprod Med Genet Stem Cell Biol.
Received: 02-Aug-2022, Manuscript No. JFIV-22-18653; Editor assigned: 08-Aug-2022, Pre QC No. JFIV-22-18653 (PQ); Reviewed: 26-Aug-2022, QC No. JFIV-22-18653; Revised: 06-Sep-2022, Manuscript No. JFIV-22-18653 (R); Published: 15-Sep-2022 , DOI: 10.35248/2375-4508.22.10:268
Copyright: © 2022 Diwekar U, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.